Abstract
Rationale: Exposure to air pollution during intrauterine development and through childhood may have lasting effects on respiratory health.
Objectives: To investigate lung function at ages 8 and 15 years in relation to air pollution exposures during pregnancy, infancy, and childhood in a UK population–based birth cohort.
Methods: Individual exposures to source-specific particulate matter ≤10 μm in aerodynamic diameter (PM10) during each trimester, 0–6 months, 7–12 months (1990–1993), and up to age 15 years (1991–2008) were examined in relation to FEV1% predicted and FVC% predicted at ages 8 (n = 5,276) and 15 (n = 3,446) years using linear regression models adjusted for potential confounders. A profile regression model was used to identify sensitive time periods.
Measurements and Main Results: We did not find clear evidence of a sensitive exposure period for PM10 from road traffic. At age 8 years, 1 μg/m3 higher exposure during the first trimester was associated with lower FEV1% predicted (−0.826; 95% confidence interval [CI], −1.357 to −0.296) and FVC% predicted (−0.817; 95% CI, −1.357 to −0.276), but similar associations were seen for exposures for other trimesters, 0–6 months, 7–12 months, and 0–7 years. Associations were stronger among boys, as well as children whose mother had a lower education level or smoked during pregnancy. For PM10 from all sources, the third trimester was associated with lower FVC% predicted (−1.312; 95% CI, −2.100 to −0.525). At age 15 years, no adverse associations with lung function were seen.
Conclusions: Exposure to road-traffic PM10 during pregnancy may result in small but significant reductions in lung function at age 8 years.
Keywords: ALSPAC, children, traffic, air pollution, respiratory health
At a Glance Commentary
Scientific Knowledge on the Subject
To date, few studies in children between the ages of 4 and 11 years have investigated the effects of air pollution exposure in each trimester on lung function, and these studies had sample sizes ranging from 171 to 788 individuals. Some studies suggested that exposure to traffic-related air pollutants, particularly during the second trimester, was associated with reduced lung function, although correlations between trimester-specific traffic-related air pollutants in each study were high. Evidence was inconsistent regarding the relative importance of early-life or more recent air pollution exposure in childhood lung function levels. Also, some studies suggested that the negative associations between early-life air pollution exposure and childhood lung function diminished or were no longer observed by adolescence.
What This Study Adds to the Field
To date, this is the largest study to investigate source-specific particulates in each pregnancy trimester, as well as in infancy and childhood, to identify potential periods of susceptibility during lung development and growth. We found that particulate exposures in each time period in pregnancy and early life were associated with reduced lung function at age 8 years. The third trimester may be a susceptible period for particulate matter ≤10 μm in aerodynamic diameter from all sources, but for particulate matter ≤10 μm in aerodynamic diameter from road traffic, no especially susceptible periods could be identified.
There is a growing awareness that early-life exposures to air pollution potentially have detrimental effects on the future respiratory health of both children and adults, with impacts on lung function trajectories throughout life (1, 2). In the developing fetus, the bronchial tree is formed by 16 weeks of gestation and alveolarization begins at approximately 28 weeks, and by age 2 years, almost the final adult number of alveoli have been formed, although the development process may continue throughout adolescence to early adulthood (3). It is biologically plausible that exposure to air pollution in early life could lead to impaired lung function growth and reduced maximal levels of FEV1 attained in early adulthood, the latter of which is associated with long-term respiratory morbidity even with physiological levels of subsequent decline (4).
There is limited information about whether exposure to air pollution during periods of rapid pulmonary development in pregnancy and infancy is related to long-term lung function independently of exposures throughout an individual’s whole life. Most previous studies have reported negative associations between air pollution exposures after birth at various life stages and childhood lung function (5–19), although which time period of exposure (early-life vs. most recent) has the strongest associations is debated. Notably, relatively few studies to date have specifically investigated prenatal air pollution exposures (20–24), and these studies have reported mixed findings in terms of susceptibility periods.
To address these gaps in knowledge, we used a large population-based birth cohort, the ALSPAC (Avon Longitudinal Study of Parents and Children) cohort (25, 26), to investigate lung function at ages 8 and 15 years in relation to modeled source-specific and all-sources air pollution exposures at residential addresses during each trimester of pregnancy, whole pregnancy, early infancy, late infancy, and childhood. Some of the results of this study were previously presented at the International Society of Environmental Epidemiology 2018 annual meeting (27).
Methods
Study Participants
Pregnant women residing in the former administrative county of Avon in southwest England, whose estimated delivery date fell between April 1, 1991 and December 31, 1992 were recruited before they gave birth, resulting in a cohort of 14,541 pregnancies (25, 26), with 13,963 children eligible for this study (Figure E1 in the online supplement). The cohort has been followed with longitudinal assessments of exposures and outcomes from pregnancy to adulthood via questionnaires and clinic visits (25). Ethical approval was obtained from the ALSPAC Ethics and Law Committee and local research ethics committees. Informed consent for the use of data collected via questionnaires and clinics was obtained from participants in accordance with the recommendations of the ALSPAC Ethics and Law Committee at the time of the study. The study website contains details regarding all of the data, which are available through a fully searchable data dictionary and variable search tool (http://www.bristol.ac.uk/alspac/researchers/our-data/).
Data Sources and Measurements
Lung function was measured by spirometry (Vitalograph 2120; Vitalograph) at age 8 years (median, 8.6 yr; range, 7.5–10.5 yr) and 15 years (median, 15.4 yr; range, 14.2–17.7 yr) according to American Thoracic Society standards (28). Both pre- and postbronchodilation measures at age 15 years were obtained; however, only prebronchodilation measures were available for age 8 years. At both ages, children who had oral steroids or chest infection in the past 3 weeks, or used either a short-acting bronchodilator in the past 6 hours or a long-acting bronchodilator in the 24 hours before the lung function tests were excluded from the analysis (Figure E1). Using measures of postbronchodilation at age 15 years and prebronchodilation at age 8 years, we derived values for FEV1% predicted, FVC% predicted, and FEV1/FVC ratio for each age using the Global Lung Function Initiative (GLI) equations (https://www.ers-education.org/guidelines/global-lung-function-initiative/spirometry-tools.aspx; accessed February 2020). We also calculated the rate of lung function growth from age 8 to 15 years (prebronchodilation measures at 15 yr − prebronchodilation measures at 8 yr / time of follow-up in yr) (29).
Sex and gestational age (categorized as preterm [<37 wk] and nonpreterm [≥37 wk]) were obtained from birth records. Questionnaires completed by the mothers provided information about maternal education level (low: educated at school for ≤16 yr; high: educated for >16 yr), maternal smoking in the first 3 months of pregnancy (yes/no), home ownership (rented or owned/mortgaged) and presence of damp and mold in the home (yes/no) when the study child was 8 months old, whether the study child was ever exposed to passive smoking up to age 6.5 years (yes/no), and whether the study child ever had breastfeeding (yes/no). Current asthma was defined at ages 8 and 15 years as a reported doctor’s diagnosis of asthma and reported wheezing, or use of asthma medication in the previous 12 months.
