Abstract
Rationale: Inhaled corticosteroids (ICS) are key treatments for controlling asthma and preventing asthma attacks. However, the responsiveness to ICS varies among individuals. MicroRNAs (miRNAs) have been lauded for their prognostic utility.
Objectives: We hypothesized that circulating miRNAs obtained at baseline/prerandomization in the Childhood Asthma Management Program (CAMP) could serve as biomarkers and biologic mediators of ICS clinical response over the 4-year clinical trial period.
Methods: We selected baseline serum samples from 462 CAMP subjects subsequently randomized to either ICS (budesonide) or placebo. Samples underwent small RNA sequencing, and read counts were normalized and filtered by depth and coverage. Linear regression was used to associate miRNAs with change in FEV1% (prebronchodilator FEV1 as a percent predicted) over the 4-year treatment period in both main effects and interaction models. We validated the function of the top associated miRNAs by luciferase reporter assays of glucocorticoid-mediated transrepression and predicted response to ICS through logistic regression models.
Measurements and Main Results: We identified 7 miRNAs significantly associated with FEV1% change (P ≤ 0.05) and 15 miRNAs with significant interaction (P ≤ 0.05) to ICS versus placebo treatments. We selected three miRNAs for functional validation, of which hsa-miR-155-5p and hsa-miR-532-5p were significantly associated with changes in dexamethasone-induced transrepression of NF-κB. Combined, these two miRNAs were predictive of ICS response over the course of the clinical trial, with an area under the receiver operating characteristic curve of 0.86.
Conclusions: We identified two functional circulating miRNAs predictive of asthma ICS treatment response over time.
Keywords: CAMP, asthma, circulating miRNA, ICS, lung function
At a Glance Commentary
Scientific Knowledge on the Subject
There is significant heterogeneity in the response to asthma medications, and the mechanisms remain unclear. Circulating microRNAs (miRNAs) are potential biologic mediators and prognostic biomarkers of asthma treatment response.
What This Study Adds to the Field
This is the largest study of circulating miRNAs in asthma to date. We identified functional miRNAs in the serum associated with subsequent differential 4-year change in FEV1 in inhaled corticosteroid (ICS) versus placebo treatments of children with asthma. Specifically, hsa-miR-155-5p and hsa-miR-532-5p were associated with long-term lung function change on ICS but not placebo in asthma, functionally modulated the actions of glucocorticoids in an in vitro model, and together can accurately predict response to ICS.
Asthma is a heterogeneous disease that affects more than 300 million people across the world, with direct cost exceeding $85 billion annually in the United States (1, 2). Currently, inhaled corticosteroids (ICS) are the most effective and commonly prescribed antiinflammatory medicines in the treatment of mild to moderate asthma (3). Corticosteroids bind to glucocorticoid receptors (GRs) in the cytoplasm and the complex translocates to the nucleus, where they not only regulate the expression of antiinflammatory genes as a transcription factor but also bind to the coactivator molecules of NF-κB (nuclear factor-κB) and transrepress the inflammatory genes (4). However, the responsiveness to ICS varies significantly among different subjects (5). We have previously demonstrated substantial interindividual variability across multiple asthma cohorts to lung function response to ICS (6). Pharmacogenomics is the study of genetic and genomic factors influencing the interindividual variable response to medications.
MicroRNAs (miRNAs) are small, single-stranded, noncoding RNAs with a length of 21 to 25 nucleotides that primarily bind to the 3′ untranslated regions of mRNAs, resulting in post-translational downregulation of gene expression (7). miRNAs are cell–cell communicators and can be released to the circulation and other extracellular compartments from disease organs, including the lung, where they are stably expressed (8). These extracellular miRNAs have been espoused as noninvasive and sensitive biomarkers in cancer, cardiovascular disease, inflammatory bowel disease, rheumatoid arthritis, and asthma (9–12).
In this study, we sought to identify pharmacogenomic miRNAs associated with ICS response by sequencing circulating miRNAs in nearly 500 subjects with asthma, representing the largest circulating miRNA analysis of asthma to date. We identified 7 miRNAs with significant main effects and 15 miRNAs with significant interaction effects on the change of lung function after ICS treatment. We subsequently functionally validated the top miRNAs and formulated prognostic models on the basis of these findings. We discovered two miRNAs strongly associated with, and prognostic of, ICS response that also functionally alter cellular glucocorticoid transrepression.
Methods
Please see online supplement for full details.
Participant Selection
We selected 462 participants from the Childhood Asthma Management Program (CAMP) (ClinicalTrials.gov Identifier: NCT00000575) subjects (188 from the budesonide treatment group and 274 from the placebo group) on the basis of availability of serum (13). These participants provided the serum samples at baseline, before randomization, and were followed up after randomization for 4 to 6 years (for details, see online supplement). Lung function, including prebronchodilator FEV1 as a percent predicted (FEV1%), was measured at randomization and at multiple times during follow-up. Equations for FEV1% for age, sex, and height were race-corrected according to Coultas and coworkers for Hispanics and Knudson and coworkers for all other ethnic groups (14, 15). Our primary outcome was defined as the difference of FEV1% between the 48-month follow-up visit and randomization; FEV1 was the primary outcome in CAMP (16).
