ABSTRACT
Background
Percutaneous endoscopic gastrostomy (PEG) can facilitate feeding and medication administration in dysphagic patients with Parkinson's disease and related disorders. Information on survival, institutionalization, and complications post PEG might inform feeding decisions.
Method
A total of 93 patients with Parkinson's disease and related disorders were identified by review of PEG registers and by searching the administrative databases in 2 large UK university hospitals (2005–2017); 83 case notes were available for retrospective review. Care processes and outcomes were assessed.
Results
The following were the diagnoses: 58 (70%) had Parkinson's disease, 10 (12%) had progressive supranuclear palsy, 5 (6%) had multiple system atrophy, 3 (4%) had dementia with Lewy bodies, and 7 (8%) had vascular parkinsonism. The median age was 78 years (interquartile range 72–82); 29 (35%) were women. Care processes included a future care plan in place prior to admission for 18 patients (22%), and PEG was placed during emergency admission in 68 patients (82%). The outcomes included median survival at 422 days; 30‐day mortality rate was 6% (5 patients); and of 56 patients admitted from home, 18 (32%) were discharged to institutions (nursing or care homes). The most common complication was aspiration pneumonia for 18 (22%) of patients. Age, sex, diagnosis, admission type, comorbidities, and place of residence did not predict survival. Discharge to own home and follow‐up by the home enteral feeding team were associated with longer survival.
Conclusion
We recommend markers of advanced disease should prompt advanced care planning. Discussions about PEG feeding should include information about post‐PEG survival, complications, and risk of institutionalization. Further research is needed on quality‐of‐life post PEG and ways to reduce aspiration pneumonia. All PEG patients should have nutrition team follow‐up.
Keywords: Parkinson's disease, PEG, percutaneous endoscopic gastroscopy, tube feeding, institutionalization
Dysphagia is common in Parkinson's disease (PD) as the disease progresses. 1 Dysphagia also occurs in atypical PD and is a poor prognostic marker in progressive supranuclear palsy. 2 Dysphagia may be complicated by weight loss and the aspiration of food and saliva, leading to pneumonia. 1 Meal times can become very prolonged and effortful. Recommendations for the initial management of dysphagia in PD include modification of food consistency and posture. 3 As dysphagia worsens, enteral tube feeding may be considered.
Percutaneous endoscopic gastrostomy (PEG) tube feeding is the most common type of long‐term enteral feeding and is likely to be effective and safe for long‐term feeding in neurogenic dysphagia. 4 PEG feeding may lead to longer survival than oral feeding in home care patients with neurological impairments, 5 but probably does not improve survival in advanced dementia. 6 Relatively few studies focus on PEG feeding in parkinsonian conditions. Two such studies estimated survival in parkinsonian conditions to be 186 days to 1.2 years, but the studies were small: the combined number of patients with PD in these 2 studies was no more than 18. 7 , 8 Worse outcomes in fully dependent patients were reported, and pneumonia was a common adverse event. Larger studies of PEG outcomes have included patients with PD, but outcomes specific to PD are not reported. 4 For example, a large British study including 10,952 cases showed a 14.6% 30‐day mortality. This study included 591 patients with PD. It did not identify PD as a risk factor for early mortality at 7 and 30 days. Outcomes specifically for PD are not further described, but in the whole study, increasing age, male sex, nonelective admission, and dementia were identified as risk factors for mortality at 30 days post PEG. 9
Enteral feeding in general (ie, not specifically in PD) can lead to improvements in nutritional markers and increased weight but can be burdensome with reductions in quality of life because of nausea, fatigue, body image, and difficulty leaving the home. 10 Complications include PEG site infections, bleeding, pain, perforation, aspiration pneumonia, ileus, and sedation‐related complications. Risk of serious complication is <2%. 11 Clinical guidelines mandate careful consideration of the wishes of the patient and the risks and benefits of tube feeding in each patient, 12 and this is especially important where patients lack capacity. 13 Recommendations include multidisciplinary team assessment prior to PEG placement, speech and language therapy assessment for swallowing problems, advanced care planning in people with PD, and the planning and coordination of future treatment and home care. 12 , 14 , 15
It is important for clinicians, patients, and families to have information about prognosis post PEG when considering PEG insertion. Information on complications, survival, and institutionalization are pertinent. We undertook a retrospective study in 2 large university hospitals to audit processes of care against these standards to estimate the frequency of PEG insertion in parkinsonism and to describe outcomes after PEG insertion in this population.