A full residential address history was constructed through the Algorithm for Generating Address-history and Exposures (ALGAE; https://smallareahealthstatisticsunit.github.io/algae/index.htmll accessed February 2020) and was geocoded for each trimester (T1, T2, and T3), early infancy (0–6 mo), late infancy (7–12 mo), and every year up to age 15 years (30). For geocoding, individuals for each exposure period must have had a valid address for at least 90% of days in trimesters and infancy, and at least 75% of days within each year of life up to age 15 years; thus, different numbers of individuals were retained for each exposure period. We used dispersion modeling to estimate residential air pollution exposures to particulate matter (PM) from different sources during each trimester and infancy (1990–1993) through childhood to adolescence up to age 15 years (1991–2008), taking account of residential mobility (31). Modeled daily estimates of PM ≤10 μm in aerodynamic diameter (PM10) from local major road traffic (hereafter, PM10_road) were based on information about traffic flows, composition, speed, tailpipe and nontailpipe emission rates, road widths, building heights for street canyons, and meteorological variables. PM10 emissions on a 1 × 1 km grid from other local combined sources, such as minor roads and industrial or domestic sources (hereafter, PM10_other), were obtained by subtracting summed emission rates from main roads from total PM10 emissions using the National Atmospheric Emissions Inventory (http://naei.beis.gov.uk/data/data-selector; accessed February 2020). Total PM10 (hereafter, PM10_total) was a combination of PM10_road, PM10_other, anthropogenic sources outside the study area and secondary formation of PM from the UK and western Europe from the Met Office’s Numerical Atmospheric dispersion Modeling Environment (NAME) III model (32), and a constant to represent local nonanthropogenic sources (e.g., wind-blown soil and dust). Each source-specific PM10 estimate was averaged for each trimester (T1, T2, and T3), whole pregnancy, and infancy period (0–6 mo and 7–12 mo) for each study child. In addition, for each child, annual average source-specific PM10 concentrations (PM10_road and PM10_other) and total sources (PM10_road, PM10_other, and anthropogenic or nonanthropogenic background concentrations) were estimated every year after birth up to age 15 years.
Statistical Analyses
Average daily PM10 exposures for each trimester, whole pregnancy, 0–6 months, and 7–12 months, and annual PM10 exposures for ages 0–7, 8–15, and 0–15 years were analyzed as continuous measures. Linear regression models were fitted to assess associations with values of lung function (FEV1% predicted, FVC% predicted, and FEV1/FVC% predicted) at ages 8 and 15 years. Effect estimates were presented as per 10 μg/m3 increment in PM10_total exposure and per 1 μg/m3 increment in PM10_road and PM10_other. All analyses were undertaken using Stata v12.1.
The main model was adjusted a priori for sex, gestational age, maternal education, home ownership, maternal smoking during pregnancy, passive smoking in childhood, presence of damp and mold in the home, and season of the clinic visit. To correct for multiple testing, we used the Simes procedure and generated q values using the “qqvalue” package in Stata.
Sensitivity analyses were conducted by adding each of the following variables into the main model: current asthma, current body mass index, ever had breastfeeding, and small for gestational age (i.e., birth weight <10th percentile for gestational age vs. ≥10th percentile).
Potential effect modifications by child’s sex, maternal education, and maternal smoking were examined by adding into the models an interaction term between PM10 exposure and each of these variables.
Missing data regarding PM10 exposures, covariates, and outcomes in the main model were imputed by using multiple imputations. Ten new datasets were created by means of imputation, resulting in a complete sample size of 13,963 for each dataset. All datasets were analyzed separately, after which the results were combined using Rubin’s rule (33). A complete-case analysis was conducted in which children were only included if information on PM10 exposures, covariates, and outcomes at both ages 8 and 15 years was available.
Spearman correlations between PM10 measures for different time periods were calculated. To investigate the independent effects of PM10_total exposure in each time period on childhood lung function, exposures from different time periods (i.e., trimesters and infancy, and trimesters and childhood) were mutually adjusted in the main model, for which potential multicollinearity was assessed by means of the variance inflation factor. Because high correlations were seen between PM10_road for different time periods, a Bayesian profile regression model (online supplement) was fitted (34) via the R package PReMiuM (35), which enables assessment of potentially collinear variables and an outcome through cluster membership.
Lung function growth rates (L/yr) from ages 8 to 15 years in relation to air pollution exposure were examined using linear regression by additionally adjusting for age and height at 15 years in the main model.
Results
Among the 13,963 children, lung function measures (FEV1% predicted, FVC% predicted, and FEV1%/FVC% predicted ratio) were calculated for 5,276 and 3,446 children at age 8 and 15 years, respectively (Table 1). The mean PM10_total exposures were 33.45 μg/m3 in the first trimester and declined in each subsequent period to 30.96 μg/m3 by 7–12 months of age (Table 2). The annual average PM10_total exposure for 0–7 years and 8–15 years was 32.99 μg/m3 and 22.98 μg/m3, respectively. The average PM exposures in each time period by child’s sex, maternal education, and maternal smoking are presented in Table E1.
Table 1.
Summary Statistics of the Study Population
| Entire Cohort (N = 13,963) |
Age 8 Years (n = 5,276*) |
Age 15 Years (n = 3,446*) |
||||
|---|---|---|---|---|---|---|
| Characteristic | N | Percentage | n | Percentage or Mean (SD) | n | Percentage or Mean (SD) |
| Sex, M | 7,213/13,963 | 52% | 2,664/5,276 | 50% | 1,682/3,446 | 49% |
| Preterm birth, <37 wk | 844/13,963 | 6% | 305/5,276 | 6% | 173/3,446 | 5% |
| Lower maternal education | 8,017/12,403 | 65% | 2,843/5,082 | 56% | 1,802/3,348 | 54% |
| Maternal smoking in pregnancy | 3,293/13,144 | 25% | 944/5,174 | 18% | 531/3,385 | 16% |
| Rented a home in the first 8 mo | 2,290/10,939 | 21% | 630/4,833 | 13% | 378/3,151 | 12% |
| Presence of damp/mold at home in the first 8 mo | 5,352/11,226 | 48% | 2,357/4,911 | 48% | 1,616/3,214 | 50% |
| Ever exposed to passive smoking in childhood | 6,654/10,188 | 65% | 2,587/4,662 | 55% | 1,574/3,044 | 52% |
| Never had breastfeeding | 2,755/11,313 | 24% | 905/4,959 | 18% | 496/3,247 | 15% |
| Current asthma | — | — | 464/4,460 | 10% | 230/2,299 | 10% |
| Current body mass index, kg/m2 | — | — | 4,979 | 17.1 (2.4) | 3,441 | 21.5 (3.5) |
| FEV1, L | — | — | 5,276 | 1.69 (0.26) | 3,446 | 3.45 (0.78) |
| FEV1% predicted | — | — | 5,276 | 99.22% (11.67) | 3,446 | 93.66% (15.65) |
| FVC, L | — | — | 5,276 | 1.92 (0.31) | 3,446 | 3.76 (0.88) |
| FVC% predicted | — | — | 5,276 | 99.04% (11.92) | 3,446 | 89.05% (14.88) |
| FEV1/FVC | — | — | 5,276 | 0.88 (0.06) | 3,446 | 0.92 (0.07) |
| FEV1/FVC% predicted | — | — | 5,276 | 99.71% (7.08) | 3,446 | 104.83% (7.72) |
Participants had data on at least FEV1% predicted or FVC% predicted values at the current age.
