Coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a real pandemic. After a median incubation period of 5 days, the disease occurs in different stages, inducing upper and lower airway responses in mild disease (80–90% of patients) and progressing to bilateral pneumonia in severe disease (10–20%) (1–3). A subgroup of patients with severe COVID-19 develops acute respiratory distress syndrome, requiring mechanical ventilation on intensive care. COVID-19 patients with preexisting comorbid conditions such as chronic respiratory diseases have worse disease outcomes, including a higher incidence of the need for hospitalization, ICU admission, and mortality (3, 4). However, it remains to be further investigated how preexisting chronic inflammatory airway diseases, such as asthma and chronic obstructive pulmonary disease (COPD), and their treatment might modify the risk for SARS-CoV-2 infection and development of COVID-19. Approximately 300 million individuals worldwide have asthma (5). Considering that a significant proportion of individuals with asthma is confronted with COVID-19, it is crucial to understand which asthma patients are particularly at risk and how inhaled corticosteroids (ICS)—the cornerstone of asthma treatment—may influence morbidity and mortality associated with COVID-19. Long-term treatment with systemic corticosteroids (e.g., in transplant patients) is immunosuppressive, increasing the risk and severity of viral infections. Because of the potential risk for worse disease outcomes, the World Health Organization does not recommend systemic corticosteroid treatment in COVID-19 (6), unless if indicated for other reasons such as acute asthma or COPD exacerbations requiring a short course of oral corticosteroids. These recommendations have caused doubt and uncertainty among patients with asthma and physicians on whether ICS therapy should be maintained during this pandemic. However, withdrawal of ICS treatment puts asthma patients at risk of severe exacerbations. A recent meta-analysis on COVID-19 outcomes in patients with chronic respiratory diseases using ICS concluded that there is currently insufficient evidence to abandon the well-established ICS treatment in asthma (7). There is thus an urgent need to elucidate demographic and clinical characteristics that determine disease outcomes of COVID-19 in asthma, to investigate the impact of ICS and to unravel the underlying pathogenic mechanisms.
In this issue of the Journal, the elegant study by Peters and colleagues (pp. 83–90) provides important insights in the complex interplay between asthma, ICS, SARS-CoV-2 infection and COVID-19 (8). The authors hypothesized that differences in the expression of ACE2 (angiotensin-converting enzyme 2) and TMPRSS2 (transmembrane protease serine 2) may modulate the individual susceptibility to and clinical course of SARS-CoV-2 infection and thus identify asthma subgroups at risk for COVID-19 morbidity. Whereas the spike protein of SARS-CoV-2 binds to ACE2 as receptor during viral attachment to host cells, viral entry is also facilitated by priming of the spike protein by the membrane-bound protease TMPRSS2 (9). By investigating induced sputum samples from 330 participants of the SARP-3 (Severe Asthma Research Program-3) program, a large and well-characterized cohort of asthma subjects (60% severe asthma) and 79 healthy controls, Peters and colleagues made three major discoveries. First, they found that there were no significant differences in gene expression of ACE2 in sputum between asthma and healthy subjects, suggesting that asthma subjects might not be at increased risk of COVID-19. This contrasts with the increased expression of ICAM-1 (intercellular adhesion molecule 1) in sputum of asthmatics. ICAM-1 is the receptor for rhinovirus, which causes only limited upper airway symptoms in healthy individuals, but can elicit protracted lower airway symptoms and severe exacerbations in asthmatics. Second, they discovered that male sex, African American ethnicity, and a history of diabetes mellitus are associated with an elevated ACE2 and TMPRSS2 mRNA expression in induced sputum. Because people with diabetes have worse outcomes in severe COVID-19 (2, 3, 10), these findings suggest that increased expression of SARS-CoV-2–associated genes may facilitate viral infection and underscore that asthmatics with one or more of these characteristics should be monitored for worse outcomes of COVID-19.
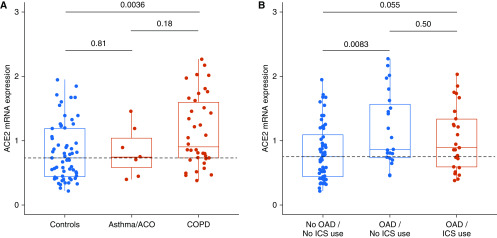
Third and most importantly, they demonstrate that—in contrast to systemic corticosteroids—the use of ICS in asthma subjects was dose-dependently associated with reduced ACE2 and TMPRSS2 mRNA expression. If these intriguing findings are confirmed at the protein level, they may have important clinical implications. ACE2 expression has been predominantly reported in epithelial cells (11–13), which are a minor fraction within induced sputum, which mainly samples inflammatory cells in the lower airways. How expression of SARS-CoV-2–associated genes is modulated in specific airway inflammatory cells in asthma and whether this affects viral entry and infectivity require further investigation. Moreover, it is important to evaluate the expression of ACE2 also in lung tissue since this is the predominant place of injury in severe COVID-19. In our lung tissue bank at Ghent University Hospital, we studied ACE2 gene expression in lung resection specimens of white subjects with and without obstructive airway disease (OAD), encompassing asthma and/or COPD, and investigated whether ACE2 gene expression was associated with ICS use (Figure 1). Whereas ACE2 mRNA expression in lung tissue was significantly increased in (current or former) smokers with COPD, it was not altered in subjects with asthma or asthma–COPD overlap (ACO) as compared with controls without OAD (Figure 1A). However, pulmonary gene expression of ACE2 did not differ in ICS-treated and non–ICS-treated OAD subjects (Figure 1B). These results need to be replicated in larger prospective studies and in other (nonwhite) ethnicities. The divergent effects of ICS use on ACE2 expression between both studies might be due to differences in respiratory compartment (induced sputum vs. lung tissue) or patient population (nonsmoking patients with asthma vs. smokers with COPD).
