Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes a range of cardiopulmonary and vascular complications, ranging from upper respiratory tract symptoms to severe acute respiratory distress syndrome (ARDS), as well as shock, acute kidney injury, and thromboembolic complications (1, 2). Although SARS-CoV-2 initially infects the upper respiratory tract epithelia, some of the most serious complications of the disease appear to arise through vascular inflammation and injury. Although further mechanistic and epidemiological studies are needed, case reports, imaging studies, and autopsy series have suggested the possibility that the SARS-CoV-2 virus, once in the lower respiratory tract, may directly infect endothelial cells, leading to a cascade of consequences including vasoplegia, vascular thromboses, pulmonary edema, endothelial sloughing, and abnormal regulation of pulmonary perfusion (2, 3). Regardless of the mechanisms, it is clear that patients often develop severe respiratory failure with hypoxemia that may be refractory to oxygen supplementation and often requires invasive mechanical ventilation. Because of the rapidity with which the virus spread, many healthcare systems were stressed by the sudden increase in coronavirus disease (COVID-19) cases, with the accompanying increased need for hospital beds, ICU beds, ventilators, and even oxygen. A high percentage of mechanically ventilated patients develop multi-organ failure syndrome, characterized by pressor-dependent shock and a high associated mortality. Even those who survive with the assistance of mechanical ventilation may require prolonged hospitalizations (4). These concerted adverse sequalae of SARS-CoV-2 infection create major strains on health care system resources.
It is with this backdrop that, in this issue of the Journal, Zamanian and colleagues (pp. 130–132) present an interesting and compelling case of a patient with pulmonary arterial hypertension (PAH) who was treated remotely in an ambulatory setting with inhaled nitric oxide (iNO) (5). This patient with well-controlled vasoreactive PAH lived in a remote area more than 300 miles away from their center and experienced symptoms of worsening breathlessness after being diagnosed with COVID-19. Considering her concerns about traveling the long distance to their center to receive care, and with recognition of her prior confirmed responsiveness to iNO, they established a plan to support her with an ambulatory iNO system while monitoring her symptoms, vital signs, and functional capacity remotely. The patient had rapid and sustained improvement in her 6-minute-walk distance, as assessed by her caregiver, and symptom score, and she recovered over several days without having to engage emergency department or hospital care.
This case report raises many questions. How might iNO have benefited this patient? Would we expect the benefit to be unique to iNO, or could other therapies that increase signaling along the NO axis also be helpful, such as NO donors, NO precursors, or phosphodiesterase 5 inhibitors? Can NO be safely administered to a patient in their own home, potentially helping to unburden overwhelmed healthcare systems?
NO is a free radial gas that functions as an important signaling molecule in human physiology. Its canonical receptor, guanylate cyclase, is highly expressed vascular smooth muscle cells, where it becomes activated once NO binds to its heme moiety, significantly increasing its enzymatic conversion of guanosine-5′-triphosphate to cyclic guanosine monophosphate, which subsequently promotes vasorelaxation. As a gas, it has unique pharmacological properties including its delivery into well-ventilated lung units where it promotes local vasodilatation. When NO enters the blood stream, it rapidly reacts with intraerythrocytic Hb, thus inactivating the NO, resulting in an extremely short half-life, which limits its systemic effects. By preferentially vasodilating pulmonary arterioles in well-ventilated lung units, it decreases the relative blood flow to poorly ventilated lung units and enhances matching, increasing oxygenation (6). NO also induces mild bronchodilation, and inhibits neutrophil-mediated oxidative burst (6). These properties have been well known for decades and have led to U.S. Food and Drug Administration approval for the treatment of persistent pulmonary hypertension of the newborn, as well as various trials of iNO for patients with myriad conditions including ARDS, right ventricular failure after cardiac surgery, acute pulmonary embolism, and more recently pulmonary fibrosis in patients requiring long-term oxygen therapy (6–10). In patients with SARS, iNO was associated with improvements in oxygenation in a severity-matched observational cohort (11). Both endogenous and exogenous NO were shown to inhibit SARS-CoV viral replication (12). While iNO has not been shown to reduce the time on mechanical ventilation or mortality in adults with ARDS, iNO does significantly improve oxygenation in ARDS patients and leads to reduction in pulmonary vascular resistance (6) (Figure 1). These therapeutic responses suggest that iNO could be used early in the course of COVID-19 infection to reduce the need for invasive mechanical ventilation. Studies of prone positioning and neuromuscular blockers in ARDS both provide a historical reminder of that potential, as clinical trials of early delivery of those therapies demonstrated benefits where prior studies had not (13, 14).