Table 2.
Distributions of Air Pollutants (μg/m3) in Different Time Periods
| PM10 Component | Exposure Period | n | Mean | SD | Median | IQR | Range |
|---|---|---|---|---|---|---|---|
| PM10_road | T1, daily average | 12,445 | 0.96 | 0.71 | 0.80 | 0.72 | 0.07–7.80 |
| T2, daily average | 12,726 | 0.96 | 0.71 | 0.80 | 0.71 | 0.10–8.37 | |
| T3, daily average | 12,670 | 0.94 | 0.69 | 0.78 | 0.70 | 0.05–7.26 | |
| Whole pregnancy, daily average | 12,315 | 0.96 | 0.69 | 0.81 | 0.72 | 0.13–7.72 | |
| 0–6 mo, daily average | 12,538 | 0.94 | 0.68 | 0.79 | 0.73 | 0.14–8.33 | |
| 7–12 mo, daily average | 12,440 | 0.89 | 0.65 | 0.75 | 0.68 | 0.13–7.17 | |
| 0–7 yr, annual average | 10,974 | 0.97 | 0.46 | 0.93 | 0.54 | 0.23–4.78 | |
| 8–15 yr, annual average | 11,181 | 0.65 | 0.28 | 0.67 | 0.33 | 0.13–3.75 | |
| 0–15 yr, annual average | 10,309 | 0.80 | 0.35 | 0.80 | 0.41 | 0.18–3.63 | |
| PM10_other | T1, daily average | 12,445 | 5.24 | 1.97 | 5.10 | 2.42 | 0.78–18.36 |
| T2, daily average | 12,726 | 5.25 | 1.96 | 5.08 | 2.41 | 0.79–19.07 | |
| T3, daily average | 12,670 | 5.17 | 1.94 | 5.06 | 2.42 | 0.86–19.32 | |
| Whole pregnancy, daily average | 12,315 | 5.24 | 1.85 | 5.20 | 2.30 | 1.12–17.14 | |
| 0–6 mo, daily average | 12,538 | 5.19 | 1.88 | 5.14 | 2.37 | 1.05–19.47 | |
| 7–12 mo, daily average | 12,440 | 4.87 | 1.76 | 4.83 | 2.23 | 1.08–18.07 | |
| 0–7 yr, annual average | 10,974 | 4.62 | 1.35 | 4.77 | 1.83 | 1.30–13.07 | |
| 8–15 yr, annual average | 11,181 | 2.70 | 0.80 | 2.93 | 1.04 | 0.66–6.41 | |
| 0–15 yr, annual average | 10,309 | 3.60 | 1.01 | 3.78 | 1.35 | 0.97–9.39 | |
| PM10_total | T1, daily average | 12,445 | 33.45 | 5.31 | 32.88 | 7.90 | 20.38–54.21 |
| T2, daily average | 12,726 | 32.97 | 5.35 | 31.97 | 7.86 | 20.65–56.78 | |
| T3, daily average | 12,670 | 31.49 | 5.26 | 30.40 | 6.87 | 17.08–67.83 | |
| Whole pregnancy, daily average | 12,315 | 32.58 | 2.98 | 32.60 | 3.79 | 22.82–48.52 | |
| 0–6 mo, daily average | 12,538 | 31.82 | 3.74 | 31.57 | 5.08 | 22.37–51.76 | |
| 7–12 mo, daily average | 12,440 | 30.96 | 3.12 | 30.83 | 4.29 | 22.51–49.72 | |
| 0–7 yr, annual average | 10,974 | 32.99 | 1.83 | 33.08 | 2.50 | 28.20–42.91 | |
| 8–15 yr, annual average | 11,181 | 22.98 | 1.05 | 23.19 | 1.44 | 20.19–27.25 | |
| 0–15 yr, annual average | 10,309 | 27.66 | 1.36 | 27.82 | 1.85 | 24.03–34.54 |
Definition of abbreviations: IQR = interquartile range; PM10_other = particulate matter ≤10 μm in aerodynamic diameter from other local combined sources, such as minor roads and industrial or domestic sources; PM10_road = PM10 from local major road traffic; PM10_total = total PM10; T1 = first trimester; T2 = second trimester; T3 = third trimester.
Spearman correlations between PM10_road measures for different time periods were highly correlated (Table E2), as was also seen for PM10_other (Table E3). In contrast, correlations between PM10_total across trimesters, 0–6 months, and 7–12 months were low to moderate (ranging from −0.16 to 0.22, except between the first trimester and 0–6 mo of age [r = 0.44] and between the second trimester and 7–12 mo [r = 0.72]) (Table E4). For the same period, correlations between each source of PM10 exposure ranged from 0.48 to 0.96 (Table E5).
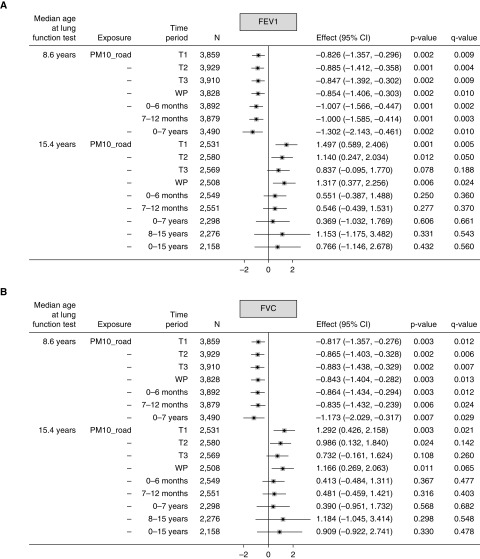
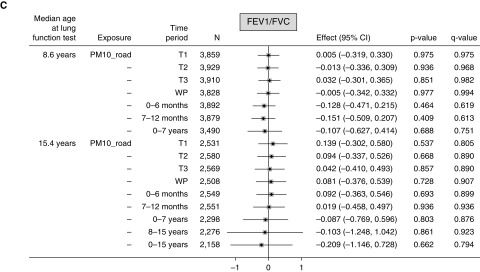
For PM10_road, each 1 μg/m3 higher exposure during the first trimester was associated with lower FEV1% predicted (−0.826; 95% confidence interval [CI], −1.357 to −0.296) (Figure 1A) and FVC% predicted (−0.817; 95% CI, −1.357 to −0.276) (Figure 1B) at age 8 years. Significant negative associations of a similar effect size were also seen for the other time periods. In contrast, at age 15 years, all associations were consistently positive but nonsignificant, except for the first and second trimesters and whole pregnancy. For example, for whole pregnancy, FEV1% predicted and FVC% predicted were higher by 1.317 (95% CI, 0.377–2.256) and 1.166 (95% CI, 0.269–2.063), respectively. No associations with FEV1/FVC ratio at either age were seen (Figure 1C).
