Abstract
Human mesenchymal stromal cells (MSCs) are a leading cell therapy candidate for the treatment of immune and inflammatory diseases due to their potent regulation of immune cells. MSC expression of indoleamine-2,3-dioxygenase (IDO) upon interferon γ (IFNγ) exposure has been proposed as both a sentinel marker and key mediator of MSC immunomodulatory potency. Rather than wait for in vivo exposure to cytokines, MSCs can be pre-licensed during manufacturing to enhance IDO expression. In this study, we systematically examine the relative role that the dose of IFNγ, the duration of pre-licensing and the donor of origin play in dictating MSC production of functional IDO. We find that across three human MSC donors, MSCs increase their expression of IDO in response to both increased dose of IFNγ and duration of pre-licensing. However, with extended pre-licensing, the expression of IDO no longer predicts MSCs ability to suppress activated peripheral blood mononuclear cells. In addition, pre-licensing dose and duration are revealed to be minor modifiers of MSCs inherent potency, and thus cannot be manipulated to boost poor donors to the levels of high-performing donors. Thus, the dose and duration of pre-licensing should be tailored to optimize performance of specific donors and an emphasis on donor selection is needed to realize significant benefits of pre-licensing.
Keywords: mesenchymal stem cells, immunosuppression, cellular therapy, priming, conditioning, biomanufacturing
1. Introduction
The immunomodulatory phenotype of mesenchymal stromal cells (MSCs) has been applied in clinical trials with notable successes in the treatment of graft versus host disease [1,2] and Crohn's disease [3,4]. MSCs modulate immune cells through diverse mechanisms including the active production of signalling molecules, release of extracellular vesicles and signalling via efferocytosis [5–7]. One potent component of this diverse immunomodulatory repertoire is indoleamine-2,3-dioxygenase (IDO), an intracellular enzyme, that has been shown to play a key role in MSC immunomodulatory function. The inhibition of IDO in vitro eliminates the suppressive action of MSCs towards activated peripheral blood mononuclear cells (PBMCs) [8]. In vivo, infusion of MSCs can induce tolerance to murine renal allografts in an IDO-dependent manner [9], and overexpression of IDO in MSCs enhances long-term tolerance in a rabbit renal allograft model [10]. As a critical facet of MSCs' immunomodulatory profile, understanding how MSCs regulate expression and activity of IDO is key to maximizing MSC therapeutic potential.
Expression of IDO in human MSCs is not constitutive, but is induced by cues in the inflammatory environment, predominately interferon γ (IFNγ) [11]. In contrast with dendritic cells, which can induce IDO in response to a variety of cytokines, MSCs are critically dependent on IFNγ exposure to stimulate IDO expression at both the mRNA and protein level [12]. The effect of IFNγ exposure on IDO protein levels is diminished as MSCs are cultured for extended periods of time [13] and reach senescence. Although senescent MSCs transcribe IDO at a similar rate as earlier passage cells, IDO protein concentration is lower due to proteasomal degradation [14]. Other cytokines, such as tumour necrosis factor α (TNFα) and interleukin-1β (IL-1β), augment IDO expression when combined with IFNγ, but are insufficient alone to induce transcription [15,16]. In addition, intact IFNγR signalling and glycolytic metabolism are also both necessary for IFNγ to induce MSCs' immunomodulatory phenotype [17]. Thus, MSCs regulate IDO expression and immunomodulatory phenotype by integrating diverse cues within their environment.
Environmental cues can be manipulated during the biomanufacturing of MSCs to prime an immunomodulatory phenotype. While multiple cues can be used to condition MSCs in culture, when MSCs are conditioned with IFNγ the process is called pre-licensing [18], as MSCs anti-inflammatory programme is activated before their first encounter with immune cells [18]. Pre-licensing has shown benefits across a variety of MSC donors [18–20], tissue sources and settings but the pre-licensing methods used have been highly variable. Pre-licensing with IFNγ doses ranging from 5 to 200 ng ml−1 and durations ranging from 2 h to 4 days have been reported [8,21–24]. In addition, Menard et al. [25] have shown that after pre-licensing with 10 ng ml−1 IFNγ and 15 ng ml−1 TNFα, MSCs isolated from different tissue sources and cultured within different growth media have differential IDO expression and immunomodulatory potency. Thus, the relative role of the source of MSCs versus the dose and duration of pre-licensing required to enhance MSC immunomodulatory function still needs to be identified to scale biomanufacturing of pre-licensed MSCs (PL-MSCs). Without optimizing these parameters, the best-case scenario is an increase in cost without improved performance, while the worst-case scenario is the loss of therapeutic efficacy. With differences in potency identified as a function of both the source of MSCs and the pre-licensing protocol, how these variables interact are critical to moving PL-MSCs into biomanufacturing systems.
While IDO expression is dictated by diverse, interacting cues, herein we sought to take a systematic approach to identify the contributors that dictate MSC expression and maintenance of IDO. After determining the effectiveness of a variety of pre-licensing strategies to enhance IDO in MSCs, these pre-licensing strategies were tested in human PBMC suppression assays to determine how they translated to immune suppression. Insight into the relative role different parameters play in the production and activity of IDO in MSCs and how this altered IDO profile translates to immunomodulation will inform the design of biomanufacturing protocols for PL-MSCs.
2. Materials and methods
2.1. Reagents
All reagents were purchased from major suppliers unless otherwise stated. IFNγ (PeproTech, 300-02), 98% ≥ l-tryptophan (Sigma-Aldrich, T0254-5G) and l-kynurenine (Sigma-Aldrich, K862). l-Kynurenine was used as a 5 mM stock concentration, solubilized in MEM-α (Biological Industries, 01-042-1A). Heated kynurenine was generated by heating a 5 mM l-kynurenine stock at 37°C for 48 h.
2.2. Mesenchymal stromal cell source and culture
Bone marrow-derived MSCs (MSC1 (00055) and MSC2 (00082)) were obtained from RoosterBio, while umbilical cord (MSC3 (4477)) MSCs were isolated using a tissue explant method, expanded and characterized in house, as we previously described [20]. All MSCs met the minimal criteria as defined by the International Society for Cellular and Gene Therapy [26]. Prior to experiments, cryopreserved MSCs were thawed and cultured to 70–90% confluence before splitting and re-plating. MSCs were used at passages P4–P6 (population doubling level (PDL) ranged from 8–12) for experimentation. All MSCs were grown in MEM-α (Biological Industries, 01-042-1A) supplemented with 15% (v/v) premium grade US Origin-ISIA traceable FBS (VWR, 97068-085), 1% (v/v) penicillin–streptomycin (Biological Industries, 03-031-1B) and 1% (v/v) l-glutamine (Gibco, 25030-081).