Methods
Cases were identified by review of a PEG register held by the nutrition nurses. Additional cases were identified by searching the hospital administrative database for admissions with codes for PD and related conditions and PEG insertion. We wanted to estimate the rate of PEG insertion and see how this varied over time. We wanted to audit processes around PEG insertion and outcomes following PEG insertion. We collected the following data: age, sex, usual place of residence, and underlying neurological diagnosis. For those with PD, we recorded if dementia had been diagnosed, and we recorded the Hoehn and Yahr stage and PD medication, including daily levodopa dose. We recorded whether an advanced care plan was in place prior to admission; admission type (emergency or elective); involvement of speech and language therapy; discussion of benefits, risks, and alternatives; and organization of community follow‐up. With regard to PEG outcomes, we recorded survival, complications, length of stay after PEG, readmissions, and place of discharge. When considering discharge destination, we recorded where there was a step up in care needs. A step up in care needs was defined as moving from home alone to home not alone, from home to residential care or to a nursing home, or from residential care to a nursing home. For calculation of proportion of individuals experiencing a step up in care, individuals whose usual place of residence was a nursing home were excluded as they were already receiving the maximum care. Individuals who died before discharge from hospital were also excluded from an analysis of step up in care needs. The audit was completed in 2 large university hospitals in the United Kingdom. Each hospital has a catchment population of 500,000. The number of patients with PD attending each PD service is about 1000 (administrative data). We reviewed notes from 2005 to 2017. We calculated survival in days and plotted Kaplan‐Meier survival curves. We calculated 30‐day mortality rate and median survival. We compared outcomes (30‐day mortality, median survival, and proportion with a step up in care) according to a variety of baseline characteristics: age, sex, underlying diagnosis, Hoehn and Yahr stage (for those with PD), comorbidity count, future (advance) care planning, and admission type. We also compared outcomes by discharge destination and by whether patients were followed up by the home enteral feeding (HEF) team. We used the χ 2 test to compare proportions having a step up in care after PEG. We used the log‐rank test to compare survival curves. We planned to use the χ 2 test to compare proportions dying within 30 days, but the numbers were too small for meaningful statistical testing. We used Wizard version 1.9.39 statistical software. The audit was approved by each host institution, and the results were fed back locally.
Results
We identified 93 PEG insertions for parkinsonism, and case records were available for review in 83 cases. Of these, the median age was 78 (interquartile range, 72–82), and 29 (35%) were women. A total of 61 patients (74%) were admitted from home, 9 patients (11%) were admitted from residential homes, and 13 patients (16%) were admitted from nursing homes. PD was the underlying diagnosis in 58 patients (70%), progressive supranuclear palsy in 10 patients (12%), vascular parkinsonism in 7 patients (8%), multisystem atrophy in 5 patients (6%), and dementia with Lewy bodies in 3 patients (4%). Of the patients with PD, 10/58 (17%) had PD dementia. For those with PD, the median levodopa dose was 400 mg, and dopamine agonists were taken prior to admission by 14 (24%). The number of PEG insertions per year is shown in Figure 1. The number varies, but there is no evidence of a trend toward increasing or declining rates of insertion. The mean number of PEG insertions per year was 6.4. The estimated number of PEG insertions per 1000 patients with PD per year was 3.2. Data about care processes are shown in Table 1. Data on