Small RNA Sequencing and Profiling
Total RNA was isolated from serum samples by the Qiagen miRNeasy Serum/Plasma extraction kit and QIAcube automation. All samples were quantified using the Nanodrop spectrophotometer (Thermo Fisher) before plating. Small RNA sequencing (RNA-seq) libraries were prepared using the Norgen Biotek Small RNA Library Prep Kit and then sequenced on the Illumina NextSeq 500 platform at 51-bp single-end reads. ExceRpt was used to assess the read quality and annotate miRNAs (17).
The read count was log transformed and normalized by quantile normalization (R package: preprocessCore) (18). Raw read counts less than five were filtered out and miRNAs with coverage <80% of all subjects were removed. Coverage refers to the number of subjects in whom a specific miRNA was detected at five or more counts. The data have been submitted to Gene Expression Omnibus (GSE134897).
Statistical Analysis
Linear regression was used to investigate the association between miRNAs and the change of FEV1% over the course of the 4-year clinical trial. Age, sex, race, total eosinophil count, and atopic dermatitis diagnosed by doctor were considered a priori as confounders in the regression model. The association between miRNAs and the change of FEV1% was studied through both univariate and multivariate linear regression model stratified by treatment groups first, and then the multivariate interaction between miRNA and treatment groups was considered. The false discovery rate (FDR) was calculated using the R package “fdrtool” (19). Permutation testing was used on significant miRNAs for additional statistical validation.
Functional Validation
We estimated the function of miRNAs on glucocorticoid-induced transrepression through an established reporter cell line A549/NF-κB-luc (20, 21). These cells are responsive to IL-1β stimulation, which is reduced by dexamethasone (Dex), a glucocorticoid receptor agonist, indicating that these cells exhibit glucocorticoid-mediated tethered transrepression of NF-κB. A549/NF-κB-luc reporter cells were transfected with either 25 nM of scramble control (AllStars Negative Control siRNA; Qiagen) or the indicated miRNA mimics (Qiagen) using RNAiMax (Life Technology) according to the manufacturer’s protocol. After 48 hours post-transfection, the cells were stimulated with 5 ng/ml IL1β ± 10 nM dexamethasone. Luciferase assays were performed after 18 hours of treatment. The luciferase activity in scramble control cells with 5 ng/ml IL-1β treatment was normalized to 1. The luciferase activities of cells treated with IL-1β + Dex and transfected with respective mimics were calculated and compared with those of cells treated with IL-1β + Dex and transfected with scramble control. Each treatment condition has four replicates.
Prediction
We built a multivariate logistic regression model to predict the ICS response in the treatment group. To decrease the intrinsic difference of FEV1%, we predicted the highest versus lowest quartiles of response. For this, 86 ICS-treated subjects, including 43 highly responsive subjects and 43 poorly responsive subjects, were selected. The normalized miRNA counts with the potential confounders, including age, sex, race, eosinophil count, and atopic dermatitis, were set as input variables, and the ICS responses were set as outcome (high lung function Y = 1 and low lung function Y = 0).
Results
Small RNA-Seq Data Results and Quality Control
The total input reads for all of our samples were nearly 10 billion, with average reads for each sample of more than 19 million. Among the reads mapped to genome, miRNA reads account for about 30%, with averaging more than 1 million reads per sample (see Table E1 in the online supplement). The miRNA reads at the expected 22-bp peak make up the largest proportion of total read count (Figures E1 and E2). About 90% of samples contain more than 1 million reads (Figure E3).
Baseline Characteristics
A total of 462 subjects were interrogated, of whom 188 subjects were selected from the ICS treatment group and 274 subjects were selected from the placebo group. The baseline characteristics of the 462 subjects are shown in Table 1. Race (P = 6.1 × 10−10, Fisher’s exact test) was significantly different between ICS treatment group and placebo group. The primary outcome of changes in FEV1% between baseline and follow-up was also significant (P = 2.8 × 10−4, t test) with a large SD. In a univariate analysis, no miRNAs were associated with race, indicating that race was not a significant confounder of our reported results (data not shown).
Table 1.
Childhood Asthma Management Program Subset Study Population Characteristics
| Characteristic | Budesonide (n = 188) | Placebo (n = 274) | P Value |
|---|---|---|---|
| Age, yr | 9.0 ± 2.1 | 8.8 ± 2.1 | 0.28 |
| Sex | 0.88 | ||
| Male | 109 (58.0) | 162 (59.1) | |
| Female | 79 (42.0) | 112 (40.9) | |
| Race | 6.1 × 10−10* | ||
| White | 127 (67.6) | 235 (85.8) | |
| Black | 41 (21.8) | 39 (14.2) | |
| Hispanic | 20 (10.6) | 0 (0) | |
| Total eosinophil count, log10 | 0.79 ± 0.3 | 0.78 ± 0.3 | 0.70 |
| Child had atopic dermatitis and saw doctor | 0.75 | ||
| Yes | 133 (70.7) | 194 (70.8) | |
| No | 48 (25.5) | 66 (24.1) | |
| Not available | 7 (3.7) | 14 (5.1) | |
| Age at first asthma symptoms, yr | 3.06 ± 2.2 | 3.0 ± 2.6 | 0.83 |
| Household income | 0.14* | ||
| <$15,000 | 17 (9.0) | 10 (3.6) | |
| $15,000–29,000 | 30 (16.0) | 42 (15.3) | |
| $30,000–49,000 | 56 (29.8) | 96 (35.0) | |
| >$50,000 | 78 (41.5) | 117 (42.7) | |
| Decline response | 5 (2.7) | 8 (2.9) | |
| Don't know | 1 (0.5) | 0 (0.0) | |
| Seen doctor for hay fever | 0.69 | ||
| Yes | 76 (40.4) | 96 (35.0) | |
| No | 27 (14.4) | 40 (14.6) | |
| Missing | 85 (45.2) | 138 (50.4) | |
| Severity of asthma | 0.27 | ||
| Mild | 80 (42.6) | 132 (48.2) | |
| Moderate | 108 (57.4) | 142 (51.8) | |
| FEV1 PC20 meth, mg/ml | 1.82 ± 2.2 | 2.12 ± 2.5 | 0.18 |
| FEV1% predicted at baseline | 93.0 ± 13.2 | 95.4 ± 13.6 | 0.06 |
| FEV1% predicted at last follow-up | 96.8 ± 12.8 | 94.4 ± 14 | 0.06 |
| Changes of FEV1% predicted between baseline and follow-up | 4.1 ± 12.8 | −0.6 ± 12.9 | 2.8 × 10−4 |
Definition of abbreviation: PC20 meth = the provocative concentration of methacholine that results in a 20% drop in FEV1.