2.3. Indoleamine-2,3-dioxygenase activity assay
IDO activity was measured by quantifying the change in the amount of kynurenine in the media via Ehrlich's reaction as previously described [27,28]. Briefly, a standard curve of known kynurenine concentrations was constructed using a 1 : 1 serial dilution ranging from 200 to 3.125 µM. Complete growth media were used for the standard curve to ensure a match between the standard and the experimental samples. Two hundred microlitres of standard kynurenine concentrations and samples were then added to a 96-well plate followed by 100 µl of 30% (w/v) trichloroacetic acid (Sigma-Aldrich, T9159-100G) to each well. The 96-well plate was heated to 52°C for 30 min to convert N-formylkynurenine to kynurenine. After heating, the plate was centrifuged at 1200g for 15 min. One hundred and fifty microlitres of supernatant were then collected, mixed and an equal volume was placed into two adjacent wells of a new 96-well plate. Seventy-five microlitres of Ehrlich's reagent (0.4% p-dimethylaminobenzaldehyde (Sigma-Aldrich, 156447-25G) in glacial acetic acid) were then added to each well for a final volume of 150 µl. Kynurenine reacts with Ehrlich's reagent to create a yellow colour proportional to the concentration of kynurenine. Absorbance at 492 nm was then immediately quantified using a plate-based spectrophotometer (Molecular Devices, 60139412). Absorbance values of the known kynurenine standards were used to construct a standard curve, from which the unknown sample concentrations were interpolated.
2.4. Mesenchymal stromal cell and peripheral blood mononuclear cell co-culture
PBMCs were obtained via isolation from leucocyte reduction cones provided by the DeGowin Blood Center at the University of Iowa Hospitals and Clinics from healthy de-identified donors. PBMC seeding was fixed at 200 K and 20 K MSCs were plated to establish a 1 : 10 MSC : PBMC. A 1 : 10 ratio in our experience typically results in naive MSCs suppressing 30–60% of PBMC proliferation, providing ample room to detect either improvement or loss of MSCs' immunomodulatory behaviour [13]. RPMI (Biological Industries, 01-100-1A-12) supplemented with 10% (v/v) FBS (VWR, 97068-085), 1% (v/v) penicillin–streptomycin (Biological Industries, 03-031-1B) and 1% (v/v) l-glutamine (Gibco, 25030-081) was used for all co-culture and control wells. MSCs were seeded first into a 24-well plate and allowed to attach for 2 h. During the 2 h MSC attachment period, previously cryopreserved PBMCs were cultured in RPMI for 1 h. After thawing, PBMCs were stained with CFSE dye at a concentration of 1 µM in PBS for 15 min (Biolegend, 423801) according to the manufacturer's protocol. Following staining and dye neutralization, PBMCs were mixed at a 1 : 1 ratio with anti-CD3/anti-CD28 Dynabeads and then added to co-culture wells. PBMCs with or without Dynabead activation were used as positive and negative controls, respectively, and used for gating in each experiment. After 6 days, PBMCs were collected, centrifuged at 500g for 5 min and then resuspended for analysis on an Accuri C6 flow cytometer.
2.5. Indoleamine-2,3-dioxygenase western blot
Cell lysate was collected in complete RIPA buffer (Santa Cruz Biotechnology, sc-24948A) and centrifuged at 8000g for 10 min. Clarified protein was then denatured with 4× LDS sample buffer (Thermo Fisher, B0007) and 10× Bolt reducing agent (Invitrogen, B0009) followed by heating at 95°C for 2 min. Loading volumes of 10–15 µg of protein per well were calculated using a MicroBCA Protein Assay Kit (Bio Basic, SK3061) according to the manufacturer's specification. Samples were run through a 4–12% Bis–Tris gradient gel followed by transfer to a PVDF membrane. Primary antibody was added which consisted of 5% (w/v) BSA (Sigma-Aldrich, A9647-10G) and either 1 : 1000 IDO primary antibody (BioLegend, 122402) or 1 : 10 000 β-actin antibody (Thermo Fisher, AM4302). Secondary antibody was then added which consisted of 5% (w/v) milk, and either 1 : 10 000 anti-rabbit HRP conjugated antibody (Santa Cruz, sc-2004) for IDO or 1 : 10 000 anti-mouse HRP conjugated antibody (Biolegend, 405306). A WesternBright Quantum mix (Advansta, K-12042-D10) was used for HRP substrate. The membrane was then scanned on a C-DiGit Blot Scanner (LI-COR Biosciences, 3600-00) on high sensitivity. The densitometry of each band was calculated using LI-COR Image Studio software. IDO intensities were normalized to β-actin intensity for each sample.
2.6. Statistics
The statistical tests used for each experiment are listed in each figure caption. GraphPad Prism v8 was used to conduct statistical analyses.
3. Results
3.1. Mesenchymal stromal cell production of indoleamine-2,3-dioxygenase is dependent on the dose and duration of interferon γ exposure
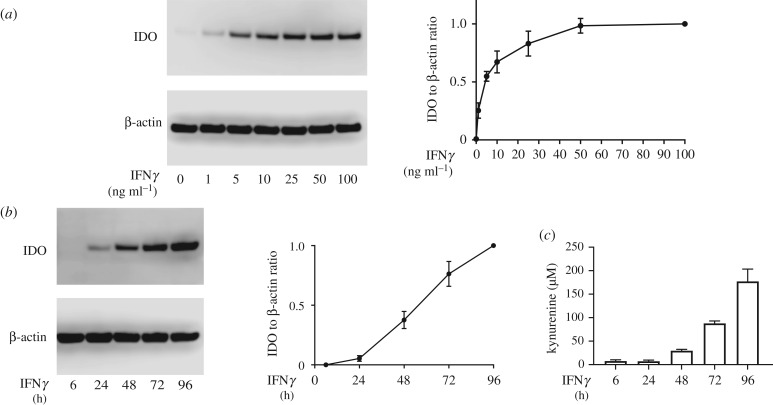
A wide variety of doses and durations of IFNγ exposure have been employed to pre-licence MSCs; however, testing both of these parameters simultaneously has made it difficult to determine the specific dose and duration dependent effects of IFNγ exposure on MSC IDO protein. To isolate the specific contribution of IFNγ dose on IDO protein, we fixed the duration of IFNγ exposure to 2 days and cultured a single bone marrow MSC donor (MSC2) with doses ranging from 0 to 100 ng ml−1. IDO protein levels were detectable at 1 ng ml−1 and continued to increase in a dose-dependent manner until reaching a plateau at 50 ng ml−1 (figure 1a).