main PEG outcomes are shown in Table 2. No patients died within 7 days of PEG, and 5 patients (6.0%) died within 30‐days of PEG insertion. Median survival after PEG was 422 days. A survival curve is shown in Figure 2A. Median survival and 30‐day mortality were compared across a number of baseline characteristics, and no statistically significant differences were noted for age, sex, diagnosis, Hoehn and Yahr stage (for patients with PD), usual place of residence, admission type (elective or nonelective), comorbidities, or advanced care planning (Table 2). For those with PD, there was a trend toward shorter survival in those with dementia (Fig. 2B). Of the patients, 5 (6%) died before discharge from hospital. All of these patients had aspiration pneumonia. The most common early complication (occurring during the admission when the PEG was inserted) was aspiration pneumonia (n = 18 [22%]). Other early complications included 7 PEG site infections (8.4%), 1 bowel perforation (1.2%), and 11 other complications (13%). The number of individuals with no early complication was 49 (59%). During follow‐up there were 2 (2.4%) buried bumpers and 2 (2.4%) accidentally removed PEG tubes. The number of patients readmitted in the first year postdischarge was 36 (47% of those discharged). The most common cause of readmission was aspiration pneumonia (n = 14 [18%]). Aspiration pneumonia at any time post PEG occurred in 28 patients (34%). A total of 38 patients (49%) were discharged to their own home, only 1 (1.2%) of those patients was discharged home alone. A total of 34 patients (44%) were discharged to nursing homes, and 6 patients (7.7%) were discharged to residential care. A step up in care was seen in 23 individuals (34%). Of 61 patients admitted from home, 5 (8%) died before discharge, 17 (28%) were moved to nursing homes, 1 (1.6%) was moved to residential care, and 38 (62%) returned home. Of the 56 patients admitted from home and surviving to discharge, 18 (32%) were institutionalized. The length of stay post PEG was 20 days (interquartile range, 8–30). Where HEF teams followed up with patients (n = 40), markers of improved nutrition were present in 33 (83%); weight increased in 18 patients (45%). The HEF team follow‐up was more likely in those discharged to their own home (66% vs. 38% discharged to nursing home/residential home; chi squared test P = 0.01). Survival was shorter for those discharged to nursing home versus home (n = 34 and n = 38, respectively; median survivals, 323 days vs. 766 days; log‐rank, P = 0.005 [P = 0.041 correcting for multiple comparisons]; Fig. 2C). Survival was shorter for those not followed up by a HEF team versus those with HEF team follow‐up (n = 38 and n = 40, respectively; median survivals, 310 days vs. 820 days; log‐rank, P = 0.005 [P = 0.047 correcting for multiple comparisons]; Fig. 2D). Outcomes for PEGs inserted in the first part of the data collection period (2005–2010) were compared with the outcomes for the later part of the data collection period (2011–2017). There were no statistically significant differences in the 2 time periods in survival or 30‐day mortality, but in 2011 to 2017 there were more individuals readmitted in the first year and fewer having a step up in care (Table 3). We have no further data on the 10 patients whose notes were unavailable, so we cannot say if these were similar to the 83 cases described here.
FIG. 1.
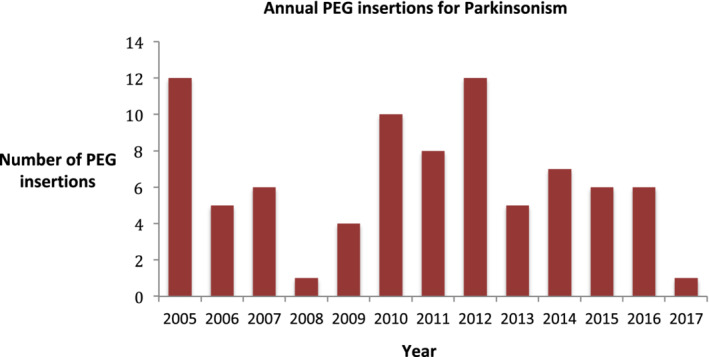
Annual percutaneous endoscopic gastrostomy (PEG) insertions for parkinsonism.
TABLE 1.
Care processes before and after PEG in patients with Parkinson's disease and related disorders
| Advanced care plan in place | 19 (23%) |
| Patient placed nil by mouth | 57 (69%) |
| Nasogastric tube used prior to PEG | 64 (77%) |
| Speech and language therapist assessment before PEG | 74 (90%) |
| Documented risk/benefit discussion | 69 (83%) |
| Community follow‐up by enteral nutrition team | 40 (52%) |
PEG, percutaneous endoscopic gastrostomy.
TABLE 2.