Data presented as n (%) or mean ± SD.
P value from Fisher’s exact test.
Significant miRNAs in the ICS Treatment Group
We performed both univariate and multivariate linear regression models to study the association between miRNAs and the change of FEV1% while on ICS, and the results are shown in Table 2.
Table 2.
Significant miRNAs in Budesonide (Inhaled Corticosteroid) Treatment Group
| miRNA | Univariate |
Multivariate* |
||
|---|---|---|---|---|
| β | P Value | β | P Value | |
| hsa-miR-155-5p | 2.02 | 0.04 | 2.90 | 0.002 |
| hsa-miR-532-5p | −3.52 | 0.001 | −3.21 | 0.002 |
| hsa-miR-4433b-5p | 2.20 | 0.003 | 2.14 | 0.003 |
| hsa-miR-345-5p | −1.51 | 0.05 | −2.21 | 0.003 |
| hsa-miR-652-3p | −2.48 | 0.03 | −2.67 | 0.02 |
| hsa-miR-126-3p | 2.64 | 0.02 | 2.61 | 0.02 |
| hsa-miR-335-5p | 2.12 | 0.04 | 2.35 | 0.03 |
Definition of abbreviation: miRNA = microRNA.
Adjusted for age, sex, race, eosinophil count, and atopic dermatitis.
In the ICS treatment group, seven miRNAs (hsa-miR-155-5p, hsa-miR-4433b-5p, hsa-miR-532-5p, hsa-miR-345-5p, hsa-miR-652-3p, hsa-miR-126-3p, and hsa-miR-335-5p) were significantly associated with the change of FEV1% (Table 2). After adjusting for age, sex, race, total eosinophil count, and atopic dermatitis in the multivariate analysis, the associations for each of the seven remained significant. Although hsa-miR-532-5p, hsa-miR-345-5p, and hsa-miR-652-3p were associated in a negative direction, the other four miRNAs had a positive association direction, which means that with increasing miRNA count, the change in FEV1% increases and patients with asthma will have improved lung function (i.e., better response to ICS). Hsa-miR-155-5p demonstrated the strongest positive effect (β = 2.90; confidence interval, 1.08 to 4.71; P = 0.002) and hsa-miR-532-5p has the strongest negative effect (β = −3.21; confidence interval, −5.23 to −1.18; P = 0.002) in the multivariate linear regression analysis. Our top four miRNAs, hsa-miR-155-5p, hsa-miR-532-5p, hsa-miR-4433b-5p, and hsa-miR-345-5p, were significant at an FDR <0.10. To further assess the significance of our results, we performed permutation tests for the seven miRNAs. After permutation, all of the miRNAs remained significant, indicating that the associations were not driven by statistical chance alone (Table E2).
Significant miRNAs by Treatment Interactions
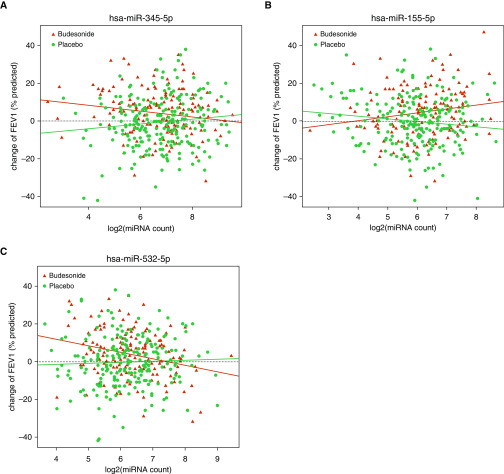
Fifteen miRNAs were associated with the change of FEV1% when considering their interaction with treatment group (Table 3). The effect modification was seen in both positive and negative directions. For example, increased expression of hsa-miR-155-5p (β = 3.88; P = 0.002) was associated with relative increase in FEV1% in ICS group versus placebo (Figure 1A), whereas increased expression of hsa-miR-345-5p (β = −3.74; P = 0.0003) and hsa-miR-532-5p (β = −3.87; P = 0.006) was associated with decreases in FEV1% in ICS group relative to placebo (Figures 1B and 1C). Our top five treatment interaction miRNAs were significant at an FDR of <0.10, with the lowest FDR-adjusted P value belonging to hsa-miR-345-5p (0.03). As above, we performed permutation analyses for the 15 significant miRNAs. All the 15 miRNAs remain significant in the permutation test (Table E3).
Table 3.