Figure 1.
IDO protein levels are dictated by both the dose and duration of IFNγ licensing. (a) Representative western blot and densitometry of MSCs licensed with increasing doses of IFNγ. IDO densitometry was normalized to housekeeping protein β-actin, and further normalized to the 100 ng ml−1 IFNγ condition (N = 3 independent experiments, reporting mean ± s.e.m.). (b) Representative western blot and densitometry of MSCs licensed for increasing durations at a fixed 50 ng ml−1 dose of IFNγ. IDO densitometry was normalized to housekeeping protein β-actin, and further normalized to the 96 h condition (N = 3 independent experiments, reporting mean ± s.e.m.). (c) Activity of IDO, measured as kynurenine concentration at the end of pre-licensing. N = 3 independent experiments, paired to (b). Reporting mean ± s.e.m.
Next, we sought to determine the contribution of the duration of IFNγ exposure on IDO protein levels and enzymatic activity. We hypothesized that increased duration of IFNγ exposure would lead to enhanced IDO protein levels, as seen with increased IFNγ dose. To test this hypothesis, MSCs were cultured in 50 ng ml−1 IFNγ for 6, 24, 72 or 96 h. IDO protein levels increased linearly as a function of the duration of IFNγ exposure with an R² value of 0.899 (p = 0.0141, figure 1b). Detectable protein levels did not occur until the 24 h mark; however, the minimum time necessary to induce IDO protein may occur anytime within the 6–24 h window. In addition, IDO activity, as measured by the conversion of tryptophan to kynurenine, mirrored what was seen with IDO protein concentration. Kynurenine production was first detectable at 6 h and continued to increase throughout the 96 h period (figure 1c), demonstrating that IDO remains active during this extended course of IFNγ exposure.
3.2. Pre-licensing strategies increase indoleamine-2,3-dioxygenase in all mesenchymal stromal cells relative to donor baseline
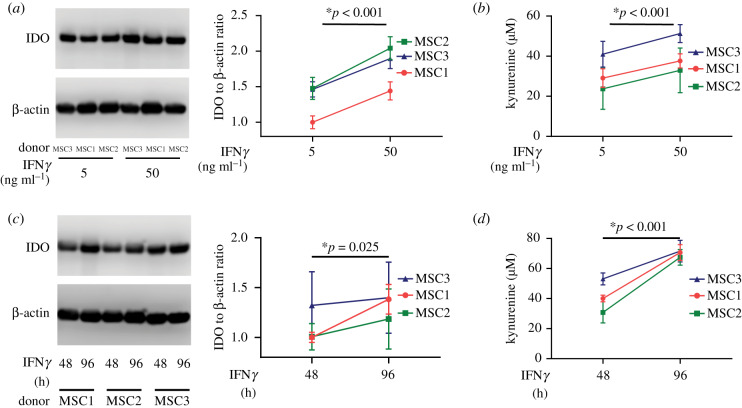
With larger doses and longer durations of IFNγ exposure shown to enhance IDO protein levels (figure 1a,b), we next wanted to determine if these parameters could be used as part of a pre-licensing strategy aimed at elevating IDO protein levels in poorer performing donors. Two pre-licensing strategies were tested: a dose-based strategy (fixed duration and variable dose) and a duration-based strategy (fixed dose and variable duration). MSC immunomodulatory potential and IDO protein concentration have been shown to vary widely between individuals and tissue sources. Therefore, we chose to test these strategies in three independent donors, two bone marrow donors (MSC1, MSC2) and one umbilical cord donor (MSC3).
First, we tested the dose-based strategy with either 5 or 50 ng ml−1 IFNγ exposure for 2 days. In our previous experiments, 5 and 50 ng ml−1 IFNγ exposure induced half and 90% of the maximal IDO protein, respectively; thus, these doses were selected for a mid- and high-level of IDO induction. At the 5 ng ml−1 dose, MSC3 and MSC2 showed a similar amount of IDO protein, while MSC1 had approximately 46% less, indicating a variability at baseline. When treated with high dose of IFNγ, all donors showed a significant increase in IDO protein relative to the low dose (figure 2a). In addition, when pre-licensed with a high dose of IFNγ, MSC1 produced roughly equivalent IDO as the other two donors at a low dose. IDO enzymatic activity also increased in all three donors when pre-licensed with high-dose IFNγ (figure 2b). Therefore, high-dose IFNγ during pre-licensing is an effective strategy to increase IDO protein concentration and IDO activity in lower producing donors.
Figure 2.
Enhanced dose and duration of IFNγ during pre-licensing is an effective strategy to increase IDO protein regardless of baseline donor profile. (a) Representative western blot and densitometry of MSCs pre-licensed in either 5 or 50 ng ml−1 IFNγ. IDO densitometry was normalized to housekeeping protein β-actin, and further normalized to the MSC1, 5 ng ml−1 IFNγ condition. N = 3 independent experiments, mean ± s.e.m. (b) Activity of IDO, measured as kynurenine concentration at the end of pre-licensing. N = 3 independent experiments, paired to figure 1b. Reporting mean ± s.e.m. (c) Representative western blot and densitometry of MSCs pre-licensed for either 48 or 96 h. IDO densitometry was normalized to housekeeping protein β-actin, and further normalized to the MSC1, 48 h condition. N = 3 independent experiments, paired to (d). Reporting mean ± s.e.m. (d) Activity of IDO, measured as kynurenine concentration at the end of pre-licensing. N = 3 independent experiments, paired to (c). Reporting mean ± s.e.m. Statistical analysis used for (a–d) was a two-way ANOVA with pairing across donors, *p < 0.05 considered significant.
Next, we sought to determine the effect of duration on IFNγ pre-licensing. To test this, we PL-MSC donors in 10 ng ml−1 IFNγ for either 48 or 96 h. All donors showed increases in IDO protein when pre-licensed for a longer duration (figure 2c). Surprisingly, when comparing the effect of the dose strategy to the duration strategy, the change in IDO protein after longer durations was less than the change observed using the dose strategy (figure 2a,c). In addition, the benefit of longer duration varied between donors. Kynurenine concentration increased from 48 to 96 h of pre-licensing across all donors demonstrating that the enzyme remains active throughout the pre-licensing period (figure 2d).