Baseline features and outcomes post PEG in patients with PD and related conditions
| Baseline Features | Number (%) | 30‐Day Mortality (%) | Median Survival in Days (Log‐Rank) | Step Up in Care (%) |
|---|---|---|---|---|
| Age, y | ||||
| <75 | 29 (35) | 4 (14) | 400 | 7 (30) |
| 75–84.9 | 39 (47) | 1 (2.6) | 532 | 11 (37) |
| ≥85 | 15 (18) | 0 | 220 (P = 0.979) | 5 (42) |
| Sex | ||||
| Female | 29 (35) | 2 (3.4) | 584 | 7 (33) |
| Male | 54 (65) | 3 (5.5) | 400 (P = 0.557) | 16 (36) |
| Diagnosis | ||||
| PD | 58 (70) | 4 (6.9) | 571 | 19 (41) |
| PSP | 10 (12) | 1 (10) | 400 | 2 (29) |
| MSA | 5 (6) | 0 | 422 | 1 (25) |
| DLB | 3 (4) | 0 | 286 | 0 |
| VP | 7 (8) | 0 | 387 (P = 0.654) | 1 (20) |
| Hoehn and Yahr stage | ||||
| 2 | 1 (2) | 0 | 761 | – |
| 3 | 14 (24) | 1 (7.1) | 344 | 4 (50) |
| 4 | 23 (40) | 1 (4.3) | 532 | 6 (30) |
| 5 | 20 (34) | 2 (10) | 422 (P = 0.853) | 4 (22) |
| Admission type | ||||
| Elective | 15 (18) | 0 | 771 | 2 (13) |
| Nonelective | 68 (82) | 5 (7.4) | 392 (P = 0.672) | 21 (40) |
| Advanced care plan | ||||
| Yes | 19 (23) | 1 (5.3) | 771 | 4 (25%) |
| No | 64 (77) | 4 (6.3) | 387 (P = 0.717) | 19 (39%) |
| Comorbidity | ||||
| 0 | 11 (13) | 1 (9.1) | 794 | 4 (44) |
| 1 | 55 (66) | 4 (7.2) | 730 | 16 (37) |
| 2 | 15 (18) | 0 | 414 | 1 (10) |
| ≥3 | 2 (2) | 0 | 392 (P = 0.270) | 2 (100) |
| Usual residence | ||||
| Home alone | 8 (9.6) | 0 | 985 | 5 (71) |
| Home not alone | 53 (64) | 4 (7.5) | 571 | 14 (29) |
| Residential care | 9 (11) | 0 | 370 | 4 (44) |
| Nursing home | 13 (16) | 1 (7.7) | 168 (P = 0.634) | ‐ |
| PD dementia | ||||
| No dementia | 48 (83) | 2 (4.2) | 584 | 17 (43) |
| Dementia | 10 (17) | 2 (20) | 344 (P = 0.105) | 2 (33) |
| All | 83 | 5 (6.0) | 422 | 23 (34) |
Baseline factors that might have influenced a step up in care were compared using chi‐squared test, and no statistically significant differences were observed. Survival curves after PEG were compared using log‐rank test. The number of deaths within 30 days were too small for statistical testing.
DLB, dementia with Lewy bodies; MSA, multisystem atrophy; PD, Parkinson's disease; PEG, percutaneous endoscopic gastrostomy; PSP, progressive supranuclear palsy; VP, vascular parkinsonism.
FIG. 2.
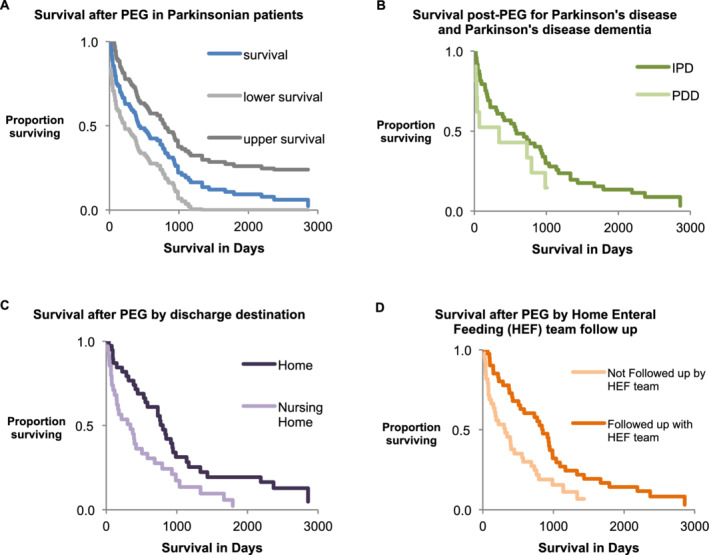
(A) Kaplan‐Meier plot for all study patients. (B) Survival plots comparing idiopathic Parkinson's disease (IPD; n = 48) and Parkinson's disease dementia (PDD; n = 10); log‐rank, P = 0.105. (C) Survival plots comparing discharge to nursing homes (n = 34; median survival, 323 days) versus discharge to own home (n = 38; median survival, 766 days); log‐rank, P = 0.005. (D) Survival plots comparing HEF team follow‐up with no HEF team follow‐up; log‐rank, P = 0.005. HEF, home enteral feeding; PEG, percutaneous endoscopic gastrostomy.
