To the Editor:
Lymphocytopenia has been identified as a common laboratory finding in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, particularly among those with more severe presentations (1); however, there are limited data on which specific lymphocyte populations may be affected or the clinical sequelae. In this report, we describe the case of a woman with hypoxemic respiratory failure found to have coinfection with SARS-CoV-2 and Pneumocystis jirovecii, a pathogen commonly seen in patients with defects in T-cell immunity.
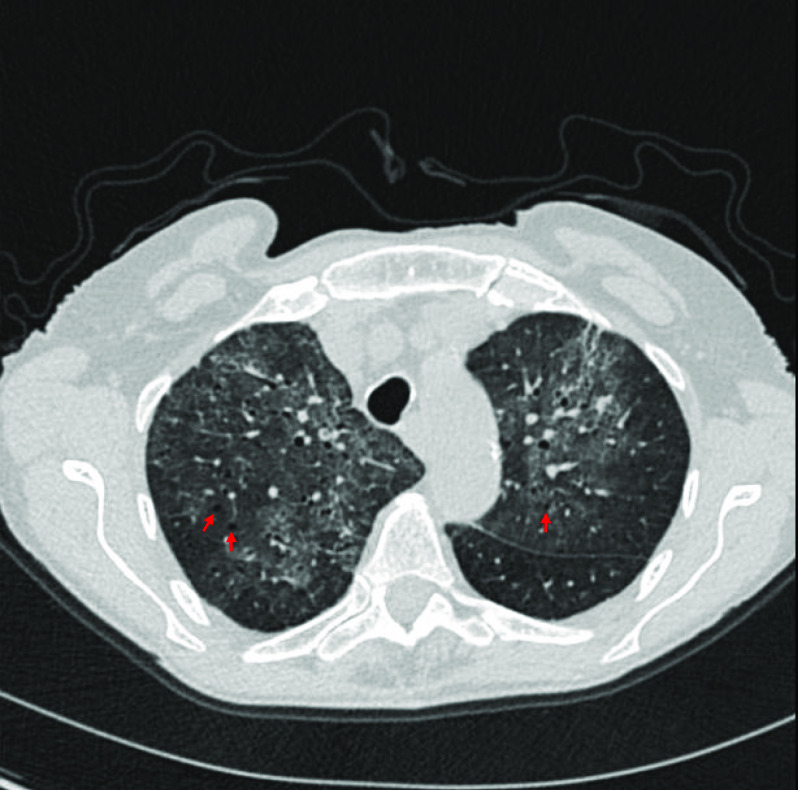
An 83-year-old female nonsmoker presented to our hospital on March 12, 2020, with fevers, malaise, headache, dry cough, and dyspnea. She had a history of mild intermittent asthma, managed with an albuterol inhaler as needed, mitral valve prolapse with moderate to severe mitral regurgitation, and mild to moderate ulcerative colitis, which was well controlled on oral budesonide (3 mg daily and being tapered) as well as maintenance-dose sulfasalazine (1,500 mg twice daily). Her symptoms had started approximately 2 weeks prior to presentation, shortly after travel from Florida to Massachusetts, and had failed to improve with courses of azithromycin and amoxicillin-clavulanate. In the emergency department, she had a fever of 39.3°C and oxygen saturation of 86% on room air, which improved to 95% on 5 L/min of supplemental oxygen by nasal cannula. Initial laboratory evaluation revealed leukocytosis and relative lymphocytopenia (absolute lymphocyte count, 1,090 cells/μl) (Table 1). Chest computed tomography was notable for diffuse bilateral ground-glass opacities with patchy bands of atelectasis and small nodular foci of consolidation with a distribution suggestive of a viral pneumonia. Subtle cystic changes were also seen in the affected regions (Figure 1). She was admitted to the medical intensive care unit and placed on strict isolation precautions given concern for community-acquired SARS-CoV-2. She developed worsening tachypnea with a respiratory rate of 40 breaths/min and hypoxia with an oxygen saturation of 80% requiring supplemental oxygen through a nonrebreather mask at a rate of 15 L/min. An arterial blood gas measurement showed a PaO2 of 63 mm Hg on 15 L/min of supplemental oxygen. She was intubated for hypoxemic respiratory failure and supported on low Vt ventilation according to the Acute Respiratory Distress Syndrome Network lower Vt protocol. Her PaO2/FiO2 was consistent with moderate acute respiratory distress syndrome.
Table 1.