3.3. Duration of pre-licensing predicts durability of indoleamine-2,3-dioxygenase protein post-withdrawal
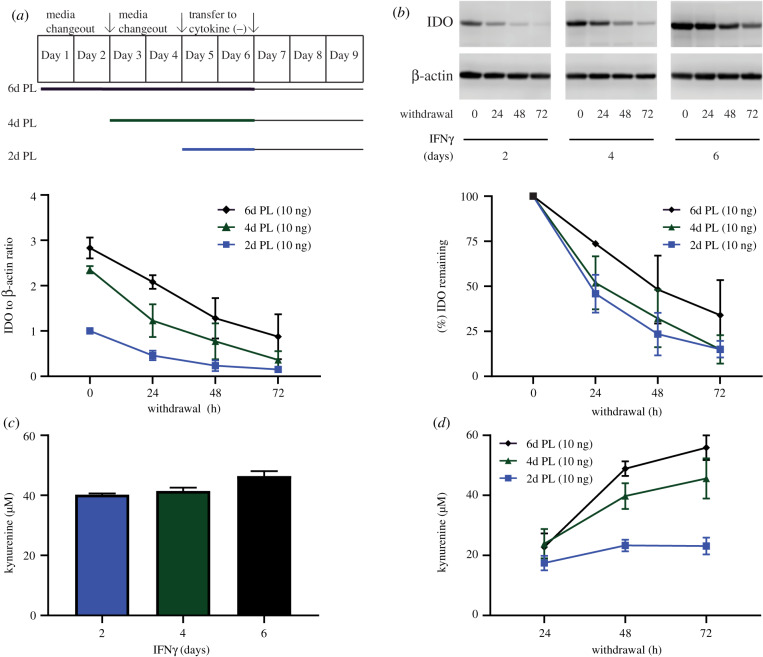
After determining the individual contribution of the dose and duration of IFNγ during pre-licensing on IDO protein levels and activity in MSCs, we next investigated if the elevated protein induced by a duration-based strategy would persist and remain functional after removal from the pre-licensing environment. Understanding how MSC potency is or is not maintained after removal from a pre-licensing environment is essential for establishing accurate predictions for therapeutic efficacy. To test this, MSCs were pre-licensed in 10 ng ml−1 IFNγ for 2, 4 or 6 days prior to being transferred to a cytokine-free environment for outgrowth (post-withdrawal period) (figure 3a). As seen from prior experiments, MSCs pre-licensed for longer durations had elevated IDO protein levels immediately after withdrawal (figure 3b). Interestingly, we found that this elevated IDO protein content persisted throughout the 72 h withdrawal period, with the per cent of IDO remaining being highest in MSCs pre-licensed for 6 days (figure 3b). In addition, we analysed kynurenine output at the end of the pre-licensing period, as well as over the course of withdrawal. Immediately after pre-licensing, all three durations resulted in a similar amount of kynurenine output (figure 3c). In the post-withdrawal period, kynurenine production was similar 24 h post-withdrawal for all tested durations; however, the output diverged significantly by 48 and 72 h, with MSCs exposed to longer durations of pre-licensing displaying higher levels of kynurenine production (figure 3d). Statistical analysis by two-way ANOVA with Tukey post hoc test revealed the 6-day PL-MSCs produced more kynurenine compared to the 2 day PL-MSCs at all three time points after withdrawal. Therefore, the duration of IFNγ exposure during pre-licensing increases IDO protein levels leading to enhanced persistence and activity of the protein even after removal from the pre-licensing environment.
Figure 3.
Longer durations of pre-licensing enhances IDO protein and activity post-withdrawal. (a) Graphical representation of the timing of media changeout, IFNγ stimulation and withdrawal from cytokines. (b) Representative western blot and densitometry of MSCs on the day of withdrawal as well as during the withdrawal period. IDO densitometry was normalized to housekeeping protein β-actin, and further normalized to the 2 day pre-licensing condition on the day of withdrawal. Per cent remaining was calculated as IDO protein for a given pre-licensing duration divided by its IDO protein level on the day of withdrawal. N = 3 independent experiments. Reporting mean ± s.e.m. (c) MSCs' kynurenine concentration for the last 48 h of pre-licensing before withdrawal. N = 4 independent experiments. Reporting mean ± s.e.m. (d) Accumulation of kynurenine 24, 48 and 72 h post-withdrawal. N = 5 independent experiments. Reporting mean ± s.e.m.
3.4. Accumulation of kynurenine metabolites during pre-licensing does not affect indoleamine-2,3-dioxygenase protein concentration or activity
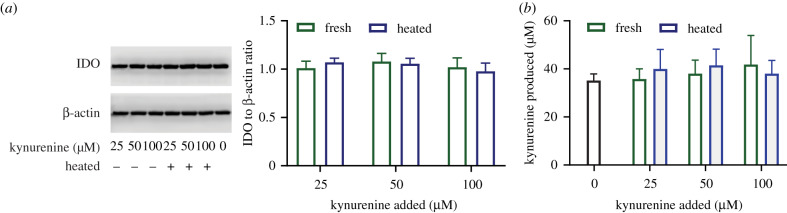
One concern with pursuing a duration-based strategy of pre-licensing is the potential accumulation of kynurenine and/or kynurenine-derived trace-extended aromatic condensation products (TEACOPs). TEACOPs are derivatives of kynurenine that spontaneously form at physiological temperatures and have been shown in other biological systems to have biological activity. Previous studies have identified both of these compounds as regulators of IDO expression in other cell types [29]; however, their effect on MSC regulation of IDO is, as of yet, unexplored. If pre-licensing is to be performed at scale during the biomanufacturing of therapeutic MSCs, it is important to determine the consequence of accumulation of these metabolites on MSCs. To determine the role of kynurenine and spontaneously formed by-products of kynurenine on IDO within MSCs, MSCs were pre-licensed with 10 ng ml−1 IFNγ with the addition of either fresh kynurenine or heated kynurenine (37°C for 48 h) for 4 days. Unlike other cell types, which show regulation of IDO activity by kynurenine and TEACOPs, neither the addition of fresh nor heated kynurenine had any appreciable impact on the production of IDO protein (figure 4a) or IDO enzymatic activity in human MSCs (figure 4b). Thus, in MSCs under the conditions examined, kynurenine metabolites do not play a critical role in modulating IDO content or activity.
Figure 4.
