ABSTRACT
Background
The head retraction reflex (HRR) is characterized by the extension of the neck after percussion stimulation of the central facial region. It is either absent or habituates in normal individuals and can become exaggerated and persistent in certain pathological conditions, having been most commonly reported in hyperekplexia and stiff‐person syndrome disorders. It has not, however, been reported in Niemann‐Pick type C (NPC), a lipid storage disorder with a variety of neurologic and systemic manifestations. The diagnosis of NPC is often delayed because of the rarity of the condition and the subtlety of clinical signs.
Cases
We present 3 consecutive cases of genetically confirmed NPC with a pathological HRR, which was not present in controls. Neurophysiological analysis showed findings suggestive of myoclonus of brainstem origin.
Conclusion
We propose that the presence of a pathological HRR, an easily performed clinical test, may provide a clue to the diagnosis of NPC.
Keywords: Niemann‐Pick C, NPC, head retraction reflex, hyperekplexia, startle response, stiff person syndrome
The head retraction reflex (HRR) is a reflex obtained by sharply tapping the midline of the face and results in the contraction of facial muscles and a withdrawal of the head. It was first described in the clinical setting, most commonly in hyperekplexia syndromes and anti‐GAD‐related disorders. 1 The neurophysiological correlate of the HRR, termed the trigemino‐cervical reflex (TCR), can be present in healthy individuals and likely serves as a protective withdrawal reflex. The TCR, however, has a longer latency and habituates in comparison with the pathological response, which has a shorter latency and persists. 2 , 3 Niemann Pick type C (NPC) is a genetic disorder characterized by the accumulation of lysosphingomyelin in various tissues and characteristically presents with a vertical supranuclear gaze palsy, ataxia, gelastic cataplexy, and cognitive dysfunction, among varied other features. Early in the disease course, however, the features may be nonspecific, and diagnosis is often delayed. We demonstrate here that the presence of a pathological HRR in patients with a vertical supranuclear gaze palsy may provide an additional clinical clue to the diagnosis of NPC.
Case Series
Case 1
A 40‐year‐old man had an insidious onset of mild dysarthria, gait disturbance, and blurred vision in his mid‐teens. He developed subtle cognitive dysfunction, with poor memory and concentration, previously being a high achiever. He had longstanding thrombocytopaenia and left leg congenital lymphoedema. There was no family history, and he was born of nonconsanguineous parents. His neurological presentation was sufficiently insidious and subtle that he was only referred for neurological review after he presented to an emergency department with cellulitis. Examination demonstrated absent saccadic downgaze and slowed saccadic upgaze with intact pursuit. There was mild limb ataxia and impaired tandem gait. There was also subtle parkinsonism with bilateral bradykinesia and decrement on finger tapping. A pathological HRR was present, with a stereotyped, nonhabituating extension of the neck to nose tap (Video S1, segment 1). There was no spontaneous myoclonus, stimulus‐sensitive limb myoclonus, or acoustic startle. Brain magnetic resonance imaging (MRI) and electroencephalogram (EEG) were normal. Lysosphingomyelin was 1.7 ng/ml (<0.4). He was a compound heterozygote with 2 known pathogenic variants in NPC1, c.3019C > G (p.Pro1007Ala) and c.2861C > T (p.Ser954Leu). Surface electromyogram (EMG) was recorded from the left orbicularis oculi, masseter, trapezius, deltoid, sternocleidomastoid (SCM), ADM, and C7 and T12 paraspinals. Nose tapping was done by a finger with a piezoelectric crystal attached. Stimulation induced a single response with an EMG response seen at a latency of 20 milliseconds, with near simultaneous onset across the muscles. In addition, there was a second discharge seen at ~100 milliseconds (Fig. 1). No reflex response was obtained following tapping of the forehead, cheek, mentalis, or sternal region.
FIG 1.