TABLE 3.
Outcomes for percutaneous endoscopic gastrostomy in different time periods
| Year of PEG Insertion | Number (%) | 30‐Day Mortality (%) | Median Survival in Days | Patients Readmitted in First Year (%) | Step Up in Care |
|---|---|---|---|---|---|
| 2005–2010 | 38 (46) | 2 (5.3) | 366 | 11 (33) | 14 (41) |
| 2011–2017 | 45 (54) | 3 (6.7) | 532 a | 25 (54) b | 9 (20) c |
Log‐rank, P = 0.298.
Chi squared test, P = 0.041.
Chi squared test, P = 0.047.
Discussion
We believe this is the largest published study of PEG outcomes in parkinsonian disorders. We report a 7‐day mortality rate of 0% and a 30‐day mortality of 6.0%. These rates are consistent with published data, although the populations studied vary. 7 , 8 , 16 Median survival is also consistent with published data. 17 PEG insertion was relatively infrequent considering how many patients with PD are seen in our clinics. Duk and colleagues 18 noted a PEG insertion rate of 1.4% in PD, with a trend toward falling insertion rates. It is noteworthy that 10 (17%) of the patients with PD had a recorded diagnosis of dementia prior to PEG insertion. The true number with dementia may have been higher as a further 7 (12%) were taking rivastigmine, a drug primarily used to treat dementia. Placing PEGs in patients with dementia is controversial. 6 In PD, a feeding tube may allow the administration of essential medication, and we speculate that this might have contributed to decisions to place PEGs in these patients. Although our data provide no insights into quality of life, the median survival in patients with PD and dementia was 344 days (554 days if those taking rivastigmine are reclassified as dementia), which suggests that PEG placement in carefully selected patients with PD and dementia is not futile. In the opinion of the authors, if dysphagia leads to significant problems taking oral dopaminergic medication, and if giving that medication by nasogastric tube leads to significant, worthwhile clinical improvements, then PEG insertion should be considered. Aspiration pneumonia occurred in 34% of our patients and was the most common early complication and the most common cause for readmission. The rate of aspiration pneumonia in our study is consistent with other recent studies, which estimate rates of 31% to 46%. 7 , 8 Aspiration pneumonia is often cited as a potential indication for PEG feeding, 14 so it is important that clinicians, patients, and staff are aware that aspiration pneumonia remains a significant problem after PEG and is a leading cause of death. The reason aspiration pneumonia still occurs is likely attributed to the aspiration of saliva, food taken orally (as not all PEG‐fed patients take their entire diet by tube), and aspiration of regurgitated PEG feed. Strategies to reduce the risk of aspiration pneumonia include a 30° to 45° head‐up positioning for feeding, jejunal feeding, and use of prokinetic agents. 19 The latter 2 strategies may be of particular relevance in PD where delayed gastric emptying is relatively common, but further research is required. An audit of the care processes showed that 23% had an advanced care plan. This is consistent with published data reporting 37% of those patients with markers of advanced disease have had discussions about advanced care plans. 20 Nonetheless, this low rate of future (advanced) care planning is disappointing considering the fairly predictable trajectory of these neurodegenerative diseases particularly as we have shown that many patients have PEG tubes inserted during nonelective admissions. This is at a stage when many patients lack the capacity to give informed consent for the procedure. 16 It is surprising that a PEG feeding tube could be placed without input from a speech and language therapist, as was the case in 10% of the patients we report. We recommend that such an assessment should always take place.
We believe the high rate of institutionalisation after PEG insertion in parkinsonian conditions has not been previously reported. In our study, nearly a third of patients admitted from home were discharged to institutional care. This is an important finding as many patients report their preferred place of care for final illness as their own home. 21 Many patients have an aversion to nursing home (NH) placement and might regard it as an adverse outcome. Information on rates of institutionalisation among PEG‐fed patients with PD should be shared with those making advanced care plans and those making decisions about PEG feeding during emergency admissions of patients with PD. The trend toward shorter survival in institutionalized (NH) patients is unsurprising and likely reflects their greater frailty. The lower follow‐up rates of NH patients by the community enteral nutrition team may in part reflect shorter exposure time and a perception that NH nursing staff have all necessary expertise and require no further support. Our data suggest that it may be wise for community enteral nutrition teams to follow‐up with all patients whether discharged home or to a nursing home.