Kynurenine and TEACOPS do not significantly regulate IDO protein or activity. (a) Representative western blot and quantified densitometry of MSCs pre-licensed with increasing doses of kynurenine with or without heating. IDO protein was normalized to the house keeping gene β-actin, and further normalized to 0 μM kynurenine condition. N = 3 independent experiments. Reporting mean ± s.e.m. (b) Activity of IDO, measured as kynurenine concentration at the end of pre-licensing. Kynurenine produced was calculated by subtracting out signal generated due to kynurenine being added at the beginning of the experiment as measured on an identical plate without MSCs. N = 4 independent experiments. Reporting mean ± s.e.m.
3.5. Peripheral blood mononuclear cell suppression is enhanced with high-dose interferon γ but diminished with longer pre-licensing
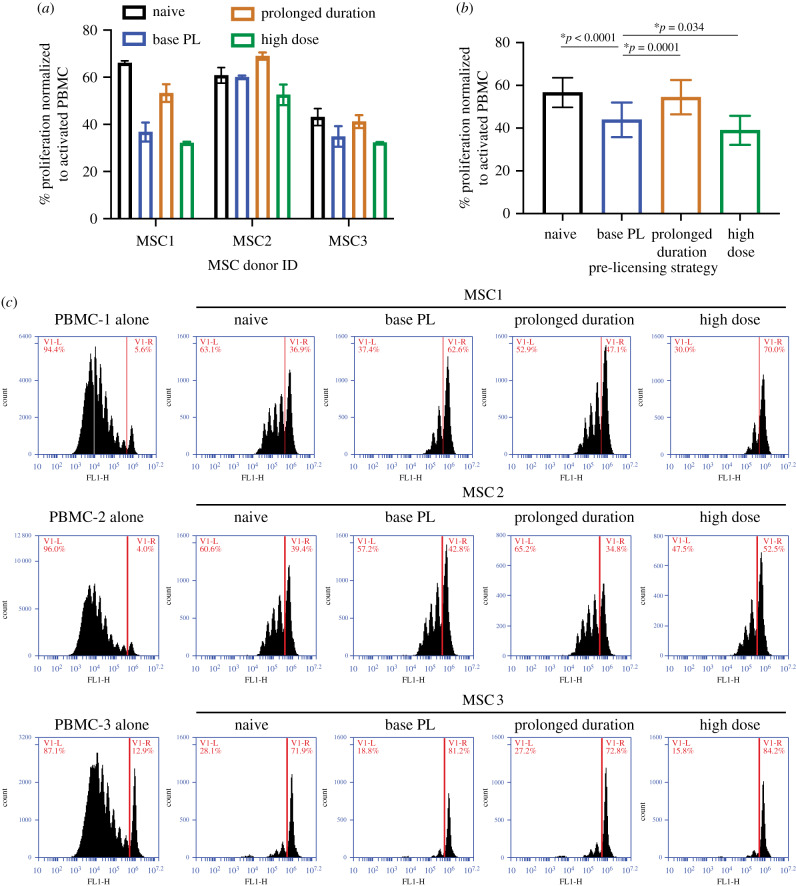
Having isolated the individual contribution of IFNγ dose and duration on IDO protein and activity, we next sought to determine if this translated to enhanced potency in a human PBMC suppression assay. To quantify the immunosuppressive ability of PL-MSCs, we pre-licensed our three donors (MSC1, MSC2, MSC3) using three unique strategies prior to direct contact co-culture with CD3/CD28-activated PBMCs, as well as a naive control, to determine baseline immunosuppressive ability for each donor. Three pre-licensing strategies used were: base pre-licensing (10 ng ml−1 IFNγ for 2 days), high-dose pre-licensing (50 ng ml−1 IFNγ for 2 days) and prolonged duration pre-licensing (10 ng ml−1 IFNγ for 4 days). Based on the results of our IDO protein and activity assays, we hypothesized that both high dose and longer duration would improve immunosuppressive potency compared to the base protocol.
Our positive control, base PL-MSCs, showed improved immunosuppressive potency compared to naive MSCs, indicating a benefit to pre-licensing as has previously been shown by us and others [20,23,25,30]. Additionally, consistent with our hypothesis, all donors benefited from high-dose pre-licensing, showing the highest suppression of proliferation across all tested strategies (figure 5a,b). Surprisingly, while IDO assays showed prolonged duration results in improved IDO levels and activity, it did not improve suppression of PBMCs compared to naive MSCs and was significantly worse than base pre-licensing (figure 5a,b). When comparing base pre-licensing with high-dose pre-licensing, although there was a significant difference between these strategies (p = 0.034), the difference was extremely modest (4.9% improvement). Using a two-way ANOVA to determine the amount of variance accounted for by donor choice versus pre-licensing strategy, most of the variance in immunomodulatory performance was attributable to donor choice (51% of variation), while pre-licensing strategy accounted for 31% of the variance and interactions between MSC donor and pre-licensing strategy accounted for 15% of the variance. Therefore, both the pre-licensing strategy and donor choice have significant roles in determining MSC immune suppression.
Figure 5.
Dose, duration and donor are regulators of MSC immune modulation. (a) Proliferation of PBMCs co-cultured with MSC donors MSC1, MSC2 and MSC3 at a 1 : 10 PBMC to MSC ratio. %Proliferation was calculated by normalizing to stimulated PBMC control within each experiment (positive control). Reporting mean ± s.d. of three replicates for each donor. (b) Proliferation data from (a) pooled and replotted to compare the effects of pre-licensing strategies independent of donor. Each bar is a pre-licensing strategy and each data point is the mean %Proliferation for an MSC donor in that strategy. N = 3 independent donors, reporting mean ± s.e.m., two-way ANOVA with Dunnett's multiple comparisons test, *p < 0.05 considered significant. (c) Representative flow plots of CFSE-stained PBMCs upon co-culture with each MSC donor under each pre-licensing condition. Red line denotes gate used to calculate %PBMC proliferation reported in (a,b).
The differences in donor potency varied widely without pre-licensing (Naive, figure 5a). Interestingly, while the trends between pre-licensing strategies were maintained across donors, the magnitude of this benefit varied substantially across donors with MSC1 benefiting the most, MSC3 receiving an intermediate benefit and MSC2 benefiting the least (figure 5a). Regardless of how pre-licensing affected the different donor's potency, MSC3 was more suppressive than either MSC1 or MSC2. Our data show that the effect of pre-licensing is not uniform, but the product of an interaction between the pre-licensing strategy and the intrinsic properties of the MSC donor.