Significant miRNAs by the Treatment Interaction Analysis
| miRNA | Univariate Interaction |
Multivariate Interaction* |
||
|---|---|---|---|---|
| β | P Value | β | P Value | |
| hsa-miR-345-5p | −2.78 | 0.01 | −3.74 | 0.0003 |
| hsa-miR-155-5p | 3.36 | 0.01 | 3.88 | 0.002 |
| hsa-miR-652-3p | −3.98 | 0.01 | −4.83 | 0.002 |
| hsa-miR-4433b-5p | 2.80 | 0.01 | 3.05 | 0.003 |
| hsa-miR-335-5p | 2.80 | 0.05 | 4.00 | 0.005 |
| hsa-let-7e-5p | 2.66 | 0.02 | 3.02 | 0.006 |
| hsa-miR-532-5p | −4.08 | 0.003 | −3.87 | 0.006 |
| hsa-miR-500a-3p | −2.96 | 0.02 | −3.44 | 0.007 |
| hsa-miR-15b-5p | 2.57 | 0.02 | 2.75 | 0.01 |
| hsa-miR-1180-3p | −2.13 | 0.02 | −2.19 | 0.02 |
| hsa-miR-186-5p | −2.78 | 0.05 | −3.13 | 0.02 |
| hsa-miR-126-3p | 3.10 | 0.04 | 3.20 | 0.03 |
| hsa-miR-424-3p | −2.30 | 0.05 | −2.44 | 0.03 |
| hsa-miR-425-3p | −3.36 | 0.02 | −3.05 | 0.03 |
| hsa-miR-502-3p | −2.76 | 0.03 | −2.60 | 0.04 |
Definition of abbreviation: miRNA = microRNA.
Adjusted for age, sex, race, eosinophil count, and atopic dermatitis.
Figure 1.
Scatter plots of (A) hsa-miR-345-5p, (B) hsa-miR-155-5p, and (C) hsa-miR-532-5p in interaction analysis. Red points denote the subjects in the inhaled corticosteroid treatment group and green points denote the subjects in the placebo group. The red line represents the regression line of the inhaled corticosteroid treatment group and the green line represents the regression line of the placebo group. miRNA = microRNA.
Effect of miR-155-5p, miR-532-5p, and miR-345-5p on the Antiinflammatory Activity of GR Function
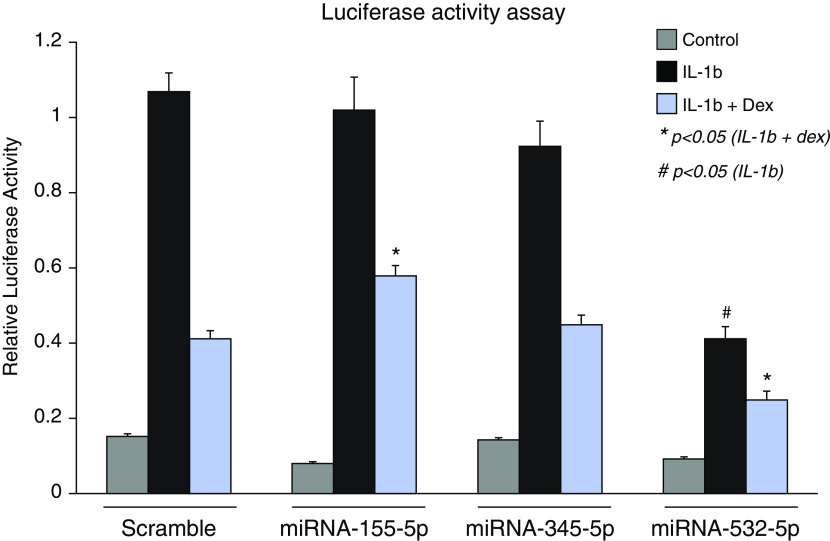
We selected the top five miRNAs (hsa-miR-155-5p, hsa-miR-4433b-5p, hsa-miR-532-5p, hsa-miR-345-5p, and hsa-miR-652-3p) in the stratified (main ICS effects) analysis as the candidates to be functionally validated, noting that these five were also significant in the interaction study. We did a literature review on the five miRNAs, and hsa-miR-4433b-5p and hsa-miR-652-3p were excluded because very few papers were found supporting a relevant functional effect or other associations related to inflammation or asthma. We therefore selected three miRNAs (hsa-miR-155-5p, hsa-miR-532-5p, and hsa-miR-345-5p) for subsequent functional validation using a cell-based GR activity assay that measures dexamethasone-mediated NF-κB repression (Figure 2).
Figure 2.
Effect of miR-345-5p, miR-155-5p, and miR-532-5p on the response of NF-κB (nuclear factor-κB) reporter cells to IL-1b ± dexamthasone (Dex). miR = microRNA.
On the basis of the luciferase activity assay results, miR-155-5p and miR-532-5p have a significant influence on GR-related NF-κB transrepression. Compared with the scramble control, miR-155-5p significantly inhibited the repressive activity of dexamethasone, whereas miR-532-5p significantly promoted the repression of dexamethasone. These observed functional effects were consistent with the clinical ICS response data, which demonstrated opposite direction of effects for miR-155-5p and miR-532-5p. miR-345-5p did not affect the GR-mediate transrepression, implying that it may act via a different mechanism. In addition to its effect on dexamethasone signaling, miR-532-5p also significantly inhibited the NF-κB proinflammatory signaling pathway even in the absence of dexamethasone.
