Abstract
EML4-ALK rearranged malignant pleural mesothelioma (MPM) is rare and its responses to anaplastic lymphoma kinase (ALK) inhibitors, including alectinib and lorlatinib, remain unexplored. In this case report, we describe a patient with EML4-ALK-rearranged stage IIIB MPM who was administered with alectinib and lorlatinib as first-line and fourth-line therapy, respectively. He had remarkable response evaluated as partial response on both regimens lasting approximately 3.5 months on each regimen. His plasma samples were collected during the treatment course and submitted for targeted sequencing to understand the molecular mechanisms of his therapeutic resistance. Sequencing analysis revealed the emergence of ALK I1171N and L1196M at alectinib progression. Meanwhile, ALK I1171N, L1196M, and G1202R mutations were identified at lorlatinib progression, wherein L1196M is confirmed to be in cis to G1202R. We speculate that these multiple mutations synergistically mediated his resistance to both alectinib and lorlatinib. Our report describes the detection of EML4-ALK rearrangement in a patient with MPM who had remarkable therapeutic response with ALK inhibitors. Moreover, our case also revealed acquired mechanisms of lorlatinib resistance mediated by multiple mutations ALK I1171N, L1196M, and G1202R, contributing an incremental step to our understanding of the complexity of acquired resistance mechanisms in sequential ALK inhibitor therapy.
The reviews of this paper are available via the supplemental material section.
Keywords: ALK mutations, lorlatinib resistance mechanism, malignant pleural mesothelioma
Background
Mesothelioma is a rare cancer, with an estimated incidence of less than 1% of all cancers diagnosed.1 Malignancies arising from the pleural lining of the thoracic cavity account for more than 90% of mesothelioma cases.1 Due to its aggressive course and limited treatment options, patients with malignant pleural mesothelioma (MPM) have poor prognosis, with stage III–IV patients having a median overall survival between 10 months and 14 months.2,3 MPM is associated with asbestos exposure and is more common in males with Caucasian or Hispanic ancestry than in African Americans or Asians1,4; however, an increase in MPM cases has been observed among Asians in recent years.2
A recent study has identified a small subset of patients (19.5%, 25/128) with protein overexpression of anaplastic lymphoma kinase (ALK).5 Preclinical studies have demonstrated the combined efficacy of ALK inhibitor crizotinib and rapamycin in simultaneously targeting ALK overexpression and mTOR to inhibit MPM tumor growth.5 Conversely, ALK rearrangements have been identified only in peritoneal mesothelioma,6 but not in MPM.7–9 A comprehensive molecular profiling study did not identify any ALK rearrangements from a cohort of 74 patients with MPM using various molecular profiling methods including whole-exome sequencing, copy-number array, mRNA sequencing, non-coding RNA profiling, and reverse-phase protein array.9
Newer generations of ALK tyrosine kinase inhibitors (TKI), including alectinib and lorlatinib, have dramatically improved the prognosis of patients with ALK-rearranged non-small cell lung cancer (NSCLC) due to the improved ability of these inhibitors to penetrate the central nervous system.10,11 Due to the rarity of ALK-rearranged MPM tumors, clinical responses of patients with MPM to ALK-TKIs remain unexplored. Herein, we describe the detection of EML4-ALK rearrangement in MPM. We further describe the clinical responses of our patient to alectinib and lorlatinib, and elucidate the molecular mechanisms of acquired ALK-TKI resistance using targeted sequencing.
Case report
In early 2018, a 33-year-old, non-smoking male with no family history of cancer and no known exposure to asbestos sought medical attention with complaints of weakness, chest pain when breathing deeply, and persistent cough with asthma. His Eastern Cooperative Oncology Group performance score (ECOG-PS) was 2. During his hospital confinement, thoracentesis was performed for 9 consecutive days to drain approximately 800 ml of pleural effusion (PE) per day. However, pathology examination of his PE samples failed to identify any malignant tumor cells, even after three repeated evaluations.