Figure 1.
(A–C) Mean changes in lung function (FEV1% predicted, FVC% predicted, and FEV1%/FVC% predicted ratio) at ages 8 and 15 years in relation to 1 μg/m3 higher particulate matter ≤10 μm in aerodynamic diameter (PM10) from local major road traffic (PM10_road) in different time periods. Fully adjusted model (adjusted for sex, gestational age, maternal education, home ownership, maternal smoking during pregnancy, passive smoking in childhood, presence of damp and mold at home, and season of clinic visit). CI = confidence interval; T1 = first trimester; T2 = second trimester; T3 = third trimester; WP = whole pregnancy.
When analyses were restricted to the 1,501 participants with lung function measurements at ages 8 and 15 years, the directions of the associations between PM10 exposures and lung function measures at both ages were generally in line with the results of the main analyses, although the effect sizes were much smaller and most associations were nonsignificant, as expected given the smaller sample (Table E6).
The profile regression model for analyses of PM10_road at age 8 years revealed a “best” partition made of three clusters of participants. The first cluster was characterized by children (n = 41) who had consistently high exposure to PM10_road throughout the in utero and early-life periods, the third cluster was characterized by children (n = 3,607) who had low exposure for the same period, and the exposure profile for the second cluster (n = 566) was somewhat in between the two. Overall, although the second and third clusters did not show substantial differences in the mean values of lung function outcomes, the first cluster was characterized by having lower mean values of FEV1 (Figure E2) or FVC (Figure E3), although the uncertainty was large because only a small number of participants were included in this cluster. At age 15 years, there was no clear association with either lung function outcome.
For PM10_other, the directions of associations at both ages were similar to those of PM10_road, although the effect sizes were smaller and most significant associations did not persist after multiple test corrections (Figures E4A–E4C).
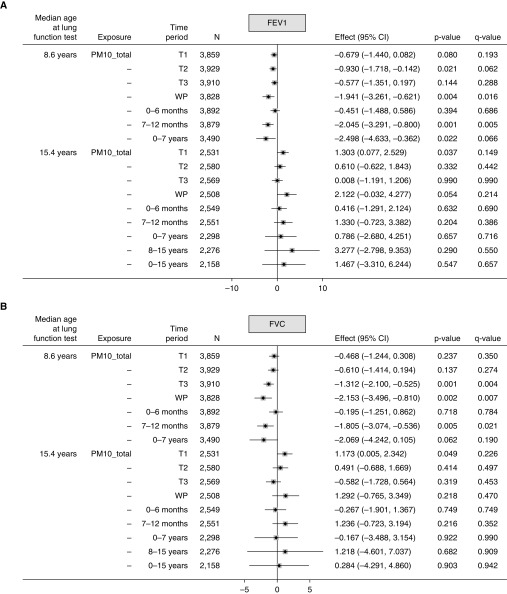
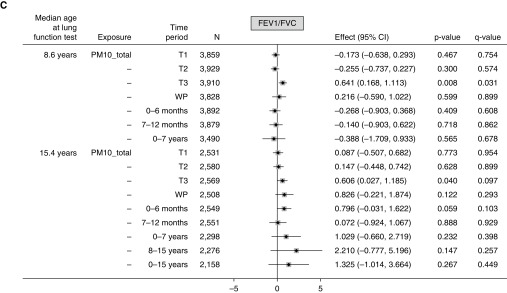
For PM10_total, associations with both FEV1% predicted and FVC% predicted at age 8 years were all negative but with varied strength across the time periods. Exposure during the second trimester, whole pregnancy, 7–12 months, and 0–7 years was significantly associated with lower FEV1% predicted (Figure 2A), and significant negative associations were observed for FVC% predicted in relation to third trimester, whole pregnancy, and 7–12 months exposure (Figure 2B). The negative association with FVC observed at the third trimester persisted in models that were mutually adjusted for exposures in other trimesters and infancy periods (Table E7), whereas multicollinearity was not detected (variance inflation factor < 10). A significant positive association (0.641; 95% CI, 0.168–1.113) in the third trimester was seen for the FEV1%/FVC% predicted ratio at age 8 years (Figure 2C), which persisted in models that were mutually adjusted for exposures in all other periods (Table E7).
Figure 2.
(A–C) Mean changes in lung function (FEV1% predicted, FVC% predicted, and ratio of FEV1%/FVC% predicted) at ages 8 and 15 years in relation to 10 μg/m3 higher total particulate matter ≤10 μm in aerodynamic diameter (PM10_total) in different time periods. Fully adjusted model (adjusted for sex, gestational age, maternal education, home ownership, maternal smoking during pregnancy, passive smoking in childhood, presence of damp and mold at home, and season of clinic visit). For definition of abbreviations, see Figure 1.
At age 15 years, there were significant positive associations between the first-trimester exposure and FEV1% predicted or FVC% predicted, and between the third-trimester exposure and the FEV1%/FVC% predicted ratio, although none of these persisted after multiple test corrections.