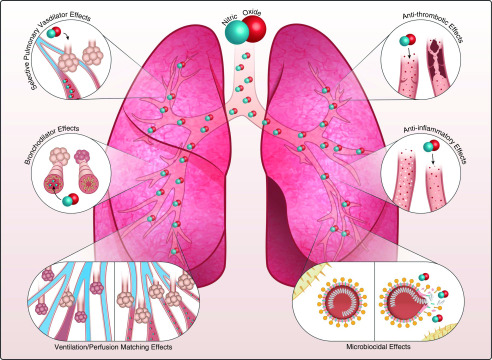
Figure 1.
Summary of major therapeutic properties of inhaled nitric oxide gas (NO). From top left: inhaled NO gas is known to be a selective pulmonary vasodilator. NO can improve right heart function and decrease pulmonary vasoconstriction in subjects with acute and chronic pulmonary hypertension. Middle left vignette: breathing NO gas is shown to improve ventilation and provide bronchodilation in mild asthmatic subjects. Bottom left vignette: NO gas in the alveolar space improves oxygenation by increasing blood flow to ventilated lung units (i.e., improvement of ventilation perfusion matching). Top and middle right vignettes: in vitro and in vivo data showed that NO gas can act as an antiinflammatory and antithrombotic agent. Bottom right vignette: NO donors and NO gas showed antibacterial and antiviral properties in in vitro studies and early clinical investigations. The extent of benefits of these six therapeutic pathways of NO gas in coronavirus disease (COVID-19) infection are now under investigation. Some of those studies testing NO therapeutic properties are highlighted in Table 1.
Zamanian’s case also highlights the feasibility of portable iNO delivery systems to treat patients at home, an option not previously available. While GENOSYL DS (VERO Biotech) is designed for the hospital intensive care setting, it has features, such as a tankless delivery system, that make it feasible to deliver at home, as demonstrated in this case. Other systems, such as INOpulse (Bellerophon Therapeutics), Nu-Med Plus (UT), and an iridium electric NO generator (Third Pole Therapeutics), have been designed with at least some degree of portability. Although there would be concerns in treating patients with a therapy like iNO at home, there is precedent. In a randomized and placebo-controlled trial of ambulatory patients with fibrotic lung disease requiring long-term oxygen, INOpulse therapy was associated with greater physical activity than placebo, and in an acute dose escalation study of patients with pulmonary hypertension associated with pulmonary fibrosis, iNO delivered through the INOpulse system lead to a 30% reduction in pulmonary vascular resistance, with improvements in and pulmonary artery compliance (15).
It is important to recognize that the experience of Dr. Zamanian’s patient is unlikely to be representative of all patients with COVID-19, or even those with PAH complicated by COVID-19. This patient had an established diagnosis of vasoreactive PAH, and as a physician herself, was uniquely qualified to engage in a complex treatment regimen. But the example serves as an interesting proof-of-concept study that supports the rationale of studying iNO therapy in patients with COVID-19 to establish if this intervention can improve oxygenation and reduce need for mechanical ventilation. In Table 1, we have summarized planned and ongoing clinical trials available that are testing NO gas therapy in COVID-19 patients. Dr. Zamanian and colleagues are to be commended for their innovative approach and important contribution to this field.
Table 1.