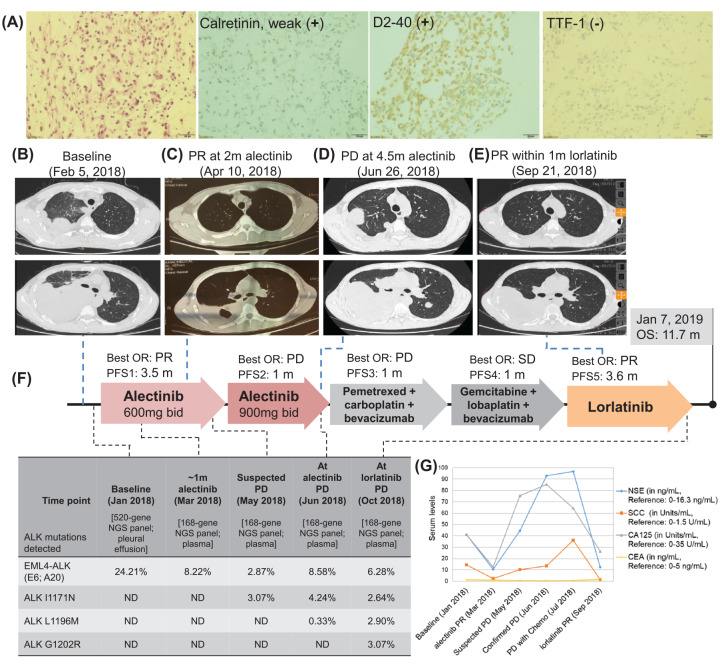
Upon referral to our department, the patient was severely ill, having weak constitution (ECOG-PS 4), intermittent fever, severe asthma, and rapid accumulation of PE. Enhanced computed tomography of the chest revealed a mass in the right lung hilum, which compressed the right pulmonary artery and blocked the tracheal carina. The presence of nodules and pleural effusion in the right pleura were also observed. Based on his comprehensive clinical workup including histopathology (Figure 1A), and imaging results (Figure 1B), he was diagnosed with stage IIIB (cT4N2M0) MPM based on recent National Comprehensive Cancer Network (NCCN) guidelines on MPM staging (v.1.2018).12 Following NCCN guidelines for first-line standard of care for MPM, he was administered a palliative chemotherapy regimen consisting of cisplatin, gemcitabine, and endostar for two cycles, which significantly reduced the PE and improved his physical mobility (ECOG-PS 2). Targeted next-generation sequencing (NGS) of the baseline PE supernatant was performed using a panel consisting of 520 cancer-related genes (OncoScreen Plus, Burning Rock Biotech, Guangzhou, China).13 He was then administered alectinib at a dose of 600 mg twice daily after the analysis revealed the detection of EML4-ALK (variant 3). After 1 month of alectinib therapy, his cough and asthma were alleviated; with a significant reduction in the blood tumor markers, particularly neuron-specific enolase (NSE) and squamous cell carcinoma antigen (SCC) (Figure 1G). After a month of therapy, plasma NGS revealed a reduction in EML4-ALK abundance, indicating treatment efficacy. Consistent with the continuous improvement in clinical symptoms (ECOG-PS 1), CT images after 2 months of therapy showed marked shrinkage of the primary lesion, evaluated as partial response (PR) based on Response Evaluation Criteria in Solid Tumors (RECIST) v.1.1 (Figure 1C). However, after 3.5 months, he again experienced increased coughing, suggesting progressive disease (PD). Blood tumor markers including NSE, SCC, and CA-125 were also observed to be increased (Figure 1G). The dose escalation of alectinib to 900 mg twice daily for a month did not improve his clinical symptoms. PD was thus confirmed after a total of 4.5 months of alectinib therapy (Figure 1D). Plasma NGS at PD revealed the emergence of two acquired missense mutations in the ALK kinase domain, I1171N and L1196M. Since third-generation ALK-TKI are not available in China yet, two chemotherapy regimens comprised of pemetrexed, carboplatin, and bevacizumab (Day 1: 0.8 g + 300 mg + 300 mg, 21 days/cycle) and gemcitabine, lobaplatin, and bevacizumab (Day 1: 1.6 g + 40 mg + 300 mg, 21 days/cycle) was administered for one cycle each. Despite a slight improvement of clinical symptoms with the second chemotherapy regimen, the patient refused to continue the regimen due to grade II vomiting and nausea. His therapy was then switched to lorlatinib, resulting in remarkable shrinkage of the primary lesion (~34%), and achieving PR within a month (Figure 1E). However, after a total of 3.6 months, he began experiencing hemoptysis and weakness, suggesting PD. In addition to the concurrent mutations detected previously, plasma NGS revealed the emergence of ALK G1202R. In late December 2018, he suffered from confusion and paralysis, suspected to be symptoms of brain progression but he was too weak to tolerate brain magnetic resonance imaging (MRI). On 3 January 2019, he was admitted to the hospital in a confused state and grave medical condition. Symptomatic treatment was administered but his condition continued to decline that led to his demise on 7 January 2019, with an overall survival of 11.7 months.