Prediction of ICS Response on the Basis of miRNAs
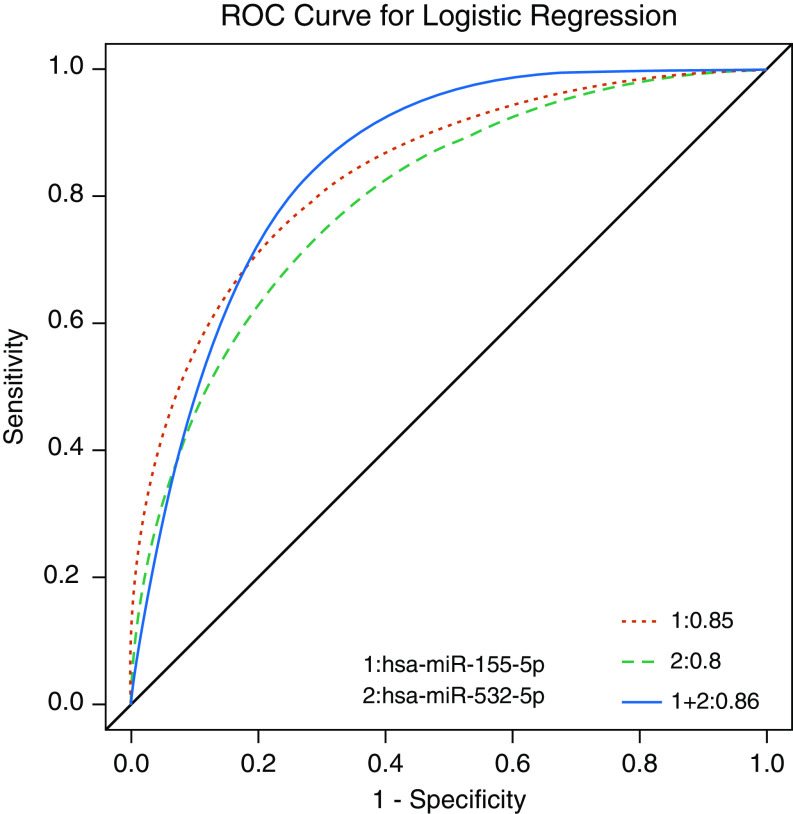
We evaluated the prognostic power of the two functionally validated miRNAs, hsa-miR-155-5p and hsa-miR-532-5p, to distinguish the highest quartile of responders (mean ± SD FEV1 change, 20.42 ± 7.27%) from the lowest (mean ± SD FEV1 change, −11.14 ± 6.84%). The area under the receiver operating characteristic (AUROC) curve was used to illustrate the performance of our logistic regression model in prediction (Figure 3). Our model demonstrated a high positive predictive value with low false-positive rates across the two candidate miRNAs. Individually, the AUROC of hsa-miR-155-5p was 0.85 and of hsa-miR-532-5p was 0.80. When combined, the AUROC for the two miRNAs reached 0.86, suggesting good to excellent prognostic ability. We also investigated the AUROC for the entire cohort, including highest quartile of response versus the lowest three quartiles (hsa-miR-155-5p: 0.77; hsa-miR-532-5p: 0.74; combination: 0.78).
Figure 3.
Prediction of hsa-miR-155-5p and hsa-miR-532-5p in logistic regression model. The red dotted curve denotes the performance of hsa-miR-155-5p and the green dashed curve denotes the performance of hsa-miR-532-5p. The blue solid curve represents the effect of combined hsa-miR-155-5p and hsa-miR-532-5p. miR = microRNA; ROC = receiver operating characteristic.
Discussion
Our study is the first to correlate long-term ICS responsiveness with circulating miRNAs and demonstrates that miRNAs are potential pharmacogenomic predictors of the response to ICS. Specifically, we identified 15 miRNAs that have significant effect modification in the ICS treatment group. We selected three miRNAs (hsa-miR-345-5p, hsa-miR-155-5p, and hsa-miR-532-5p) for functional validation, and two of them (hsa-miR-155-5p and hsa-miR-532-5p) were significantly associated with the cortisol-dependent NF-κB transrepression. We predicted the 4-year longitudinal ICS response in the treatment group through a logistic regression model and the AUROC of the two miRNAs reached 0.86. These miRNAs may explain the variability of subjects in response to ICS treatment and thus serve as powerful pharmacogenomic biomarkers.
According to both our main effects and interaction analyses, for subjects on ICS, hsa-miR-155-5p had a positive effect on lung function over time, whereas hsa-miR-532-5p had a negative effect, with little effect on the placebo lung function change over time (Figure 1). Notably, our functional data also demonstrated an effect that was in opposite directions in how these miRNAs modulate GR-mediated transrepression, with miR-155-5p decreasing transrepression and miR-532-5p increasing it (Figure 2).
We note that, on the surface, the functional effects reported may appear to be opposite of that expected with higher circulating miR-155-5p associated with improved ICS response, but miR-155-5p mimics resulting in decreased transrepression (increased inflammation) in our in vitro airway epithelial cell model and vice versa for miR-532-5p. miR-155-5p is widely expressed in most human tissues and strongly associated with immune system functioning (22, 23); less is known about miR-532-5p, and ours is the first description of miR-532-5p in asthma. To delineate potential explanations for our observed findings, we therefore focus on known studies involving miR-155.