Clinical Laboratory Results
| Measure | Result | Reference |
|---|---|---|
| Hematology and chemistry | ||
| Hb, g/dl | 8.9 | 11.5–16.4 |
| Hematocrit, % | 27.3 | 36–48 |
| Leukocytes, ×103/μl | 15.2 | 4–10 |
| Differential, % | ||
| Neutrophils | 89.5 | 40–70 |
| Lymphocytes | 7.2 | 22–44 |
| Monocytes | 2.4 | 4–11 |
| Eosinophils | 0.0 | 0–8 |
| Basophils | 0.2 | 0–3 |
| Platelets, ×103/μl | 562 | 150–450 |
| Ferritin, μg/L | 54 | 13–150 |
| Procalcitonin, ng/ml | 0.1 | 0.00–0.08 |
| Lactate dehydrogenase, U/L | 348 | 135–225 |
| Microbiology | ||
| Respiratory culture (tracheal aspirate) | 3+ neutrophils, negative Gram stain, and no growth on culture | No growth |
| Blood culture | No growth | No growth |
| SARS-CoV-2 (COVID-19 PCR) | Positive | Negative |
| Influenza A and B PCR | Negative | Negative |
| Parainfluenza PCR | Negative | Negative |
| Adenovirus PCR | Negative | Negative |
| Respiratory syncytial virus PCR | Negative | Negative |
| Human metapneumovirus PCR | Negative | Negative |
| Rhinovirus PCR | Negative | Negative |
| S. pneumoniae urine antigen | Negative | Negative |
| Legionella urine antigen | Negative | Negative |
| Histoplasma urine antigen | Negative | Negative |
| Coccidioides urine antigen | Negative | Negative |
| Blastomyces urine antigen | Negative | Negative |
| Cryptococcal antigen | Negative | Negative |
| Galactomannan antigen | 0.08 | 0–0.49 |
| (1,3)-β-d-glucan, pg/ml | 305 | <80 |
| Pneumocystis jirovecii PCR | Positive | Negative |
| Immunology | ||
| CD4+ T lymphocytes (absolute) | 291 | 441–2,156 |
Definition of abbreviations: COVID-19 = coronavirus disease; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; S. pneumoniae = Streptococcus pneumoniae.
Figure 1.
Chest computed tomographic image. Shown is a representative axial image from the patient’s chest computed tomography scan. Red arrows indicate cystic changes.
A broad infectious workup for viral, bacterial, and fungal organisms (Table 1) confirmed the diagnosis of SARS-CoV-2 infection based on positive detection for the presence of SARS-CoV-2 RNA from a nasopharyngeal swab (Nucleocapsid [N]1 target [Cycle threshold (Ct) 31.33]; N2 target [Ct 33.38]; RNase P control [Ct 25.66]), a qualitative test result (positive when Ct <40.00 for N1 and N2 targets) reported by the Massachusetts State Public Health Laboratory, using its Food and Drug Administration Emergency Use Authorization–approved CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel. In addition, a serum (1,3)-β-d-glucan level was markedly elevated at 305 pg/ml (reference value <80 pg/ml), prompting additional testing for P. jirovecii with a qualitative real-time PCR assay from a tracheal aspirate, which was positive (fluorescent value 0.160 at melting temperature of 62.4°C; minimum fluorescent signal intensity for positive test ≥0.020). Notably, she had no apparent clinical characteristics associated with false-positive (1,3)-β-d-glucan measurements, such as exposure to hemodialysis membranes, intravenous immunoglobulin, albumin, gauze packing, or intravenous β-lactam antibiotics. HIV-1/2 antibody/antigen testing was nonreactive. However, a CD4+ T lymphocyte count was low at 291 cells/μl (reference value, 441–2,156 cells/μl), as was the CD4+/CD8+ ratio (1.18; reference value, 1.20–5.30). She was treated with trimethoprim-sulfamethoxazole and successfully extubated on hospital day 7. A follow-up serum (1,3)-β-d-glucan level obtained 1 week after initiating treatment was significantly reduced (90 pg/ml). Moreover, a follow-up CD4+ T lymphocyte count obtained 10 days after initial presentation demonstrated improvement (730 cells/μl).
CD4+ T lymphocytes play a critical role in the immune response against P. jirovecii. Classically, when patients with untreated HIV develop severe CD4+ lymphocytopenia (<200 cells/μl), the risk of Pneumocystis pneumonia increases significantly (2). In the present case, we hypothesize that SARS-CoV-2 infection led to a state of functional immune suppression related to CD4+ lymphocytopenia, which then predisposed the patient to P. jirovecii infection. Although the patient’s CD4+ T-cell count was >200 cells/μl, the sample was collected nearly a week into her course after her total lymphocyte count had started to recover. It is also possible that an underlying immune defect predisposed the patient independently to SARS-CoV-2 and P. jirovecii infection; however, the patient did not have a known underlying immunodeficiency, nor did she have any classical risk factors for Pneumocystis pneumonia, such as malignancy, organ transplantation, or prolonged exposure to systemic corticosteroids. Although patients with inflammatory bowel disease on systemic corticosteroids, biologics, and other immunosuppressants may be at increased risk of Pneumocystis pneumonia (3), the overall incidence in ulcerative colitis is low (approximately 8/100,000 person-years) (4) and has not been associated with oral budesonide use (5). Given the high sensitivity of P. jirovecii PCR (6), Pneumocystis colonization cannot be completely excluded. However, taken together, the highly positive PCR test, significant elevation in (1,3)-β-d-glucan, cystic lesions on chest imaging, progressive hypoxemia in the setting of CD4+ lymphocytopenia, and response to trimethoprim-sulfamethoxazole therapy are highly supportive of a diagnosis of Pneumocystis pneumonia.