Figure 1.
Clinical summary of the patient. (A) Pathology results including hematoxylin-eosin staining, positivity with immunohistochemical markers of MPM including calretinin and D2-40 and negative TTF-1 expression. (B–E) Thoracic CT scans of primary lung lesions at (B) baseline (15.03 × 9.8 cm); (C) PR at 2 months of alectinib therapy (10.7 × 5.7 cm); (D) PD after a total of 4.5 months of alectinib therapy (17.59 × 12.09 cm); (E) PR within 1 month of lorlatinib therapy (14.14 × 9.94 cm). (F) An illustrated summary of the treatment received by the patient, including the best OR and PFS in each line of treatment. Mutations and their corresponding allelic fractions detected during the course of treatment using targeted NGS are also tabulated at the bottom of the figure. (G) Plot summarizing the serum levels of the tumor biomarkers including NSE, SCC, CA-125, and CEA during the treatment course.
ALK, anaplastic lymphoma kinase; CA, carbohydrate antigen; CEA, carcinoembryonic antigen; CT, computed tomography; MPM, malignant pleural mesothelioma; ND, not detected; NGS, next-generation sequencing; NSE, neuron-specific enolase; OR, objective response; PD, progressive disease; PFS, progression-free survival; PR, partial response; SCC, squamous cell carcinoma antigen; TTF-1, thyroid transcription factor
Discussion
To the best of our knowledge, our study is the first to identify EML4-ALK rearrangements in a patient with MPM and describes his clinical response and molecular mechanisms of resistance to alectinib and lorlatinib. Furthermore, our report is also the first to provide clinical evidence of the mechanism of lorlatinib resistance mediated by acquired multiple mutations ALK I1171N, L1196M, and G1202R.
Thus far, no study has identified EML4-ALK in MPM.7–9 Instead of genetic alterations in classic NSCLC oncogenic drivers, comprehensive genomic studies in MPM have revealed mutations in four pathways including the TP53/DNA repair, cell cycle, mitogen-activated protein kinase (MAPK), and phosphoinositide 3-kinase (PI3K)/AKT pathways.8 The remarkable clinical responses of our patient, including the significant tumor shrinkage and improvement in clinical symptoms were associated with the reduction in the allelic fraction (AF) of EML4-ALK fusion, suggesting that the EML4-ALK fusion was a major oncogenic driver that contributed to the sensitivity to alectinib-mediated inhibition of the MPM of our patient. Despite the remarkable clinical responses he achieved with alectinib and lorlatinib, resistance to these inhibitors developed within 3.5 months through the emergence of multiple secondary missense mutations in the kinase domain of ALK. His resistance to alectinib was potentially mediated by the acquisition of ALK I1171N and L1196M (Figure 1). He responded to lorlatinib until the emergence of ALK G1202R confirmed to be in cis to L1196M (Figure 2) and concurrent to I1171N. These three missense mutations are commonly acquired, albeit as single or double mutation(s), during ALK-TKI therapy, and mediate drug resistance.14 ALK I1171N has been shown to be resistant to crizotinib and alectinib, but sensitive to ceritinib and lorlatinib, whereas L1196M is resistant to crizotinib and sensitive to alectinib, ceritinib, and lorlatinib; G1202R is resistant to crizotinib, alectinib, and ceritinib, and is sensitive to lorlatinib.14,15 Whereas single mutations of I1171N, L1196M, and G1202R are sensitive to lorlatinib, preclinical evidence had demonstrated lorlatinib resistance in compound mutations between G1202R and either L1196M or I1171N.15,16 Ceritinib is potentially effective in targeting ALK I1171N/L1196M double mutations,16 whereas ALK G1202R/L1196M is resistant to all available ALK-TKI.15,16 The resistance to alectinib and lorlatinib therapy in our patient was potentially mediated by multiple mutations ALK I1171N/L1196M and ALK I1171N/L1196M/G1202R, respectively. A synergistic effect among these acquired multiple mutations contributes to the complexity of ALK-TKI resistance mechanism. This evidence emphasizes the need for molecular profiling during the treatment course to monitor genetic alterations that could potentially mediate resistance to ALK-TKIs, particularly when administered sequentially, to improve the clinical management of patients with ALK-rearranged tumors.