Panganiban and colleagues (24) compared extracellular (plasma) miRNAs between 35 patients with asthma and 19 nonallergic subjects without asthma. They demonstrated that miR-155-5p was substantially elevated in healthy subjects (4.36-fold change healthy subjects vs. subjects with asthma; P < 0.001). In contrast, Jardim and colleagues (25) evaluated miRNA expression in human bronchial epithelial cells from 23 patients with asthma and 23 healthy control subjects. They noted that miR-155-5p was elevated in patients with asthma (2.3-fold change; P < 0.01). Thus, healthy subjects express more miR-155-5p in the extracellular circulatory system and less in airway epithelial cells, with the converse true for patients with asthma. Two additional epidemiologic studies also demonstrated that hsa-miR-155-5p was decreased in extracellular exhaled breath condensate in asthma (26), as well as symptomatic asthma (27), compared with healthy control subjects. Extrapolating, it is reasonable to conclude that miR-155-5p concentrations would be increased extracellularly (here, in the serum) in association with milder asthma, which is more responsive to ICS medications (Figure E4).
Our in vitro studies suggest that miR-155-5p is an intracellular proinflammatory mediator of asthma. This is also further confirmed by other in vitro and in vivo studies. miR-155-5p knockout mice demonstrate decreased allergic inflammation in the airways (28). In addition, cockroach extract–treated human and murine bronchial epithelial cells result in significantly increased miR-155-5p, a response that is abrogated in the miR-155 knockout mouse (29). Antagonism of miR-155-5p has been postulated to have corticosteroid-like effects in the treatment of asthma, on the basis of similar resulting immunophenotypes (30); conversely, an abundance of miR-155-5p intracellularly can overcome the antiinflammatory role of glucocorticoids (31, 32). Furthermore, in a study by Mann and colleagues (33), NF-κB activated miR-155-5p expression, and miR-155-5p also amplified and positively regulated NF-κB proinflammatory activity. Although this study was performed in mouse bone marrow–derived macrophages, it implies that miR-155 could strengthen the inflammatory activity of NF-κB intracellularly. Together, these studies are fully consistent with our functional studies in airway epithelial cells demonstrating that miR-155 decreases glucocorticoid-induced NF-κB tethered transrepression.
miR-532-5p has been previously found to be highly expressed in patients with asthma in bronchial smooth muscle cells (34). A recent study demonstrated that miR-532-5p could downregulate expression of SESTD1 and TAB3 in human cells and play an important role in controlling RNA virus infections (35). It was also shown to be upregulated in good responders to leukemia treatment when compared with chemotherapy-resistant subjects, implying an antiinflammatory effect (36). Our analysis showed that miR-532-5p could inhibit IL-1β–induced NF-κB even in the absence of dexamethasone, which suggests that miR-532-5p expression may be a potential antiinflammatory mediator to attenuate the symptoms of asthma.
miR-345-5p could also be involved in NF-κB signaling pathway, although it was not significant in our luciferase activity assay. In a study by Dang and colleagues (37), miR-345-5p directly targets the 3′ untranslated regions of RelA in human monocytes, and RelA is known as a p65 activator and is a subunit of NF-κB.
We have predicted the improvement of lung function in the ICS treatment group with hsa-miR-155-5p and hsa-miR-532-5p through a logistic regression model. When we combined the two miRNAs together, the AUROC reached 0.86, which suggests that they may be potential serum biomarkers to characterize the response to ICS treatment among different patients with asthma. According to our study, relative to the placebo group, hsa-miR-155-5p was associated with an improvement of 5.24% in the change of FEV1% within the interquartile range of normalized read count, whereas hsa-miR-532-5p demonstrated a decrement of 6.27%.
Corticosteroids have a complex set of mechanisms to promote an antiinflammatory response, and the effect is markedly variable in patients even with similar clinical features. In this scenario, pharmacogenomics emerges by combining pharmacology and genomics to help explain the drug response (38, 39). Our study has successfully correlated the miRNA expression with the variability in response to ICS, yielding biomarkers with good to excellent prognostic capabilities. Although multiple other studies have investigated the pharmacogenomic response to ICS through genetic variants (40, 41), the predictive capability for SNPs alone has been modest at best (42). We believe that pharmacogenomics of miRNAs is a promising way to advance personalized, precision medicine.
Our study has several potential limitations. First, our study is based on the CAMP cohort, so the subjects are all patients with childhood asthma. Therefore, the results of our study may be difficult to generalize to adult asthma without additional testing. We acknowledge the lack of a replication cohort, as very few asthma clinical trial cohorts of this long follow-up duration with the detailed phenotypic and sample availability of CAMP are available. However, the sample size used for this study is an order of magnitude larger than any prior extracellular miRNA study of asthma, helping to support the validity of these results. Finally, although the two functionally validated miRNAs demonstrated opposite directions of effect for response to corticosteroids both clinically and in vitro, it is difficult to precisely quantitate the equivalent in vivo to in vitro “miRNA dose” to make further determinations related to translational targeting.
In summary, our findings show that circulating miRNAs are associated with, and predictive of, asthma treatment–specific responses over time. Detailing these and other miRNAs may lead to novel mechanistic understanding behind the improvements noted with ICS and other asthma therapies or to potential alterative therapeutic strategies for asthma.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank all members of the Childhood Asthma Management Program (CAMP) research group and volunteers who have participated in this study.
Footnotes
Supported by NIH grants R01 HL127332, R01 HL129935, R01 HL092197, U01HL065899, and R01 HL139634.