The strengths of this study are its size compared with other studies of outcomes for PEG feeding in PD and that it includes data from more than 1 center. Also, our audit assessed pre‐PEG and post‐PEG care and not just outcomes such as mortality, complications, and readmissions. These data may help inform difficult discussions about the risks and benefits of PEG feeding in parkinsonian disorders.
The main weakness of the study is its retrospective nature with incomplete data capture. Complications may have been incompletely recorded, so more complications may have occurred than we report. When considering the utility of our data in informing PEG placement decisions in dysphagic patients with PD, it would be better to also have outcome data on those managed without PEG, but we have no such data. We had to go back 13 years in 2 large university hospitals to get data on 83 PEG‐fed parkinsonian patients. This suggests that patients are being carefully selected for the procedure. Clinicians, patients, and family should be cautious about applying these data to all dysphagic patients with PD. The data span 13 years during which time practice may have changed. A further weakness was the absence of patient‐reported outcome measures that reflected quality of life.
We recommend that markers of advanced disease (onset of swallowing problems, care home placement, admission for aspiration pneumonia, diagnosis of dementia, unplanned weight loss) should prompt advanced care discussions, including preference with regard to tube feeding. Discussions about PEG feeding in parkinsonian conditions should include information about post‐PEG survival and complications as described in this report. The risk of institutionalization (32%) should be disclosed and considered. HEF teams should follow‐up with all patients discharged with PEG feeding tubes and educate about head‐up positioning for feeding. Further research should establish the impact of PEG feeding on health‐related quality of life in patients with PD and related conditions. Research should also be undertaken into strategies to reduce aspiration in PEG‐fed patients with PD.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
L.B.: 1A, 1B, 1C, 3B
M.O.: 1B, 1C, 3B
A.‐D.S.: 1C, 3B
H.M.: 1B, 1C, 3B N.B.: 1C, 3B
J.C.: 1B, 3B
A.L.: 1A, 1B, 3B
R.S.: 1A, 2A, 2B, 3A, 3B
Disclosures
Ethical Compliance Statement: The study was approved as audit at the two host institutions. Ethical approval and informed patient consent was not necessary for this work. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: No specific funding was received for this work. The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the previous 12 months: We believe we have no conflicts of interest relevant to this manuscript but make the following declarations. L.B. has received research grants from Parkinson's UK. She has received service development grants from Parkinson's UK and the Parkinson's Foundation. R.S. has received research grants from Parkinson's UK and the Health Foundation. He has received service development grants from Parkinson's UK and the Parkinson's Foundation. A.L. has received honoraria from Britannia, The Neurology Academy, and Medicine for Old Age Psychiatrists in the last year. HM has received honoraria from Britannia Pharmaceuticals, Bial and EVER Pharma.