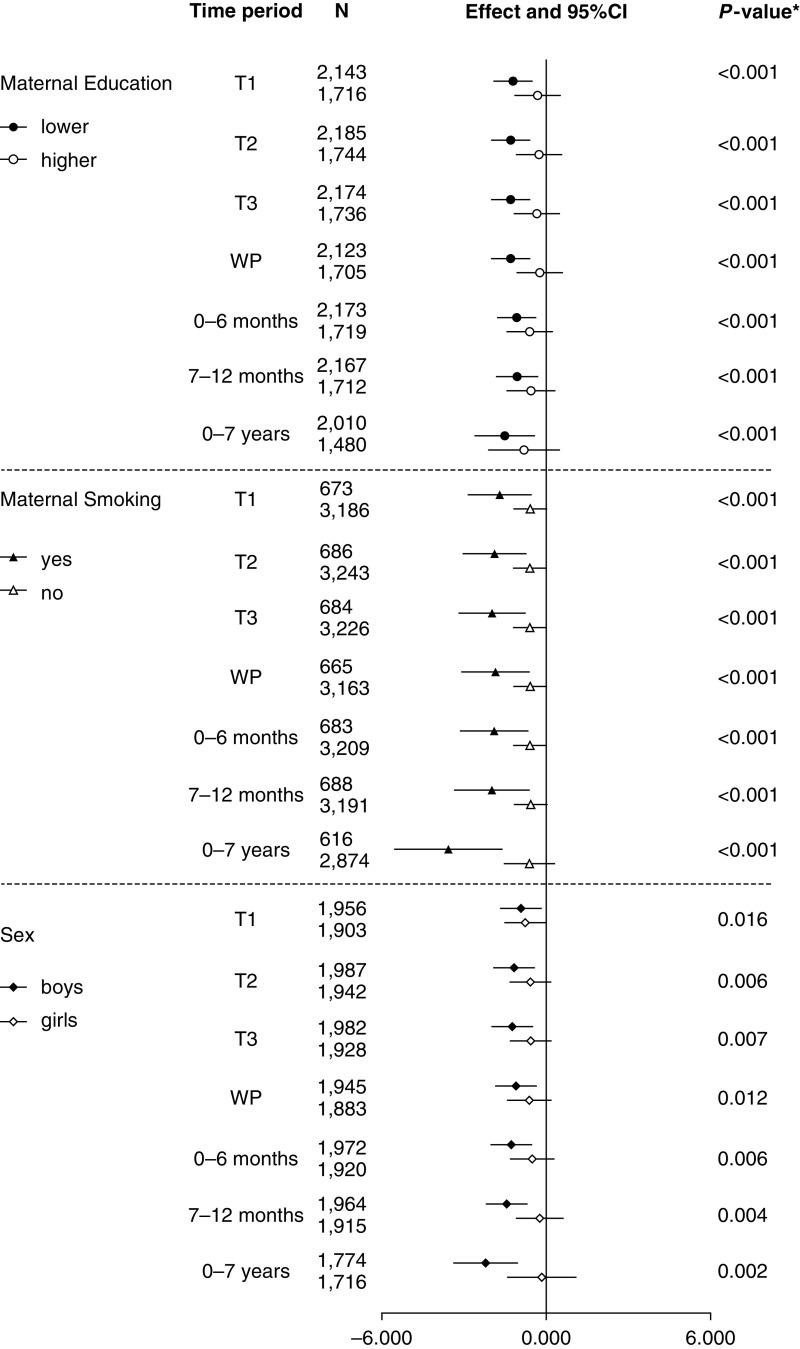
At age 8 years, for the associations between PM10_road and FVC% predicted at all time periods, negative associations were larger among boys, and children whose mother had a lower education level or smoked during pregnancy (Figure 3). Similar patterns were also observed for PM10_total (Figure E5).
Figure 3.
Mean changes in FVC% predicted at age 8 years in relation to per 1 μg/m3 higher particulate matter ≤10 μm in aerodynamic diameter (PM10) from local major road traffic (PM10_road) in different time periods by maternal education, smoking, and sex. Fully adjusted model (*adjusted for sex, gestational age, maternal education, home ownership, maternal smoking during pregnancy, passive smoking in childhood, presence of damp and mold at home, and season of clinic visit). CI = confidence interval; T1 = first trimester; T2 = second trimester; T3 = third trimester; WP = whole pregnancy.
Sensitivity analyses and analyses using imputed datasets (Tables E8 and E9) did not materially change the main findings. Most associations between air pollution exposure in different periods and lung function growth from ages 8 to 15 years were nonsignificant, except that positive associations were found between PM10 exposure from each source during the first trimester and FEV1 growth (Table E10).
Discussion
In this large cohort of children who were followed from the antenatal period to age ≥15 years, PM10 exposures during each pregnancy trimester, infancy, and childhood were modeled specifically for road-traffic and non–road-traffic sources. We found that road-traffic PM10 appeared to be particularly harmful for mid-childhood lung function. Specific susceptibility time periods in pregnancy and early life were not identified, possibly because exposures to road-traffic PM10 were highly correlated across the time periods. Associations were larger among boys, as well as children whose mother had a lower education level or smoked during pregnancy. Associations with total PM10 (i.e., from all sources) were less consistent but pointed to susceptibility periods during the third trimester in relation to lower FVC levels in mid-childhood. However, these negative associations were not observed at age 15 years.
The observed reduction in our study of 0.8% of predicted lung function levels in relation to early-life road-traffic PM10 exposure equates to an average decrement of ∼14 ml FEV1 and ∼16 ml FVC loss at age 8 years. These deficits, although small for individuals, may unfavorably shift the distribution of average lung function in the population (i.e., the number of individuals with lower lung function will increase) and this may have long-term implications for lung health (1).
It is difficult to make direct comparisons with previous studies owing to different exposure assessments (e.g., nitrogen dioxide [NO2] or PM2.5) and the small numbers and different characteristics of the study children (e.g., children with asthma and preschoolers). We did not model PM2.5 estimates in our study because PM2.5 monitoring data only became available from 2008 in Bristol and the surrounding areas (31). Nevertheless, our modeled estimates of PM10_road should be qualitatively comparable to NO2, nitrogen oxide, or PM2.5, as all are likely to be primarily derived from road-traffic sources.
The negative association between PM10 derived from road traffic and mid-childhood lung function in our study is consistent with results of previous studies that investigated trimester-specific or prenatal exposures to NO2 or PM2.5. In the Spanish INMA (Infancia y Medio Ambiente) birth cohort of 620 preschool children 4.5 years of age, an interquartile range higher exposure in the second trimester to Land Use Regression–derived NO2 was significantly associated with a 28-ml and 33-ml reduction in FEV1 and FVC, respectively (22). Similarly, an earlier study in California of 232 children with asthma 6–11 years of age reported that FEV1 and FVC were reduced by 1.2% and 7.1%, respectively, for each interquartile range higher second-trimester exposure to NO2, assigned from the nearest monitoring station (21). Although these two studies pointed to susceptibility periods during the second trimester, another study (n = 171) from Boston found that prenatal exposure to modeled PM2.5 in late pregnancy (≥35 wk gestation) was significantly associated with impaired lung function at age 7 years, particularly among boys (24). More recently, a study of the PARIS (Pollution and Asthma Risk: An Infant Study) cohort of 788 children with an average age of 8.5 years did not report such significant negative associations with either FEV1 or FVC in relation to monitoring-based nitrogen oxide exposures in each pregnancy trimester (23).