4. Discussion
While MSCs’ immunomodulatory properties have been extensively documented, these properties are often assumed to be innate characteristics rather than dynamic cell states. Here, we focused on MSC regulation of IDO, a critical immunoregulatory enzyme shown to correlate with MSC suppression of PBMCs [31], and systematically separated the role of the dose of IFNγ stimulation, the duration of pre-licensing and the MSC donor. These studies were motivated both by a desire to uncover the dynamics of MSC activation into an immunomodulatory state and to identify parameters that need to be tuned to manufacture PL-MSCs at a therapeutic scale. Previous studies by Klinker and Marklein have shown that MSCs' morphology changes drastically in response to 10 ng ml−1 IFNγ but does not significantly change further as the dose is escalated, suggesting there is a minimal dose that elicits a fully primed response [30,32]. In agreement with this prior work, we found MSCs increase in IDO content to be dose dependent but approach a plateau where further increases to dose did not increase IDO content (figure 1a). Interestingly, increasing the dose of IFNγ had a more consistent effect across MSC donors than increasing the duration of pre-licensing. Increasing the dose of IFNγ from 5 to 50 ng ml−1 led to an increase in IDO protein and production of kynurenine to a very similar degree across all donors tested (figure 2a,b). By contrast, the benefit of a duration-based strategy was found to be more donor dependent (figure 2c). Thus, at the time of withdrawal, a high-dose pre-licensing strategy appears to favour higher levels of IDO compared to an extended duration strategy.
Next, we wished to determine how pre-licensing affected the durability of IDO. While identification of pre-licensing protocols that maximize IDO activity is desirable, they are only useful if IDO remains functional once the cells are transplanted. If IDO is degraded immediately after removal, pre-licensing is of little use. We hypothesized that increased duration of pre-licensing would enhance IDO protein levels, not only on the day of withdrawal, but also throughout the days following withdrawal. Both of our predictions, that IDO protein would be elevated on the day of withdrawal as well as on subsequent days after removal of IFNγ, were supported by the data (figure 3b). In addition, IDO enzymatic activity was maintained longer after withdrawal for MSCs that were pre-licensed for longer durations (figure 3d). Thus, prolonged pre-licensing appears beneficial both for the levels of IDO protein and the longevity of IDO after removal from the pre-licensing environment.
Next, we examined the role of kynurenines on MSC pre-licensing. Both kynurenine and kynurenine derivatives (TEACOPs) are known ligands for the aryl hydrocarbon receptor (AhR), which has been shown to regulate IDO expression in other cell types [29]. Thus, depending on the type of regulation, accumulation of such by-products could be either beneficial or detrimental to the manufacturing of potent PL-MSCs. Seok et al. showed that TEACOPs spontaneously form at physiological temperatures and are potent ligands for AhR. Once bound, the AhR–ligand complex translocates to the nucleus and regulates IDO transcription [33]. AhR regulation can either augment or inhibit transcription of target genes and is dependent upon cell type and the ligand that binds AhR [33]. However, the role of AhR and AhR ligands on MSCs has not been explored. While Seok et al. showed TEACOPS are potent AhR activators in COS-1 cells from African green monkey, exposure of human MSCs to kynurenine or kynurenine that had been heated to 37°C for 4 days did not have an appreciable effect on IDO protein or activity in human MSCs (figure 4a,b). Thus, selective removal or addition of these compounds during biomanufacturing does not appear to be a strategy that would augment IDO protein concentration or activity in PL-MSCs.
With the effect of IFNγ dose and duration of pre-licensing on IDO protein and activity tested, we next determined how these different pre-licensing strategies translate to MSC immune suppression. Based on our observations of MSC IDO, we expected both the high-dose strategy and the extended duration strategy to lead to a significant increase in MSC-mediated suppression of PBMCs. To our surprise, enhancing the dose of IFNγ fivefold only moderately increased the level of PBMC suppression (figure 5a,b). Even more surprising was that extending the duration of pre-licensing actually had a detrimental effect on MSC potency (figure 5a,b). Thus, we found that MSCs immunomodulatory phenotype cannot be captured using a single surrogate marker, in this case IDO, highlighting the need for multiple metrics to be used to predict MSC function [34]. In addition, while we initially thought the dose and duration of pre-licensing would be the most critical parameters to optimize, we found the donor itself had the largest influence on the potency of PL-MSCs. While base and high-dose pre-licensing had a consistent effect across donors, the donor of MSCs itself was responsible for most of the variance in immune suppression observed, highlighting the critical role of the starting cellular material (figure 5a). Pre-licensing with IFNγ is not always enough to overcome inherent donor impotency and screening of donors for allogeneic use is necessary to maximize the immunomodulatory benefit of pre-licensing.
The systematic approach taken here can serve as a template to study MSC dynamics after exposure to factors other than IFNγ or compare different lots of IFNγ and can help refine the control of MSC products for specific disease applications from specific manufacturing conditions. Such a strategy could even be employed for cryopreserved MSCs, as IDO protein expression is retained through the cryopreservation process [35]. The strengths of this study lie in our methodical approach to separate the influence of dose, duration and donor on MSC regulation of IDO and the analysis of MSC immunomodulatory potency using three surrogate markers: IDO protein, IDO activity and PBMC suppression. However, several limitations should be noted. As the donor source was the largest contributor to MSC immunomodulatory function, MSCs collected from different donors, tissues and cell culture systems may require a different ‘optimal’ dose of IFNγ and duration of pre-licensing to maximize their therapeutic potency. The strategy outlined here can, thus, serve as a template for analysing a range of doses and durations for specific lots of MSCs. Second, all experiments in this study were performed in a classic MSC media formulation (MEM-α, 15% FBS, 1% l-glutamine, 1% penicillin–streptomycin). We used the highest quality US Origin-ISIA certified FBS available. This was done to make our work relevant to both the large body of MSC literature using FBS and current MSC products that are being tested in clinical studies [36]. MSCs grown in alternate media formulations could generate MSCs that respond distinctly to cytokine stimulation [37,38].
5. Conclusion
The source of MSCs and the process used to manufacture them prior to transplantation have a major influence on the immunosuppressive properties of MSCs. MSCs dynamically upregulate IDO in an IFNγ dose-dependent manner and continue to upregulate IDO during extended periods of IFNγ exposure. However, despite elevated levels of IDO and maintained IDO activity, prolonged exposure to IFNγ can lead to MSCs immunosuppressive potency reverting to levels similar to naive MSCs. In addition, while both dose and duration can be used to modulate MSC production of IDO, the MSC donor played the biggest role in determining MSCs' immunosuppressive potency. Thus, the dose and duration of pre-licensing should be tailored to optimize the performance of specific donors and an emphasis on donor selection is needed to realize significant benefits of pre-licensing.