Acknowledgment
We acknowledge the assistance of the nutrition team at Royal Derby Hospital for help in identifying cases.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Suttrup I, Warnecke T. Dysphagia in Parkinson's disease. Dysphagia 2016;31(1):24–32. 10.1007/s00455-015-9671-9 [DOI] [PubMed] [Google Scholar]
- 2. Glasmacher SA, Leigh PN, Saha RA. Predictors of survival in progressive supranuclear palsy and multiple system atrophy: a systematic review and meta‐analysis. J Neurol Neurosurg Psychiatry 2017;88(5):402–411. 10.1136/jnnp-2016-314956 [DOI] [PubMed] [Google Scholar]
- 3. Langmore SE, Grillone G, Elackattu A, Walsh M. Disorders of swallowing: palliative care. Otolaryngol Clin North Am 2009;42(1):87–105, ix. 10.1016/j.otc.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 4. Lonnen JSM, Adler BJA. Systematic review of the evidence for percutaneous gastrostomy tube feeding or nasogastric tube feeding in patients with dysphagia due to idiopathic Parkinson's disease. Mov Disord 2011;26:S170–S171. [Google Scholar]
- 5. Shintani S. Efficacy and ethics of artificial nutrition in patients with neurologic impairments in home care. J Clin Neurosci 2013;20(2):220–223. 10.1016/j.jocn.2012.01.054 [DOI] [PubMed] [Google Scholar]
- 6. Murphy LM, Lipman TO . percutaneous endoscopic gastrostomy does not prolong survival in patients with dementia. Arch Intern Med 2003;163(11):1351–1353. 10.1001/archinte.163.11.1351 [DOI] [PubMed] [Google Scholar]
- 7. Sarkar P, Cole A, Scolding NJ, Rice CM. Percutaneous endoscopic gastrostomy tube insertion in neurodegenerative disease: a retrospective study and literature review. Clin Endosc 2017;50(3):270–278. 10.5946/ce.2016.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marois C, Del Mar Amador M, Payan C, et al. Outcome of gastrostomy in parkinsonism: A retrospective study. Parkinsonism Relat Disord 2017;43:110–113. 10.1016/j.parkreldis.2017.06.012 [DOI] [PubMed] [Google Scholar]
- 9. Bowering K. Analysis Of Routine Hospital Administrative Data (Including Hospital Episode Statistics) to Assess Variation in Process and Outcomes in Gastroenterology [thesis]. https://livrepository.liverpool.ac.uk/15135/1/BoweringKat_Jan2014_15135.pdf.
- 10. Brotherton AM, Judd PA. Quality of life in adult enteral tube feeding patients. J Acad Nutr Diet 2007;20(6):513–522. [DOI] [PubMed] [Google Scholar]
- 11. Skelly R. Are we using percutaneous endoscopic gastrostomy appropriately in the elderly? Curr Opin Clin Nutr Metab Care 2002;5:35–42. [DOI] [PubMed] [Google Scholar]
- 12. Löser C, Aschl G, Hébuterne X, et al. ESPEN Guidelines on artificial enteral nutrition—percutaneous endoscopic gastrostomy (PEG). Clin Nutr 2005;24:848–861. [DOI] [PubMed] [Google Scholar]
- 13. British Medical Association/Royal College of Physicians . Clinically‐Assisted Nutrition and Hydration (CANH) and Adults Who Lack the Capacity to Consent. Guidance for Decision‐Making in England and Wales. London, UK: British Medical Association/Royal College of Physicians; 2018. [Google Scholar]
- 14.National Confidential Enquiry into Patient Outcomes and Death. Scoping our practice. The 2004 report of the National Confidential Enquiry into Patient Outcomes and Death. https://www.ncepod.org.uk/2004report/Full_Report_2004.pdf. Accessed October 19, 2019.
- 15. National Institute of Health and Care Excellence . Parkinson's disease in adults. NICE Guideline NG71. https://www.nice.org.uk/guidance/ng71. Accessed October 19, 2019.
- 16. Fairclough H, Bashir H, Beekman R, Lee M, Johns E, McDonald C. Single centre experience of PEG feeding in Parkinson's disease [abstract]. Mov Disord 2019;34(suppl 2). https://www.mdsabstracts.org/abstract/single-centre-experience-of-peg-feeding-in-parkinsons-disease/. [Google Scholar]
- 17. Malmgren A, Hede GW, Karlstrom B, et al. Indications for percutaneous endoscopic gastrostomy and survival in old adults. Food Nutr Res 2011;55(1):6037 10.3402/fnr.v55i0.6037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim Duk Soo, Jones R, D'Abreu A, Friedman J, Akbar U. Trend in PEG placement in Parkinson's disease compared to Alzheimer dementia and ALS (P5.8‐034). Neurology 2019;92(15 suppl):P5.8‐034. [Google Scholar]
- 19. Metheny NA. Preventing respiratory complications of tube feedings: evidence‐based practice. Am J Crit Care 2006;15(4):360–369. [PubMed] [Google Scholar]
- 20. Parkinson's UK. 2017. UK Parkinson's Audit Summary Report. https://www.parkinsons.org.uk/sites/default/files/2018‐05/CS2257%20UK%20Parkinson%27s%20Audit%20Summary_Report_FINAL_0.pdf. Accessed December 30, 2019.
- 21. Snell K, Pennington S, Lee M, Walker R. The place of death in Parkinson's disease. Age Ageing 2009;38 (5):617–619. 10.1093/ageing/afp123 [DOI] [PubMed] [Google Scholar]