Unlike previous studies (21, 22, 24), we did not observe an effect from PM10_road limited only to the second trimester or late pregnancy, but given the very high correlations across time periods in these other studies (an issue we also encountered in our study for PM10_road), it is difficult to be certain of a critical time window. We did not identify a specific sensitive trimester in relation to PM10_road in the profile regression, but found lower mean FEV1 and FVC in children with consistently high exposure to PM10_road throughout the in utero and early-life periods. Given that our work and the above-mentioned studies did not consistently identify a pregnancy trimester or early-life period that is particularly susceptible to the effects of road-traffic air pollution on the developing lung (as measured by lung function), there may not be a truly trimester-specific effect. The public-health implication of this is that it is important to reduce exposures to road-traffic air pollution throughout pregnancy and early childhood.
Our findings are also consistent with results from the Swedish BAMSE (Children, Allergy, Milieu, Stockholm, Epidemiological Survey) cohort (2,278 children) (15) and the GINIplus (German Infant Study on the Influence of Nutrition Intervention Plus Environmental and Genetic Influences on Allergy Development) and LISAplus (Influence of Lifestyle Factors on the Development of the Immune System and Allergies in East and West Germany Plus the Influence of Traffic Emissions and Genetics) cohorts in Germany (2,266 children) (17), which showed negative associations between air pollution exposures in infancy and lung function at age 8 years, but no significant or even positive associations at 15 years. Two other studies, however, did not find associations between exposures at birth or first year of life and lung function levels at 6–11 years (7, 12), partly due to uncertainty in modeling historical exposures or a lack of power. Our findings complement those from the Southern California Children’s Health Study, which showed that children living closer to a freeway had significant deficits in lung function growth from 10 to 18 years, based on a “within-community” analysis (5). Recently, the PIAMA (Prevention and Incidence of Asthma and Mite Allergy) birth cohort study reported that higher exposures to PM10, PM2.5, or NO2 at birth and age 4 years were significantly associated with reduced growth in FEV1, but not FVC, from ages 8 to 16 years (19). However, we did not replicate this finding in the longitudinal analysis using lung function at both ages 8 and 15 years.
As in a previous study (12), the effect sizes of air pollutants on FEV1 and FVC were similar in our study, indicating that traffic-related air pollution exposure during pregnancy may potentially result in a restrictive pattern of lung function in mid-childhood, but not necessarily predispose children for chronic airflow obstruction.
We found that PM10 exposure from all sources, in particular during the third trimester, was negatively associated with FVC at age 8 years. During late fetal life and infancy, the developing lungs undergo structural and functional growth, mainly in the formation of small airways that range from terminal bronchioles to alveolar sacs (36). It is plausible that during this period, exposure to particulate air pollution, associated with oxidative stress and proinflammatory activities, may have an impact on the development of the small airways, such as slower growth and/or closure (12), which may lead to a decrease in FVC in childhood.
However, because the decrements in lung function did not persist to age 15 years, the possible effects on the lung from early exposure to PM (especially from road traffic) may be transitory or reflect the toxicity of the air pollution mix in the fetal and early-life periods.
General air quality improved over our study period (31), and the proportional contribution of diesel emissions became progressively smaller. It is likely that diesel emissions, which are believed to be particularly toxic to developing lungs (5), contributed largely to PM concentrations in our study area during the pregnancy periods, because regulations on PM emissions from diesel vehicles were first introduced in the United Kingdom in July 1992 (i.e., Euro 1). Before the Euro 1 regulations were established, PM10 emissions from diesel vehicles were ∼0.17 g per kilometer driven. PM10 emissions were reduced by many orders of magnitude (i.e., <0.01 g/km) by 2009 with progressively tightening of regulations, for example, through the fitting of diesel particulate filters (37). Thus, it is possible that the negative effects of early-life exposure to higher, more toxic air pollution were offset by increased lung growth up to age 15 years in the absence of continued cumulative high exposure. This is supported by results from the Southern California Children’s Health Study, in which long-term air quality improvements in the increase of both FEV1 and FVC in subjects aged 11 to 15 years (6).
The mechanisms underlying the effects of air pollution (particularly during pregnancy) on childhood lung function remain unclear. One likely central pathway is oxidative stress (i.e., overproduction of reactive oxygen species). It is believed that particles inhaled by the mother during pregnancy could cross the placental barrier (38) and directly disturb in utero lung development by promoting oxidative stress (9). Some recent studies have suggested that epigenetic changes induced by prenatal air pollution exposure may also play an important role (24, 39).
We found an increased susceptibility to the effects of air pollution on lung function in mid-childhood among boys, consistent with several studies (6, 7, 14, 24); however, other studies reported no differences between the sexes (12, 22). Another finding of public health concern is that children from a lower socioeconomic background tended to be more susceptible, possibly because they were likely exposed to some other unfavorable exposures related to their socioeconomic disadvantage.
Our study has limitations. We relied on modeling of air pollution exposures at residential addresses and did not make allowance for travel patterns or indoor pollution sources. The downward time trends in PM10 levels during follow-up may have affected our results; however, as we previously documented that declines in PM10 levels during measurement of lung function in both 1999–2002 and 2006–2008 were relatively small (33), substantial confounding due to time trends seems unlikely. As would be expected in any long-running birth cohort, there was loss to follow-up, which may have been differential. For example, children who were more socially deprived were more likely to drop out than socially advantaged children, although PM10_road exposures in pregnancy and infancy, as well as lung function measures at age 8 years, were similar between the groups (Table E11). This potential selection bias may in part explain the nonsignificant findings at age 15 years, and affects the generalizability of our findings.
We used separate models for each trimester-averaged exposure, but estimates may be biased if exposure in other trimesters act as unmeasured confounders (e.g., through seasonal trends in particulate exposure) (40). This can be reduced by including all trimester-averaged exposures in a single model (which is not possible if there are high correlations between trimesters, such as PM10_road and PM10_other) or by using other statistical models. Wilson and colleagues used a distributed lag model that considered exposures in each week of pregnancy (40), but we were unable to implement this model in our current analysis because we had too few data points and because the model requires the data points to be of equal temporal length. Therefore, we used a profile regression model that was designed to deal with correlated data points and does not have a requirement about temporal spacing of data. This did not identify a specific susceptible trimester.
Only two time points of lung function measurements were available for our analyses. It is more difficult to study the effects of early-life air pollution exposure on lung function in adolescence than in mid-childhood. Throughout adolescence, the rapid growth of lung function may follow a nonlinear pattern and is dependent on sex, as puberty plays a major role. Generally, lung volume growth in boys tends to occur rapidly toward the end of puberty, whereas in girls this process starts earlier in puberty (41). Tobacco smoking is also an important factor that impacts lung function growth; however, our results did not change after we made a further adjustment for smoking at age 15 years. We did not adjust for dietary patterns during pregnancy or throughout childhood, which may also impact lung function.