Acknowledgements
We would like to acknowledge Dr Donna Santillan and the University of Iowa Maternal-Fetal Tissue Bank and the DeGowin Blood Center for providing de-identified and coded tissue and blood that enabled the completion of this study.
Data accessibility
The data used to support the findings of this study are available from the corresponding author upon request.
Authors' contributions
D.T.B.: concept and design, data collection, data design, data analysis and interpretation, writing of original manuscript, editing of original manuscript and approval of final manuscript. L.K.B.: concept and design, data collection, data design, data analysis and interpretation, editing of original manuscript and approval of final manuscript. A.J.Bu.: concept and design, data design, data analysis and interpretation, editing of original manuscript and approval of final manuscript. A.J.Br.: concept and design, writing of original manuscript and approval of final manuscript. J.A.A.: concept and design, data design, data analysis and interpretation, financial support, writing of original manuscript, editing of original manuscript and approval of final manuscript
Competing interests
The authors declare no potential conflicts of interest.
Funding
A.J.Bu. was supported through an NIH training grant (no. 1T32NS045549) and L.K.B. was supported in part by NIH training grant no. 5T32GM007337. Support from the Straub Foundation, Diabetes Action Research and Education Foundation and the Fraternal Order of Eagles Diabetes Research Foundation awarded to J.A.A. were used to complete the project. Several of the chemical/media reagents used for this work were provided through a Biological Industries USA Research Award to J.A.A. Funders were not involved in the study design, collection, analysis or interpretation of data, writing of the manuscript or decision to publish.
References
- 1.Kurtzberg J, et al. 2014. Allogeneic human mesenchymal stem cell therapy (remestemcel-L, Prochymal) as a rescue agent for severe refractory acute graft-versus-host disease in pediatric patients. Biol. Blood Marrow Transplant. 20, 229–235. ( 10.1016/j.bbmt.2013.11.001) [DOI] [PubMed] [Google Scholar]
- 2.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O.. 2004. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363, 1439–1441. ( 10.1016/S0140-6736(04)16104-7) [DOI] [PubMed] [Google Scholar]
- 3.Forbes GM, Sturm MJ, Leong RW, Sparrow MP, Segarajasingam D, Cummins AG, Phillips M, Herrmann RP. 2014. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn's disease refractory to biologic therapy. Clin. Gastroenterol. Hepatol. 12, 64–71. ( 10.1016/j.cgh.2013.06.021) [DOI] [PubMed] [Google Scholar]
- 4.Mayer L, Pandak WM, Melmed GY, Hanauer SB, Johnson K, Payne D, Faleck H, Hariri RJ, Fischkoff SA. 2013. Safety and tolerability of human placenta-derived cells (PDA001) in treatment-resistant Crohn's disease: a phase 1 study. Inflamm. Bowel Dis. 19, 754–760. ( 10.1097/MIB.0b013e31827f27df) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galleu A, et al. 2017. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 9, eaam7828 ( 10.1126/scitranslmed.aam7828) [DOI] [PubMed] [Google Scholar]
- 6.Kusuma GD, Carthew J, Lim R, Frith JE. 2017. Effect of the microenvironment on mesenchymal stem cell paracrine signaling: opportunities to engineer the therapeutic effect. Stem Cells Dev. 26, 617–631. ( 10.1089/scd.2016.0349) [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal S, Pittenger MF. 2004. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822. ( 10.1182/blood-2004-04-1559) [DOI] [PubMed] [Google Scholar]
- 8.Ryan JM, Barry F, Murphy JM, Mahon BP. 2007. Interferon-γ does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin. Exp. Immunol. 149, 353–363. ( 10.1111/j.1365-2249.2007.03422.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge W, Jiang J, Arp J, Liu W, Garcia B, Wang H. 2010. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation 90, 1312–1320. ( 10.1097/TP.0b013e3181fed001) [DOI] [PubMed] [Google Scholar]
- 10.He Y, Zhou S, Liu H, Shen B, Zhao H, Peng K, Wu X. 2015. Indoleamine 2, 3-dioxgenase transfected mesenchymal stem cells induce kidney allograft tolerance by increasing the production and function of regulatory T cells. Transplantation 99, 1829–1838. ( 10.1097/TP.0000000000000856) [DOI] [PubMed] [Google Scholar]
- 11.fcCroitoru-Lamoury J, Lamoury FMJ, Caristo M, Suzuki K, Walker D, Takikawa O, Taylor R, Brew BJ. 2011. Interferon-y regulates the proliferation and differentiation of mesenchymal stem cells via activation of indoleamine 2,3 dioxygenase (IDO). PLoS ONE 6, e14698 ( 10.1371/journal.pone.0014698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rovira Gonzalez YI, Lynch PJ, Thompson EE, Stultz BG, Hursh DA. 2016. In vitro cytokine licensing induces persistent permissive chromatin at the indoleamine 2,3-dioxygenase promoter. Cytotherapy 18, 1114–1128. ( 10.1016/j.jcyt.2016.05.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ankrum JA, Dastidar RG, Ong JF, Levy O, Karp JM. 2014. Performance-enhanced mesenchymal stem cells via intracellular delivery of steroids. Sci. Rep. 4, 4645 ( 10.1038/srep04645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loisel S, et al. 2017. Brief report: proteasomal indoleamine 2,3-dioxygenase degradation reduces the immunosuppressive potential of clinical grade-mesenchymal stromal cells undergoing replicative senescence. Stem Cells 35, 1431–1436. ( 10.1002/stem.2580) [DOI] [PubMed] [Google Scholar]
- 15.Jin P, et al. 2016. Interferon-γ and tumor necrosis factor-α polarize bone marrow stromal cells uniformly to a Th1 phenotype. Sci. Rep. 6, 1–11. ( 10.1038/srep26345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Y, Yoo SM, Park HH, Baek SY, Kim Y, Lee S, Kim YL, Seo K, Kang K. 2019. Preconditioning with interleukin-1 beta and interferon-gamma enhances the efficacy of human umbilical cord blood-derived mesenchymal stem cells-based therapy via enhancing prostaglandin E2 secretion and indoleamine 2,3-dioxygenase activity in dextran sulfate sodium-induced colitis. J. Tissue Eng. Regen. Med. 10, 1792–1804. ( 10.1002/term.2930) [DOI] [PubMed] [Google Scholar]