Respiratory viral infections, particularly influenza, predispose patients to the development of secondary bacterial infections (7) and invasive fungal infections, including aspergillosis, most notably in immunocompromised patients (8). Although no cases of Pneumocystis pneumonia have been reported in patients infected with SARS-CoV-1 or Middle East respiratory syndrome coronavirus, coinfection with P. jirovecii has been reported in HIV and hematopoietic stem cell transplant patients with influenza A infection (9, 10). Furthermore, two cases of Pneumocystis pneumonia and H1N1 influenza A coinfection have been reported in immunocompetent patients, possibly secondary to influenza-induced CD4+ lymphocytopenia (11).
There is emerging evidence that patients with SARS-CoV-2 are at high risk for coinfection (12), and this case highlights the importance of being vigilant about excluding treatable respiratory pathogens, including P. jirovecii. Because COVID-19 and Pneumocystis pneumonia may share common clinical features (e.g., bilateral multifocal infiltrates and profound hypoxemia), coinfection with P. jirovecii may not be appreciated in patients with severe SARS-CoV-2 infection. It may therefore be reasonable to consider additional diagnostic testing for P. jirovecii in patients with SARS-CoV-2 infection, particularly when there are other clinical characteristics that may support coinfection (e.g., elevated lactate dehydrogenase, cystic findings on chest computed tomography), even in the absence of classical P. jirovecii risk factors. Finally, this case extends the potential utility of (1,3)-β-d-glucan testing for diagnosing Pneumocystis pneumonia (13) in patients with suspected SARS-CoV-2 infection, which is particularly relevant given concerns about healthcare transmission associated with performing bronchoscopy in these patients.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Sandra C. Smole, Ph.D., Director of the Massachusetts State Public Health Laboratory, Bureau of Infectious Disease and Laboratory Sciences, for assistance with interpretation of the results of the SARS-CoV-2 real-time RT-PCR assay.
Footnotes
Author Contributions: A.A.M., D.D.B., E.B.G., and L.E.F. contributed to the literature review and data collection and drafted the manuscript. All authors participated in the clinical care of the patient and read, revised, and approved the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202003-0766LE on May 15, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas CF, Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487–2498. doi: 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 3.Cotter TG, Gathaiya N, Catania J, Loftus EV, Jr, Tremaine WJ, Baddour LM, et al. Low risk of pneumonia from Pneumocystis jirovecii infection in patients with inflammatory bowel disease receiving immune suppression. Clin Gastroenterol Hepatol. 2017;15:850–856. doi: 10.1016/j.cgh.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long MD, Farraye FA, Okafor PN, Martin C, Sandler RS, Kappelman MD. Increased risk of Pneumocystis jiroveci pneumonia among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1018–1024. doi: 10.1097/MIB.0b013e3182802a9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lichtenstein GR, Bengtsson B, Hapten-White L, Rutgeerts P. Oral budesonide for maintenance of remission of Crohn’s disease: a pooled safety analysis. Aliment Pharmacol Ther. 2009;29:643–653. doi: 10.1111/j.1365-2036.2008.03891.x. [DOI] [PubMed] [Google Scholar]
- 6.Arcenas RC, Uhl JR, Buckwalter SP, Limper AH, Crino D, Roberts GD, et al. A real-time polymerase chain reaction assay for detection of Pneumocystis from bronchoalveolar lavage fluid. Diagn Microbiol Infect Dis. 2006;54:169–175. doi: 10.1016/j.diagmicrobio.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Martin-Loeches I, van Someren Gréve F, Schultz MJ. Bacterial pneumonia as an influenza complication. Curr Opin Infect Dis. 2017;30:201–207. doi: 10.1097/QCO.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 8.Schauwvlieghe AFAD, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Van Tienen C, et al. Dutch-Belgian Mycosis study group. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 9.Franconi I, Monari C, Tutone M, Ciusa G, Corradi L, Franceschini E, et al. Pneumocystosis as a complication of H1N1 influenza A infection in an HIV-positive patient on effective cART. Open Forum Infect Dis. 2019;6:ofz105. doi: 10.1093/ofid/ofz105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke J, Soubani AO. Influenza and Pneumocystis jirovecii pneumonia in an allogeneic hematopoietic stem cell transplantation recipient: coinfection or superinfection? Transpl Infect Dis. 2018;20:e12802. doi: 10.1111/tid.12802. [DOI] [PubMed] [Google Scholar]
- 11.Marwah V, Singh S, Govind R, Shergill S, Vasan A. Pneumocystis jiroveci pneumonia and H1N1 co-infection in an immunocompetent patient. Indian J Chest Dis Allied Sci. 2019;61:219–220. [Google Scholar]
- 12.Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. doi: 10.1001/jama.2020.6266. [online ahead of print] 15 Apr 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karageorgopoulos DE, Qu JM, Korbila IP, Zhu YG, Vasileiou VA, Falagas ME. Accuracy of β-D-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: a meta-analysis. Clin Microbiol Infect. 2013;19:39–49. doi: 10.1111/j.1469-0691.2011.03760.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.