In conclusion, exposure to road-traffic PM10 in pregnancy and early life was associated with small but significant reductions in lung function in mid-childhood. Although PM10 emissions from diesel vehicles have been declining over the years in the United Kingdom, other traffic-derived pollutants, such as NO2, a good proxy of diesel emissions, are still found in high concentrations in many UK cities (42). A stringent policy for controlling road-traffic–related air pollution is required to protect the respiratory health of children, and may have long-term benefits for lung health throughout the life course (1).
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank all of the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
The authors dedicate this work to the memory of Professor A. John Henderson, a highly respected clinical expert and academic who led the program of respiratory follow-up in the ALSPAC cohort and made enormous contributions to research into pediatric respiratory medicine and the epidemiology of respiratory disease in childhood.
Footnotes
Funded by the UK Medical Research Council (MRC) (grant number G0700920). The UK MRC and Wellcome Trust (grant number 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. The MRC Centre for Environment and Health is funded by the UK MRC (grant number MR/S019669/1). Y.C. was supported by an MRC Early-Career Research Fellowship awarded through the MRC Centre for Environment and Health (grant number MR/M501669/1). This article was completed with support from the PEAK Urban program, funded by UK Research and Innovation’s Global Challenge Research Fund (grant number ES/P011055/1). P.E. received funding from the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre, the NIHR Health Protection Research Unit in Health Impact of Environmental Hazards (HPRU-2012-10141), and the UK Dementia Research Institute supported by UK DRI Ltd., which is funded by the UK MRC, Alzheimer’s Society, and Alzheimer’s Research UK. P.E. is associate director of Health Data Research UK-London, which receives funding from a consortium led by the UK MRC. The views expressed are those of the authors and not necessarily those of the Department of Health, the National Health Service, or the NIHR.
Author Contributions: P.E. and A.J.H. conceived the study idea and design and supervised the work. D.F. and J.G. provided input on exposure modeling. R.G. and M.B. provided input on the methodology for analyzing the data. Y.C. and A.L.H. did the main data analyses and wrote the manuscript. M.Z. provided input on the profile regression analysis. All authors provided substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work; revised the manuscript for important intellectual content; approved the final version; and agreed to be accountable for all aspects of the work. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201902-0286OC on March 6, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Belgrave DCM, Granell R, Turner SW, Curtin JA, Buchan IE, Le Souëf PN, et al. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir Med. 2018;6:526–534. doi: 10.1016/S2213-2600(18)30099-7. [DOI] [PubMed] [Google Scholar]
- 2.Bui DS, Lodge CJ, Burgess JA, Lowe AJ, Perret J, Bui MQ, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med. 2018;6:535–544. doi: 10.1016/S2213-2600(18)30100-0. [DOI] [PubMed] [Google Scholar]
- 3.Royal College of Physicians. Every breath we take: the lifelong impact of air pollution, report of a working party. London: Report of a working party, RCP; 2016 [published 2016 Feb 23; accessed 2020 Apr 20]. Available from: https://www.rcplondon.ac.uk/projects/outputs/every-breath-we-take-lifelong-impact-air-pollution. [Google Scholar]
- 4.Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 5.Gauderman WJ, Vora H, McConnell R, Berhane K, Gilliland F, Thomas D, et al. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet. 2007;369:571–577. doi: 10.1016/S0140-6736(07)60037-3. [DOI] [PubMed] [Google Scholar]
- 6.Gauderman WJ, Urman R, Avol E, Berhane K, McConnell R, Rappaport E, et al. Association of improved air quality with lung development in children. N Engl J Med. 2015;372:905–913. doi: 10.1056/NEJMoa1414123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gehring U, Gruzieva O, Agius RM, Beelen R, Custovic A, Cyrys J, et al. Air pollution exposure and lung function in children: the ESCAPE project. Environ Health Perspect. 2013;121:1357–1364. doi: 10.1289/ehp.1306770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang BF, Chen YH, Lin YT, Wu XT, Leo Lee Y. Relationship between exposure to fine particulates and ozone and reduced lung function in children. Environ Res. 2015;137:382–390. doi: 10.1016/j.envres.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Korten I, Ramsey K, Latzin P. Air pollution during pregnancy and lung development in the child. Paediatr Respir Rev. 2017;21:38–46. doi: 10.1016/j.prrv.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Mölter A, Agius RM, de Vocht F, Lindley S, Gerrard W, Lowe L, et al. Long-term exposure to PM10 and NO2 in association with lung volume and airway resistance in the MAAS birth cohort. Environ Health Perspect. 2013;121:1232–1238. doi: 10.1289/ehp.1205961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oftedal B, Brunekreef B, Nystad W, Madsen C, Walker SE, Nafstad P. Residential outdoor air pollution and lung function in schoolchildren. Epidemiology. 2008;19:129–137. doi: 10.1097/EDE.0b013e31815c0827. [DOI] [PubMed] [Google Scholar]
- 12.Rice MB, Rifas-Shiman SL, Litonjua AA, Oken E, Gillman MW, Kloog I, et al. Lifetime exposure to ambient pollution and lung function in children. Am J Respir Crit Care Med. 2016;193:881–888. doi: 10.1164/rccm.201506-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rojas-Martinez R, Perez-Padilla R, Olaiz-Fernandez G, Mendoza-Alvarado L, Moreno-Macias H, Fortoul T, et al. Lung function growth in children with long-term exposure to air pollutants in Mexico City. Am J Respir Crit Care Med. 2007;176:377–384. doi: 10.1164/rccm.200510-1678OC. [DOI] [PubMed] [Google Scholar]
- 14.Schultz ES, Gruzieva O, Bellander T, Bottai M, Hallberg J, Kull I, et al. Traffic-related air pollution and lung function in children at 8 years of age: a birth cohort study. Am J Respir Crit Care Med. 2012;186:1286–1291. doi: 10.1164/rccm.201206-1045OC. [DOI] [PubMed] [Google Scholar]
- 15.Schultz ES, Hallberg J, Bellander T, Bergström A, Bottai M, Chiesa F, et al. Early-life exposure to traffic-related air pollution and lung function in adolescence. Am J Respir Crit Care Med. 2016;193:171–177. doi: 10.1164/rccm.201505-0928OC. [DOI] [PubMed] [Google Scholar]