- 17.Jitschin R, et al. 2019. Inflammation-induced glycolytic switch controls suppressivity of mesenchymal stem cells via STAT1 glycosylation. Leukemia 33, 1783–1796. ( 10.1038/s41375-018-0376-6) [DOI] [PubMed] [Google Scholar]
- 18.Krampera M. 2011. Mesenchymal stromal cell ‘licensing’: a multistep process. Leukemia 9, 1408–1414. ( 10.1038/leu.2011.108) [DOI] [PubMed] [Google Scholar]
- 19.Chinnadurai R, Copland IB, Garcia MA, Petersen CT, Lewis CN, Waller EK, Kirk AD, Galipeau J. 2016. Cryopreserved mesenchymal stromal cells are susceptible to T-cell mediated apoptosis which is partly rescued by IFNγ licensing. Stem Cells 34, 2429–2442. ( 10.1002/stem.2415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boland L, Burand AJ, Brown AJ, Boyt D, Lira VA, Ankrum JA. 2018. IFN-γ and TNF-α pre-licensing protects mesenchymal stromal cells from the pro-inflammatory effects of palmitate. Mol. Ther. 26, 860–873. ( 10.1016/j.ymthe.2017.12.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.François M, Copland IB, Yuan S, Romieu-Mourez R, Waller EK, Galipeau J. 2012. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-γ licensing. Cytotherapy 14, 147–152. ( 10.3109/14653249.2011.623691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi JZ, Chen ZH, Xu FH, Wang ZY, Zhang HQ, Jiang GS, Luan XY. 2018. Interferon-γ suppresses the proliferation and migration of human placenta-derived mesenchmal stromal cells and enhances their ability to induce the generation of CD4+ CXCR5+ Foxp3+ Treg subset. Cell. Immunol. 326, 42–51. ( 10.1016/j.cellimm.2017.07.009) [DOI] [PubMed] [Google Scholar]
- 23.Kim DS, Jang IK, Lee MW, Ko YJ, Lee DH, Lee JW, Sung KW, Koo HH, Yoo KH. 2018. Enhanced immunosuppressive properties of human mesenchymal stem cells primed by interferon-γ. EBioMedicine 28, 261–273. ( 10.1016/j.ebiom.2018.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Witte SFH, et al. 2017. Cytokine treatment optimises the immunotherapeutic effects of umbilical cord-derived MSC for treatment of inflammatory liver disease. Stem Cell Res. Ther. 8, 140 ( 10.1186/s13287-017-0590-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menard C, et al. 2013. Clinical-grade mesenchymal stromal cells produced under various good manufacturing practice processes differ in their immunomodulatory properties: standardization of immune quality controls. Stem Cells Dev. 22, 1789–1801. ( 10.1089/scd.2012.0594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dominici M, et al. 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317. ( 10.1080/14653240600855905) [DOI] [PubMed] [Google Scholar]
- 27.Däubener W, Wanagat N, Pilz K, Seghrouchni S, Georg Fischer H, Hadding U. 1994. A new, simple, bioassay for human IFN-γ. J. Immunol. Methods. 168, 39–47. ( 10.1016/0022-1759(94)90207-0) [DOI] [PubMed] [Google Scholar]
- 28.Gramlich OW, Burand AJ, Brown AJ, Deutsch RJ, Kuehn MH, Ankrum JA. 2016. Cryopreserved mesenchymal stromal cells maintain potency in a retinal ischemia/reperfusion injury model: toward an off-the-shelf therapy. Sci. Rep. 6, 26463 ( 10.1038/srep26463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seok SH, et al. 2018. Trace derivatives of kynurenine potently activate the aryl hydrocarbon receptor (AHR). J. Biol. Chem. 293, 1994–2005. ( 10.1074/jbc.RA117.000631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klinker MW, Marklein RA, Lo Surdo JL, Wei C-H, Bauer SR. 2017. Morphological features of IFN-γ-stimulated mesenchymal stromal cells predict overall immunosuppressive capacity. Proc. Natl Acad. Sci. 114, E2598–E2607. ( 10.1073/pnas.1617933114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.François M, Romieu-Mourez R, Li M, Galipeau J. 2012. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 20, 187–195. ( 10.1038/mt.2011.189) [DOI] [PubMed] [Google Scholar]
- 32.Marklein RA, Klinker MW, Drake KA, Polikowsky HG, Lessey-Morillon EC, Bauer SR. 2019. Morphological profiling using machine learning reveals emergent subpopulations of interferon-γ-stimulated mesenchymal stromal cells that predict immunosuppression. Cytotherapy 21, 17–31. ( 10.1016/j.jcyt.2018.10.008) [DOI] [PubMed] [Google Scholar]
- 33.Lewis HC, Chinnadurai R, Bosinger SE, Galipeau J. 2017. The IDO inhibitor 1-methyl tryptophan activates the aryl hydrocarbon receptor response in mesenchymal stromal cells. Oncotarget 8, 91 914–91 927. ( 10.18632/oncotarget.20166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chinnadurai R, et al. 2018. Potency analysis of mesenchymal stromal cells using a combinatorial assay matrix approach. Cell Rep. 22, 2504–2517. ( 10.1016/j.celrep.2018.02.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burand AJ, Di L, Boland LK, Boyt DT, Schrodt MV, Santillan DA, Ankrum JA.. 2020. Aggregation of human mesenchymal stromal cells eliminates their ability to suppress human T cells. Front. Immunol. 11, 1–14. ( 10.3389/fimmu.2020.00143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burand AJ, Gramlich O, Brown AJ, Ankrum JA. 2017. Function of cryopreserved mesenchymal stromal cells with and without interferon-γ prelicensing is context dependent. Stem Cells 35, 1437–1439. ( 10.1002/stem.2528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin JQ, Zhu J, Ankrum JA. 2019. Manufacturing of primed mesenchymal stromal cells for therapy. Nat. Biomed. Eng. 3, 90–104. ( 10.1038/s41551-018-0325-8) [DOI] [PubMed] [Google Scholar]
- 38.Boland LK, et al. 2019. Nature vs. nurture: defining the effects of mesenchymal stromal cell isolation and culture conditions on resiliency to palmitate challenge. Front. Immunol. 10, 1080 ( 10.3389/fimmu.2019.01080) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.