Author Contributions: J.L. performed the main analysis and wrote the manuscript. R.P. and Q.L. validated the candidate microRNAs through luciferase activity assay. A.T.K. assisted the analysis and revised the manuscript. M.J.M. and S.T.W. gave comments and revised the manuscript. L.F. and R.P.C. prepared the performance study data. K.G.T. conceived the study, revised the manuscript, and was responsible for funding of the study. All authors have read and approved the final manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201907-1454OC on April 9, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Martinez FD, Vercelli D. Asthma. Lancet. 2013;382:1360–1372. doi: 10.1016/S0140-6736(13)61536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010 NCHS Data Brief 2012. 94):1–8. [PubMed] [Google Scholar]
- 3.Barnes PJ. Inhaled corticosteroids. Pharmaceuticals (Basel) 2010;3:514–540. doi: 10.3390/ph3030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 5.Szefler SJ, Martin RJ, King TS, Boushey HA, Cherniack RM, Chinchilli VM, et al. Asthma Clinical Research Network of the National Heart Lung, and Blood Institute. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol. 2002;109:410–418. doi: 10.1067/mai.2002.122635. [DOI] [PubMed] [Google Scholar]
- 6.Tantisira KG, Lake S, Silverman ES, Palmer LJ, Lazarus R, Silverman EK, et al. Corticosteroid pharmacogenetics: association of sequence variants in CRHR1 with improved lung function in asthmatics treated with inhaled corticosteroids. Hum Mol Genet. 2004;13:1353–1359. doi: 10.1093/hmg/ddh149. [DOI] [PubMed] [Google Scholar]
- 7.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 8.Turchinovich A, Samatov TR, Tonevitsky AG, Burwinkel B. Circulating miRNAs: cell-cell communication function? Front Genet. 2013;4:119. doi: 10.3389/fgene.2013.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kishikawa T, Otsuka M, Ohno M, Yoshikawa T, Takata A, Koike K. Circulating RNAs as new biomarkers for detecting pancreatic cancer. World J Gastroenterol. 2015;21:8527–8540. doi: 10.3748/wjg.v21.i28.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viereck J, Thum T. Circulating noncoding RNAs as biomarkers of cardiovascular disease and injury. Circ Res. 2017;120:381–399. doi: 10.1161/CIRCRESAHA.116.308434. [DOI] [PubMed] [Google Scholar]
- 11.Zahm AM, Thayu M, Hand NJ, Horner A, Leonard MB, Friedman JR. Circulating microRNA is a biomarker of pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2011;53:26–33. doi: 10.1097/MPG.0b013e31822200cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kho AT, Sharma S, Davis JS, Spina J, Howard D, McEnroy K, et al. Circulating MicroRNAs: association with lung function in asthma. PLoS One. 2016;11:e0157998. doi: 10.1371/journal.pone.0157998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Covar RA, Fuhlbrigge AL, Williams P, Kelly HW the Childhood Asthma Management Program Research Group. The childhood asthma management Program (CAMP): contributions to the understanding of therapy and the natural history of childhood asthma. Curr Respir Care Rep. 2012;1:243–250. doi: 10.1007/s13665-012-0026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coultas DB, Howard CA, Skipper BJ, Samet JM. Spirometric prediction equations for Hispanic children and adults in New Mexico. Am Rev Respir Dis. 1988;138:1386–1392. doi: 10.1164/ajrccm/138.6.1386. [DOI] [PubMed] [Google Scholar]
- 15.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 16.Szefler S, Weiss S, Tonascia J, Adkinson NF, Bender B, Cherniack R, et al. Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 17.Rozowsky J, Kitchen RR, Park JJ, Galeev TR, Diao J, Warrell J, et al. exceRpt: a comprehensive analytic platform for extracellular RNA profiling. Cell Syst. 2019;8:352–357, e3. doi: 10.1016/j.cels.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolstad B. preprocessCore: a collection of pre-processing functions. R package, version 1.44. 0. 2018 [accessed 2019 Jan 15]. Available from: https://rdrr.io/bioc/preprocessCore/
- 19.Strimmer K. fdrtool: a versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics. 2008;24:1461–1462. doi: 10.1093/bioinformatics/btn209. [DOI] [PubMed] [Google Scholar]
- 20.Jiang X, Dahlin A, Weiss ST, Tantisira K, Lu Q. A high-throughput chemical screen identifies novel inhibitors and enhancers of anti-inflammatory functions of the glucocorticoid receptor. Sci Rep. 2017;7:7405. doi: 10.1038/s41598-017-07565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGeachie MJ, Clemmer GL, Hayete B, Xing H, Runge K, Wu AC, et al. Systems biology and in vitro validation identifies family with sequence similarity 129 member A (FAM129A) as an asthma steroid response modulator. J Allergy Clin Immunol. 2018;142:1479–1488, e12. doi: 10.1016/j.jaci.2017.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin MM, Lee EJ, Buckenberger JA, Schmittgen TD, Elton TS. MicroRNA-155 regulates human angiotensin II type 1 receptor expression in fibroblasts. J Biol Chem. 2006;281:18277–18284. doi: 10.1074/jbc.M601496200. [DOI] [PubMed] [Google Scholar]