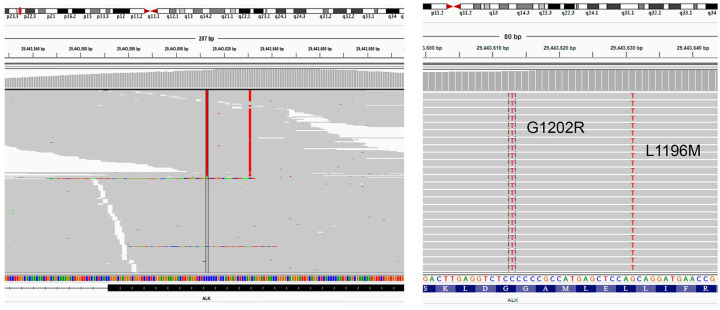
Figure 2.
Screenshots from integrated genome viewer illustrating the compound in cis mutations ALK L1196M and G1202R detected from the patient at PD from lorlatinib therapy. Left panel is a zoom-out view that illustrates the sequencing reads for the region; the right panel is a base-level view that illustrates the base change. Colors represent the base change, wherein red represents the nucleotide threonine (T). The red column on the left represents reads with c.3604G>A (p.G1202R), while the red column on the right represents reads with c.3586C>A (p.L1196M), indicating substitution of C or G with A on the antisense strand. Exon 23 of ALK is depicted at the bottom left panel as a black box with repeating less-than symbols (<) indicating its antisense direction. Each gray row represents the sequencing read from a DNA fragment and the presence of both red bars on the same gray row represents their detection on the same strand of DNA. Two bars located at the bottom respectively indicate the nucleotide and amino acid sequence annotation of ALK.
ALK, anaplastic lymphoma kinase; PD, progressive disease.
Conclusion
Our report provides clinical evidence of the identification of EML4-ALK rearrangement in a patient with MPM, which is very rare, as well as his therapeutic response to ALK-TKIs. Moreover, our case also identified the acquired mechanisms of lorlatinib resistance mediated by multiple missense mutations in ALK kinase domain, I1171N, L1196M, and G1202R. Our report contributes an incremental step to our understanding of the clinical responses of ALK-rearranged MPM to ALK-TKI therapy and the complex molecular mechanisms of acquired resistance to sequential ALK-TKI therapy. Moreover, this report also highlights the importance of targeted sequencing in elucidating the molecular mechanism of treatment response and disease progression.
Supplemental Material
Supplemental material, Author_Response_1 for Acquired multiple mutations ALK I1171N, L1196M and G1202R mediate lorlatinib resistance in EML4-ALK-rearranged malignant pleural mesothelioma: a case report by Jia Hu, Baoshi Zhang, Fangfang Yao, Yan Fu, Dianjun Chen, Donghui Li, Nan Du, Analyn Lizaso, Jinlei Song, Lu Zhang and Xiaosong Li in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Acquired multiple mutations ALK I1171N, L1196M and G1202R mediate lorlatinib resistance in EML4-ALK-rearranged malignant pleural mesothelioma: a case report by Jia Hu, Baoshi Zhang, Fangfang Yao, Yan Fu, Dianjun Chen, Donghui Li, Nan Du, Analyn Lizaso, Jinlei Song, Lu Zhang and Xiaosong Li in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.2 for Acquired multiple mutations ALK I1171N, L1196M and G1202R mediate lorlatinib resistance in EML4-ALK-rearranged malignant pleural mesothelioma: a case report by Jia Hu, Baoshi Zhang, Fangfang Yao, Yan Fu, Dianjun Chen, Donghui Li, Nan Du, Analyn Lizaso, Jinlei Song, Lu Zhang and Xiaosong Li in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Acquired multiple mutations ALK I1171N, L1196M and G1202R mediate lorlatinib resistance in EML4-ALK-rearranged malignant pleural mesothelioma: a case report by Jia Hu, Baoshi Zhang, Fangfang Yao, Yan Fu, Dianjun Chen, Donghui Li, Nan Du, Analyn Lizaso, Jinlei Song, Lu Zhang and Xiaosong Li in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.2 for Acquired multiple mutations ALK I1171N, L1196M and G1202R mediate lorlatinib resistance in EML4-ALK-rearranged malignant pleural mesothelioma: a case report by Jia Hu, Baoshi Zhang, Fangfang Yao, Yan Fu, Dianjun Chen, Donghui Li, Nan Du, Analyn Lizaso, Jinlei Song, Lu Zhang and Xiaosong Li in Therapeutic Advances in Respiratory Disease
Acknowledgments
The authors thank the patient and his family. We also thank the investigators, study coordinators, operation staff, and the whole project team who worked on this case.