Figure 1.
ACE2 (angiotensin-converting enzyme 2) mRNA expression in human lung tissue and effect of ICS. (A) ACE2 gene expression in lung tissue from controls (n = 61), asthma/ACO (n = 7), and COPD (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage II) (n = 38). (B) ACE2 gene expression in controls (no OAD, no ICS use, n = 56), in subjects with OAD not using ICS (n = 23) and in subjects with OAD using ICS (n = 25). Lung resection samples were obtained with approval from the ethical committee of Ghent University Hospital (2016/0132); all participants provided written informed consent. Processing for RNA and quantitative RT-PCR analysis was performed as described previously (14, 15). ACE2 mRNA expression was normalized to the expression of three reference genes. ACO = asthma–COPD overlap; COPD = chronic obstructive pulmonary disease; ICS = inhaled corticosteroids; OAD = obstructive airway diseases (asthma, ACO, or COPD).
In conclusion, the crucial findings from Peters and colleagues support the recommendation that in patients with asthma using ICS, this treatment should be continued since ICS are the cornerstone of asthma management, reducing exacerbations and asthma mortality, and are associated with decreased expression of ACE2, the receptor of SARS-CoV-2, in induced sputum. To what extent up- or downregulation of ACE2 expression in sputum, airways, or lungs has clinical consequences on infectivity or outcomes of COVID-19 needs to be elucidated. In subjects without asthma (or exacerbation-prone COPD), ICS should not be started since ICS use does not seem to influence ACE2 expression in lung tissue. However, we eagerly await the results of randomized controlled trials assessing the efficacy and safety of ICS in treating COVID-19 in patients with and without chronic airway diseases (see https://clinicaltrials.gov).
Supplementary Material
Acknowledgments
Acknowledgment
The authors acknowledge S. Wijnant, M. Jacobs, H. Van Eeckhoutte, and the technical staff of the Department of Respiratory Medicine for their efforts in collecting the data that are presented in this manuscript.
Footnotes
The Department of Respiratory Medicine (Ghent University) is funded by a concerted research action of Ghent University (BOF-/GOA-01G00819) and the Fund for Scientific Research in Flanders (Projects G053516N, G052518N, and G041819N and EOS project G0G2318N).
Author Contributions: T.M. and G.G.B. have jointly written the editorial. K.B. coordinates the human lung tissue bank at Ghent University Hospital and has performed the ACE2 gene expression analysis. All authors have given final approval for the manuscript to be submitted.
Originally Published in Press as DOI: 10.1164/rccm.202005-1651ED on May 21, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020;55:2000607. doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. doi: 10.1001/jama.2020.2648. [online ahead of print] 24 Feb 2020; DOI: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4. Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, et al. COVID-19 with different severity: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201:1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Geneva: World Health Organization; Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. 2020 [accessed 2020 Apr 29]. Available from: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novelcoronavirus-(ncov)-infection-is-suspected. [Google Scholar]
- 7. Halpin DMG, Singh D, Hadfield RM. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J. 2020;55:2001009. doi: 10.1183/13993003.01009-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peters MC, Sajuthi S, Deford P, Christenson S, Rios CL, Montgomery MT, et al. NHLBI Severe Asthma Research Program-3 Investigators. COVID-19–related genes in sputum cells in asthma: relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020;202:83–90. doi: 10.1164/rccm.202003-0821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280, e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. China Medical Treatment Expert Group for COVID-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leung JM, Yang CX, Tam A, Shaipanich T, Hackett TL, Singhera GK, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55:2000688. doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brake SJ, Barnsley K, Lu W, McAlinden KD, Eapen MS, Sohal SS. Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19) J Clin Med. 2020;9:841. doi: 10.3390/jcm9030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cai G, Bossé Y, Xiao F, Kheradmand F, Amos CI. Tobacco smoking increases the lung gene expression of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med. doi: 10.1164/rccm.202003-0693LE. [online ahead of print] 24 Apr 2020; DOI: 10.1164/rccm.202003-0693LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seys LJM, Widagdo W, Verhamme FM, Kleinjan A, Janssens W, Joos GF, et al. DPP4, the Middle East respiratory syndrome coronavirus receptor, is upregulated in lungs of smokers and chronic obstructive pulmonary disease patients. Clin Infect Dis. 2018;66:45–53. doi: 10.1093/cid/cix741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seys LJ, Verhamme FM, Schinwald A, Hammad H, Cunoosamy DM, Bantsimba-Malanda C, et al. Role of B cell-activating factor in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192:706–718. doi: 10.1164/rccm.201501-0103OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.