- 23.O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci USA. 2007;104:1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panganiban RP, Wang Y, Howrylak J, Chinchilli VM, Craig TJ, August A, et al. Circulating microRNAs as biomarkers in patients with allergic rhinitis and asthma. J Allergy Clin Immunol. 2016;137:1423–1432. doi: 10.1016/j.jaci.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 25.Jardim MJ, Dailey L, Silbajoris R, Diaz-Sanchez D. Distinct microRNA expression in human airway cells of asthmatic donors identifies a novel asthma-associated gene. Am J Respir Cell Mol Biol. 2012;47:536–542. doi: 10.1165/rcmb.2011-0160OC. [DOI] [PubMed] [Google Scholar]
- 26.Pinkerton M, Chinchilli V, Banta E, Craig T, August A, Bascom R, et al. Differential expression of microRNAs in exhaled breath condensates of patients with asthma, patients with chronic obstructive pulmonary disease, and healthy adults. J Allergy Clin Immunol. 2013;132:217–219. doi: 10.1016/j.jaci.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Mendes FC, Paciência I, Ferreira AC, Martins C, Rufo JC, Silva D, et al. Development and validation of exhaled breath condensate microRNAs to identify and endotype asthma in children. PLoS One. 2019;14:e0224983. doi: 10.1371/journal.pone.0224983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malmhall C, Alawieh S, Lu Y, Sjostrand M, Bossios A, Eldh M, et al. MicroRNA-155 is essential for T(H)2-mediated allergen-induced eosinophilic inflammation in the lung. J Allergy Clin Immunol. 2014;133:1429–1438, 1438, e1-7. doi: 10.1016/j.jaci.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Qiu L, Zhang Y, Do DC, Ke X, Zhang S, Lambert K, et al. miR-155 modulates cockroach allergen- and oxidative stress-induced cyclooxygenase-2 in asthma. J Immunol. 2018;201:916–929. doi: 10.4049/jimmunol.1701167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou H, Li J, Gao P, Wang Q, Zhang J. miR-155: a novel target in allergic asthma. Int J Mol Sci. 2016;17:E1773. doi: 10.3390/ijms17101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang ZH, Liang YB, Tang H, Chen ZB, Li ZY, Hu XC, et al. Dexamethasone down-regulates the expression of microRNA-155 in the livers of septic mice. PLoS One. 2013;8:e80547. doi: 10.1371/journal.pone.0080547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng Y, Xiong S, Jiang P, Liu R, Liu X, Qian J, et al. Glucocorticoids inhibit lipopolysaccharide-mediated inflammatory response by downregulating microRNA-155: a novel anti-inflammation mechanism. Free Radic Biol Med. 2012;52:1307–1317. doi: 10.1016/j.freeradbiomed.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 33.Mann M, Mehta A, Zhao JL, Lee K, Marinov GK, Garcia-Flores Y, et al. An NF-κB-microRNA regulatory network tunes macrophage inflammatory responses. Nat Commun. 2017;8:851. doi: 10.1038/s41467-017-00972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexandrova E, Miglino N, Hashim A, Nassa G, Stellato C, Tamm M, et al. Small RNA profiling reveals deregulated phosphatase and tensin homolog (PTEN)/phosphoinositide 3-kinase (PI3K)/Akt pathway in bronchial smooth muscle cells from asthmatic patients. J Allergy Clin Immunol. 2016;137:58–67. doi: 10.1016/j.jaci.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 35.Slonchak A, Shannon RP, Pali G, Khromykh AA. Human MicroRNA miR-532-5p exhibits antiviral activity against west nile virus via suppression of host genes SESTD1 and TAB3 required for virus replication. J Virol. 2015;90:2388–2402. doi: 10.1128/JVI.02608-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosakhani N, Räty R, Tyybäkinoja A, Karjalainen-Lindsberg ML, Elonen E, Knuutila S. MicroRNA profiling in chemoresistant and chemosensitive acute myeloid leukemia. Cytogenet Genome Res. 2013;141:272–276. doi: 10.1159/000351219. [DOI] [PubMed] [Google Scholar]
- 37.Dang TM, Wong WC, Ong SM, Li P, Lum J, Chen J, et al. MicroRNA expression profiling of human blood monocyte subsets highlights functional differences. Immunology. 2015;145:404–416. doi: 10.1111/imm.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tse SM, Tantisira K, Weiss ST. The pharmacogenetics and pharmacogenomics of asthma therapy. Pharmacogenomics J. 2011;11:383–392. doi: 10.1038/tpj.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park HW, Tantisira KG, Weiss ST. Pharmacogenomics in asthma therapy: where are we and where do we go? Annu Rev Pharmacol Toxicol. 2015;55:129–147. doi: 10.1146/annurev-pharmtox-010814-124543. [DOI] [PubMed] [Google Scholar]
- 40.Tantisira KG, Hwang ES, Raby BA, Silverman ES, Lake SL, Richter BG, et al. TBX21: a functional variant predicts improvement in asthma with the use of inhaled corticosteroids. Proc Natl Acad Sci USA. 2004;101:18099–18104. doi: 10.1073/pnas.0408532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tantisira KG, Lasky-Su J, Harada M, Murphy A, Litonjua AA, Himes BE, et al. Genomewide association between GLCCI1 and response to glucocorticoid therapy in asthma. N Engl J Med. 2011;365:1173–1183. doi: 10.1056/NEJMoa0911353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGeachie MJ, Wu AC, Chang HH, Lima JJ, Peters SP, Tantisira KG. Predicting inhaled corticosteroid response in asthma with two associated SNPs. Pharmacogenomics J. 2013;13:306–311. doi: 10.1038/tpj.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.