Footnotes
Author contribution(s): Jia Hu: Conceptualization; Data curation; Formal analysis; Investigation; Project administration; Validation; Writing-original draft; Writing-review & editing.
Baoshi Zhang: Data curation; Investigation; Methodology; Validation; Writing-review & editing.
Fangfang Yao: Data curation; Formal analysis; Investigation; Validation; Writing-review & editing.
Yan Fu: Data curation; Formal analysis; Investigation; Project administration; Validation; Writing-review & editing.
Dianjun Chen: Conceptualization; Data curation; Formal analysis; Investigation; Validation; Writing-review & editing.
Donghui Li: Data curation; Formal analysis; Visualization; Writing-review & editing.
Nan Du: Data curation; Investigation; Validation; writing-review & editing.
Analyn Lizaso: Formal analysis; Validation; Visualization; Writing-original draft; Writing-review & editing.
Jinlei Song: Formal analysis; Software; Validation; Visualization; Writing-review & editing.
Lu Zhang: Formal analysis; Investigation; Project administration; Validation; Writing-review & editing.
Xiaosong Li: Conceptualization; Methodology; Project administration; Supervision; Visualization; Writing-original draft; Writing-review & editing.
Availability of data and materials: All data generated or analyzed in this study are available on reasonable request.
Conflict of interest statement: Analyn Lizaso, Jinlei Song, and Lu Zhang are employees of Burning Rock Biotech. All other authors report no conflict of interest.
Ethical consent: The patient’s next of kin provided written informed consent for the use of biological samples and publication of case details of the patient.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Xiaosong Li  https://orcid.org/0000-0002-5731-8488
https://orcid.org/0000-0002-5731-8488
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Jia Hu, Department of Oncology, Fourth Medical Center of Chinese PLA General Hospital, Beijing, China.
Baoshi Zhang, Department of Cardiothoracic Surgery, Fourth Medical Center of Chinese PLA General Hospital, Beijing, China.
Fangfang Yao, Department of Oncology, Fourth Medical Center of Chinese PLA General Hospital, Beijing, China.
Yan Fu, Department of Oncology, Fourth Medical Center of Chinese PLA General Hospital, Beijing, China.
Dianjun Chen, Department of Oncology, Fourth Medical Center of Chinese PLA General Hospital, Beijing, China.
Donghui Li, Department of Oncology, Fourth Medical Center of Chinese PLA General Hospital, Beijing, China.
Nan Du, Department of Oncology, Fourth Medical Center of Chinese PLA General Hospital, Beijing, China.
Analyn Lizaso, Burning Rock Biotech, Guangzhou, China.
Jinlei Song, Burning Rock Biotech, Guangzhou, China.
Lu Zhang, Burning Rock Biotech, Guangzhou, China.
Xiaosong Li, Department of Oncology, Fourth Medical Center of Chinese PLA General Hospital, No. 51 Fucheng Road, Haidian District, Beijing, 100037, China.