Ongoing Clinical Trials Registered on clinicaltrials.gov Testing NO Gas in COVID-19 Infection
| Short Title | PI | Coordinating Center | Study Design | Drug | Dose (ppm) | Duration | Subjects (n) | Study Status | Follow-up (d) | Detailed Protocol | Primary Endpoint | Secondary Endpoint | NCT Number |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NO Therapy: Healthcare Providers | Lorenzo Berra | MGH, Boston | Multicenter, open-label RCT | NO gas | 160 | 15 min twice daily | 470 | Recruiting | 14 | https://www.medrxiv.org/content/10.1101/2020.04.05.20054544v1 | Prevention of COVID-19 in healthcare providers | (I) Prevention to become positive | NCT04312243 |
| (II) Number of quarantine days | |||||||||||||
| NO Therapy: COVID-19 Infection in ED | Stuart Harris | MGH, Boston | RCT | NO gas | 250 | 30 min, single dose | 260 | Recruiting | 28 | Not available | Rates of return visits to the ED | (I) Inpatient hospitalization | NCT04338828 |
| (II) Rates of intubation | |||||||||||||
| (III) Mortality | |||||||||||||
| NO Therapy: Spontaneous Breathing COVID-19 Infection | Lorenzo Berra | MGH, Boston | Multicenter, open-label RCT | NO gas | 160 | 30 min twice daily | 240 | Recruiting | 28 | https://www.medrxiv.org/content/10.1101/2020.03.10.20033522v1 | Prevention of progression of the disease | (I) Antimicrobial effect | NCT04305457 |
| (II) Other clinical outcomes | |||||||||||||
| NO Therapy: Ventilated Patients with COVID-19 | Lorenzo Berra | MGH, Boston | Multicenter, open-label RCT | NO gas | Initial dose 80 | Continuous until extubation | 200 | Recruiting | 90 | https://www.medrxiv.org/content/10.1101/2020.03.09.20033530v1 | Sustained improved oxygenation | (I) Time to reach normoxia | NCT04306393 |
| (II) Other clinical outcome | |||||||||||||
| High-Dose NO for COVID-19 (ICU Patients) | Jennifer Lister | University Health Network, Toronto | Multicenter, open-label RCT | NO gas | 160 | 6 h for 2 d | 20 | Not yet recruiting | 3 | Not available | Rate of PCR positivity | Not available | NCT04383002 |
| The NO-COVID-19 Study | Marvin Kostam | Tufts Medical Center, Boston | Open-label RCT | NO gas | 20 | Not available | 42 | Not yet recruiting | 28 | Not available | Prevention of progression of the disease | (I) Prevention of progression | NCT04388683 |
| (II) Clinical improvement | |||||||||||||
| Pulsed NO in Mild or Moderate COVID-19 | Hunter Gilles | Not available | Expanded access | NO gas | 20 | 14 d | Not available | Recruiting | 28 | Not available | Prevention of progression of the disease | Not available | NCT04358588 |
| Randomized Trial of INOpulse for COVID-19 | Roger Alvarez | Miller School of Medicine, Miami | Placebo- controlled RCT | NO gas | 40 | To resolution of acute hypoxemia | 30 | Not yet recruiting | 2 | Not available | Safety and tolerability | (I) Prevention of progression | NCT04398290 |
| (II) Clinical improvement | |||||||||||||
| NO Releasing Solutions to Prevent and Treat COVID-19 | Jeremy Road | BC Diabetes Vancouver | Multicenter RCT | NORS | Not available | 14 | 200 | Recruiting | 21 | Not available | Prevention of COVID-19 and progression of the disease | (I) Prevention of progression | NCT04337918 |
| (II) Antimicrobial effect | |||||||||||||
| NO Treatment for Lung Infections | Jeremy Road | Diamond Centre Vancouver | Sequential assignment | NO gas | 160 | Not available | 20 | Active, not recruiting | 26 | Not available | Safety | (I) Lung function | NCT03331445 |
| (II) Antimicrobial effect | |||||||||||||
| (III) Quality of life |
Definition of abbreviations: BC = British Columbia; COVID-19 = coronavirus disease; ED = emergency department; MGH = Massachusetts General Hospital; NO = nitric oxide; NORS = NO-releasing solution; PI = principal investigator; RCT = randomized controlled trial.