- 16.Urman R, McConnell R, Islam T, Avol EL, Lurmann FW, Vora H, et al. Associations of children’s lung function with ambient air pollution: joint effects of regional and near-roadway pollutants. Thorax. 2014;69:540–547. doi: 10.1136/thoraxjnl-2012-203159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuertes E, Bracher J, Flexeder C, Markevych I, Klümper C, Hoffmann B, et al. Long-term air pollution exposure and lung function in 15 year-old adolescents living in an urban and rural area in Germany: the GINIplus and LISAplus cohorts. Int J Hyg Environ Health. 2015;218:656–665. doi: 10.1016/j.ijheh.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Barone-Adesi F, Dent JE, Dajnak D, Beevers S, Anderson HR, Kelly FJ, et al. Long-term exposure to primary traffic pollutants and lung function in children: cross-sectional study and meta-analysis. PLoS One. 2015;10:e0142565. doi: 10.1371/journal.pone.0142565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milanzi EB, Koppelman GH, Smit HA, Wijga AH, Oldenwening M, Vonk JM, et al. Air pollution exposure and lung function until age 16 years: the PIAMA birth cohort study. Eur Respir J. 2018;52:1800218. doi: 10.1183/13993003.00218-2018. [DOI] [PubMed] [Google Scholar]
- 20.Jedrychowski WA, Perera FP, Maugeri U, Mroz E, Klimaszewska-Rembiasz M, Flak E, et al. Effect of prenatal exposure to fine particulate matter on ventilatory lung function of preschool children of non-smoking mothers. Paediatr Perinat Epidemiol. 2010;24:492–501. doi: 10.1111/j.1365-3016.2010.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mortimer K, Neugebauer R, Lurmann F, Alcorn S, Balmes J, Tager I.Air pollution and pulmonary function in asthmatic children: effects of prenatal and lifetime exposures Epidemiology 200819550–557.[Discussion, pp. 561–562.] [DOI] [PubMed] [Google Scholar]
- 22.Morales E, Garcia-Esteban R, de la Cruz OA, Basterrechea M, Lertxundi A, de Dicastillo MD, et al. Intrauterine and early postnatal exposure to outdoor air pollution and lung function at preschool age. Thorax. 2015;70:64–73. doi: 10.1136/thoraxjnl-2014-205413. [DOI] [PubMed] [Google Scholar]
- 23.Bougas N, Rancière F, Beydon N, Viola M, Perrot X, Gabet S, et al. Traffic-related air pollution, lung function, and host vulnerability: new insights from the PARIS birth cohort. Ann Am Thorac Soc. 2018;15:599–607. doi: 10.1513/AnnalsATS.201711-900OC. [DOI] [PubMed] [Google Scholar]
- 24.Lee AG, Le Grand B, Hsu HL, Chiu YM, Brennan KJ, Bose S, et al. Prenatal fine particulate exposure associated with reduced childhood lung function and nasal epithelia GSTP1 hypermethylation: sex-specific effects. Respir Res. 2018;19:76. doi: 10.1186/s12931-018-0774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, et al. Cohort profile: the ‘children of the 90s’. The index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42:111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, et al. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42:97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai Y, Hansell A, Granell R, Blangiardo M, Fecht D, Gulliver J, et al. Prenatal, early-life and lifetime exposure to air pollution and childhood lung function and asthma: the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort study. ISEE Conference Abstracts; 2018. 10.1289/isesisee.2018.P03.1140.
- 28.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 29.Peralta GP, Fuertes E, Granell R, Mahmoud O, Roda C, Serra I, et al. Childhood body composition trajectories and adolescent lung function: findings from the ALSPAC study. Am J Respir Crit Care Med. 2019;200:75–83. doi: 10.1164/rccm.201806-1168OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyd A, Thomas R, Hansell AL, Gulliver J, Hicks LM, Griggs R, et al. Data resource profile: the ALSPAC birth cohort as a platform to study the relationship of environment and health and social factors. Int J Epidemiol. 2019;48:1038–1039k. doi: 10.1093/ije/dyz063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gulliver J, Elliott P, Henderson J, Hansell AL, Vienneau D, Cai Y, et al. Local- and regional-scale air pollution modelling (PM10) and exposure assessment for pregnancy trimesters, infancy, and childhood to age 15 years: Avon Longitudinal Study of Parents And Children (ALSPAC) Environ Int. 2018;113:10–19. doi: 10.1016/j.envint.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones A, Thomson D, Hort M, Devenish B.The U.K. Met Office’s next-generation atmospheric dispersion model, NAME III Borrego C, Norman AL.editors. Air pollution modeling and its application XVII Boston, MA: Springer; 2007580–589. [Google Scholar]
- 33.Li KH, Raghunathan TE, Rubin DB. Large-sample significance levels from multiply imputed data using moment-based statistics and an F reference distribution. J Am Stat Assoc. 1991;86:1065–1073. [Google Scholar]
- 34.Molitor J, Papathomas M, Jerrett M, Richardson S. Bayesian profile regression with an application to the National Survey of Children’s Health. Biostatistics. 2010;11:484–498. doi: 10.1093/biostatistics/kxq013. [DOI] [PubMed] [Google Scholar]
- 35.Liverani S, Hastie DI, Azizi L, Papathomas M, Richardson S. PReMiuM: an R package for profile regression mixture models using dirichlet processes. J Stat Softw. 2015;64:1–30. doi: 10.18637/jss.v064.i07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kajekar R. Environmental factors and developmental outcomes in the lung. Pharmacol Ther. 2007;114:129–145. doi: 10.1016/j.pharmthera.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Department for Environment, Food and Rural Affairs, UK. Emissions of air quality pollutants 1990–2012. 2015 [reported 2015 Oct 15; accessed 2020 Apr 21]. Available from: https://uk-air.defra.gov.uk/assets/documents/reports/cat07/1511261124_AQPI_Summary_1990-2012_Issue_v1.1.pdf.
- 38.Bové H, Bongaerts E, Slenders E, Bijnens EM, Saenen ND, Gyselaers W, et al. Ambient black carbon particles reach the fetal side of human placenta. Nat Commun. 2019;10:3866. doi: 10.1038/s41467-019-11654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plusquin M, Chadeau-Hyam M, Ghantous A, Alfano R, Bustamante M, Chatzi L, et al. DNA methylome marks of exposure to particulate matter at three time points in early life. Environ Sci Technol. 2018;52:5427–5437. doi: 10.1021/acs.est.7b06447. [DOI] [PubMed] [Google Scholar]
- 40.Wilson A, Chiu YM, Hsu HL, Wright RO, Wright RJ, Coull BA. Potential for bias when estimating critical windows for air pollution in children’s health. Am J Epidemiol. 2017;186:1281–1289. doi: 10.1093/aje/kwx184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nève V, Girard F, Flahault A, Boulé M. Lung and thorax development during adolescence: relationship with pubertal status. Eur Respir J. 2002;20:1292–1298. doi: 10.1183/09031936.02.00208102. [DOI] [PubMed] [Google Scholar]
- 42.Mudway IS, Dundas I, Wood HE, Marlin N, Jamaludin JB, Bremner SA, et al. Impact of London’s low emission zone on air quality and children’s respiratory health: a sequential annual cross-sectional study. Lancet Public Health. 2019;4:e28–e40. doi: 10.1016/S2468-2667(18)30202-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.