References
- 1. van der Bij S, Koffijberg H, Burgers JA, et al. Prognosis and prognostic factors of patients with mesothelioma: a population-based study. Br J Cancer 2012; 107: 161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu TH, Lee LJ, Yuan CT, et al. Prognostic factors and treatment outcomes of malignant pleural mesothelioma in Eastern Asian patients—a Taiwanese study. J Formos Med Assoc 2019; 118: 230–236. [DOI] [PubMed] [Google Scholar]
- 3. Wald O, Sugarbaker DJ. New concepts in the treatment of malignant pleural mesothelioma. Annu Rev Med 2018; 69: 365–377. [DOI] [PubMed] [Google Scholar]
- 4. Shavelle R, Vavra-Musser K, Lee J, et al. Life Expectancy in pleural and peritoneal mesothelioma. Lung Cancer Int 2017; 2017: 2782590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Monch D, Bode-Erdmann S, Kalla J, et al. A subgroup of pleural mesothelioma expresses ALK protein and may be targetable by combined rapamycin and crizotinib therapy. Oncotarget 2018; 9: 20781–20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hung YP, Dong F, Watkins JC, et al. Identification of ALK rearrangements in malignant peritoneal mesothelioma. JAMA Oncol 2018; 4: 235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Varesano S, Leo C, Boccardo S, et al. Status of anaplastic lymphoma kinase (ALK) in malignant mesothelioma. Anticancer Res 2014; 34: 2589–2592. [PubMed] [Google Scholar]
- 8. Hylebos M, Van Camp G, van Meerbeeck JP, et al. The genetic landscape of malignant pleural mesothelioma: results from massively parallel sequencing. J Thorac Oncol 2016; 11: 1615–1626. [DOI] [PubMed] [Google Scholar]
- 9. Hmeljak J, Sanchez-Vega F, Hoadley KA, et al. Integrative molecular characterization of malignant pleural mesothelioma. Cancer Discov 2018; 8: 1548–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 2017; 377: 829–838. [DOI] [PubMed] [Google Scholar]
- 11. Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018; 19: 1654–1667. [DOI] [PubMed] [Google Scholar]
- 12. Rice D, Chansky K, Nowak A, et al. The IASLC mesothelioma staging project: proposals for revisions of the N descriptors in the forthcoming eighth edition of the TNM classification for pleural mesothelioma. J Thorac Oncol 2016; 11: 2100–2111. [DOI] [PubMed] [Google Scholar]
- 13. Guo Z, Xie Z, Shi H, et al. Malignant pleural effusion supernatant is an alternative liquid biopsy specimen for comprehensive mutational profiling. Thorac Cancer 2019; 10: 823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bayliss R, Choi J, Fennell DA, et al. Molecular mechanisms that underpin EML4-ALK driven cancers and their response to targeted drugs. Cell Mol Life Sci 2016; 73: 1209–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoda S, Lin JJ, Lawrence MS, et al. Sequential ALK inhibitors can select for lorlatinib-resistant compound ALK mutations in ALK-positive lung cancer. Cancer Discov 2018; 8: 714–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okada K, Araki M, Sakashita T, et al. Prediction of ALK mutations mediating ALK-TKIs resistance and drug re-purposing to overcome the resistance. EBioMedicine 2019; 41: 105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response_1 for Acquired multiple mutations ALK I1171N, L1196M and G1202R mediate lorlatinib resistance in EML4-ALK-rearranged malignant pleural mesothelioma: a case report by Jia Hu, Baoshi Zhang, Fangfang Yao, Yan Fu, Dianjun Chen, Donghui Li, Nan Du, Analyn Lizaso, Jinlei Song, Lu Zhang and Xiaosong Li in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Acquired multiple mutations ALK I1171N, L1196M and G1202R mediate lorlatinib resistance in EML4-ALK-rearranged malignant pleural mesothelioma: a case report by Jia Hu, Baoshi Zhang, Fangfang Yao, Yan Fu, Dianjun Chen, Donghui Li, Nan Du, Analyn Lizaso, Jinlei Song, Lu Zhang and Xiaosong Li in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.2 for Acquired multiple mutations ALK I1171N, L1196M and G1202R mediate lorlatinib resistance in EML4-ALK-rearranged malignant pleural mesothelioma: a case report by Jia Hu, Baoshi Zhang, Fangfang Yao, Yan Fu, Dianjun Chen, Donghui Li, Nan Du, Analyn Lizaso, Jinlei Song, Lu Zhang and Xiaosong Li in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Acquired multiple mutations ALK I1171N, L1196M and G1202R mediate lorlatinib resistance in EML4-ALK-rearranged malignant pleural mesothelioma: a case report by Jia Hu, Baoshi Zhang, Fangfang Yao, Yan Fu, Dianjun Chen, Donghui Li, Nan Du, Analyn Lizaso, Jinlei Song, Lu Zhang and Xiaosong Li in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.2 for Acquired multiple mutations ALK I1171N, L1196M and G1202R mediate lorlatinib resistance in EML4-ALK-rearranged malignant pleural mesothelioma: a case report by Jia Hu, Baoshi Zhang, Fangfang Yao, Yan Fu, Dianjun Chen, Donghui Li, Nan Du, Analyn Lizaso, Jinlei Song, Lu Zhang and Xiaosong Li in Therapeutic Advances in Respiratory Disease