Footnotes
R.A.A. receives research support from the University of Miami Office of the Vice Provost for Research, under the COVID-19 Rapid Response Grant UM 2020-2240. M.T.G. receives research support from NIH grants 5R01HL098032, 2R01HL125886, and 5P01HL103455, 5T32HL110849, and UG3HL143192; the Burroughs Wellcome Foundation; Globin Solutions, Inc.; and the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania. L.B. receives research support from NIH grant K23HL128882.
Originally Published in Press as DOI: 10.1164/rccm.202005-1906ED on May 21, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. doi: 10.1007/s00330-020-06801-0. [online ahead of print] 19 Mar 2020; DOI: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. doi: 10.1001/jama.2020.6775. [online ahead of print] 22 Apr 2020; DOI: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zamanian RT, Pollack CV, Jr., Gentile MA, Rashid M, Fox JC, Mahaffey KW, et al. Outpatient inhaled nitric oxide in a patient with vasoreactive idiopathic pulmonary arterial hypertension and COVID-19 infection [letter] Am J Respir Crit Care Med. 2020;202:130–132. doi: 10.1164/rccm.202004-0937LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Griffiths MJ, Evans TW. Inhaled nitric oxide therapy in adults. N Engl J Med. 2005;353:2683–2695. doi: 10.1056/NEJMra051884. [DOI] [PubMed] [Google Scholar]
- 7. Gladwin MT, Kato GJ, Weiner D, Onyekwere OC, Dampier C, Hsu L, et al. DeNOVO Investigators. Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial. JAMA. 2011;305:893–902. doi: 10.1001/jama.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hu J, Spina S, Zadek F, Kamenshchikov NO, Bittner EA, Pedemonte J, et al. Effect of nitric oxide on postoperative acute kidney injury in patients who underwent cardiopulmonary bypass: a systematic review and meta-analysis with trial sequential analysis. Ann Intensive Care. 2019;9:129. doi: 10.1186/s13613-019-0605-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kline JA, Puskarich MA, Jones AE, Mastouri RA, Hall CL, Perkins A, et al. Inhaled nitric oxide to treat intermediate risk pulmonary embolism: a multicenter randomized controlled trial. Nitric Oxide. 2019;84:60–68. doi: 10.1016/j.niox.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nathan SD, Flaherty KR, Glassberg MK, Raghu G, Swigris J, Alvarez R, et al. A randomized, double-blind, placebo-controlled study to assess the safety and efficacy of pulsed, inhaled nitric oxide at a dose of 30 μg/Kg ideal body weight/hr in subjects at risk of pulmonary hypertension associated with pulmonary fibrosis receiving oxygen therapy. Chest. doi: 10.1016/j.chest.2020.02.016. [online ahead of print] 21 Feb 2020; DOI: 10.1016/j.chest.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 11. Chen L, Liu P, Gao H, Sun B, Chao D, Wang F, et al. Inhalation of nitric oxide in the treatment of severe acute respiratory syndrome: a rescue trial in Beijing. Clin Infect Dis. 2004;39:1531–1535. doi: 10.1086/425357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akerström S, Mousavi-Jazi M, Klingström J, Leijon M, Lundkvist A, Mirazimi A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J Virol. 2005;79:1966–1969. doi: 10.1128/JVI.79.3.1966-1969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guérin C, Reignier J, Richard J-C, Beuret P, Gacouin A, Boulain T, et al. PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 14. Papazian L, Forel J-MM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 15. Alvarez RA, Dudenhofer R, Ahmad K, Csete MKG, Lancaster L, Raghu G, et al. An acute dose escalation study to assess the safety and hemodynamic efficacy of pulsed inhaled nitric oxide (iNO) in subjects with pulmonary hypertension associated with pulmonary fibrosis (PF) or sarcoidosis [abstract] Am J Respir Crit Care Med. 2020;201:A3818. [Google Scholar]