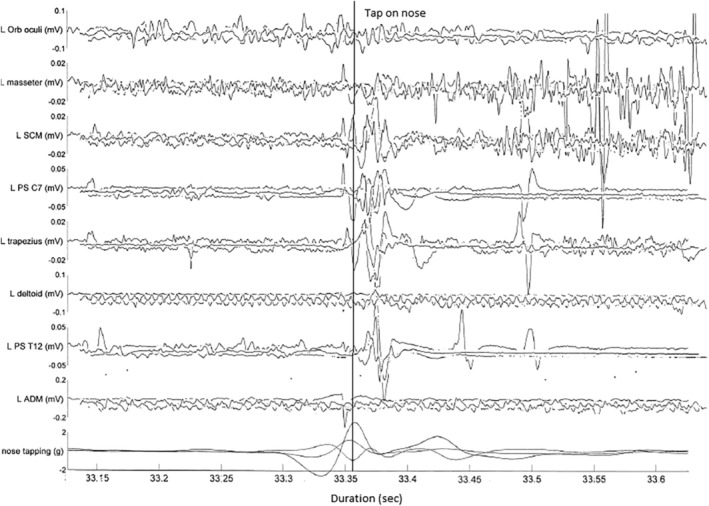
Patient 1: multichannel surface electromyogram (EMG) recording after a tap to the nose, representative superimposed trace of 3 trials to demonstrate lack of habituation. Bottom channel represents accelerometer attached to finger, measuring in triaxial planes. Note only a single triaxial accelerometer recording (hence the 3 traces, representing the 3 axes) was illustrated for clarity. Note also the sweep duration 50 milliseconds per division. Initial movement represents finger withdrawal, with tap occurring at the peak of the curve (as marked). The first EMG response is consistently seen at latency 20 milliseconds, with earliest onset at lower brainstem innervated muscles (SCM, trapezius), with a second discharge seen at ~100 milliseconds. A small EMG response seen prior to nose tap is artefactual. Note that given a single accelerometer trace is included for clarity, some EMG responses may appear earlier in this figure. ADM, abductor digiti minimi; L, left; Orb, orbicularis; PS, paraspinals; SCM, sternocleidomastoid.
Case 2
A 39‐year‐old woman presented with a 5‐year to 6‐year history of cognitive decline associated with slurring of speech and gait disturbance. She had a history of severe depression, schizophrenia, and postpartum psychosis. She had suffered with learning difficulties from a young age and had delayed motor milestones. There was no family history, and she was born of a nonconsanguineous marriage. Examination showed a vertical supranuclear gaze palsy, with normal horizontal eye movements. There was also limb and truncal ataxia with a broad‐based gait. There was no spontaneous myoclonus, stimulus‐sensitive limb myoclonus, or acoustic startle. A pathological HRR was present (Video S1, segment 2). There was no spontaneous or stimulus‐sensitive limb myoclonus. Brain MRI and EEG were normal. Lysosphingomyelin was elevated at 1.2 ng/ml (<0.4). Massive parallel sequencing of NPC1 and NPC2 showed heterozygous variants in NPC1 at c.743G > A(p(Glyc248Asp), which has not been reported previously, and a known pathogenic variant in c.1298C > 5 (Pro433Leu). Multi‐EMG recordings of the left orbicularis oculi, masseter, trapezius, deltoid, SCM, ADM, and C7 and T12 paraspinals following nose tapping, as in patient 1, induced a single response, with the earliest onset of EMG response seen in the SCM, with a latency of 20 milliseconds, followed by near simultaneous onset across muscles (Fig. 2). A similar reflex response was obtained from tapping the sternal region, but not the forehead, cheek, or mentalis.
FIG 2.
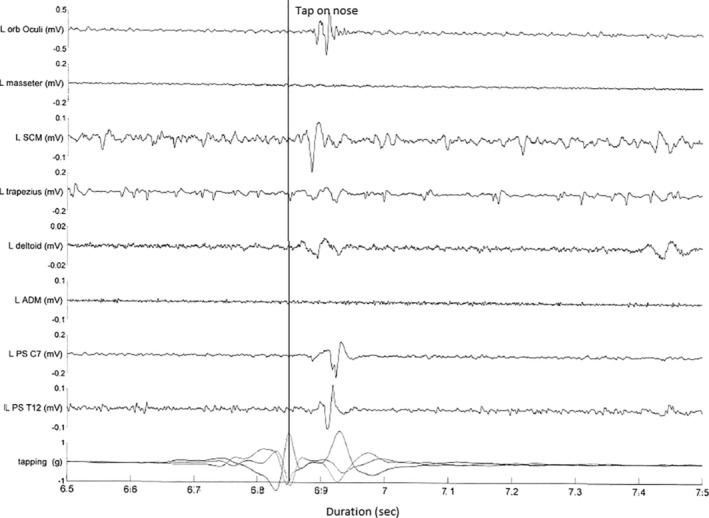
Patient 2: multichannel surface electromyogram recording after a tap to the nose. Bottom channel represents accelerometer attached to finger. Note the sweep duration 100 milliseconds per division. Initial movement represents finger withdrawal, with tap occurring at the peak of the curve (as marked). The earliest onset of electromyogram response is seen in the SCM with a latency of 20 milliseconds followed with spread to the rostral and caudal muscles. ADM, abductor digiti minimi; L, left; Orb, orbicularis; PS, paraspinals; SCM, sternocleidomastoid.
Case 3
A 44‐year‐old man had an onset of subtle cognitive dysfunction in his mid to late teens. Speech disturbance 2 to 3 years prior to presentation brought the patient to medical attention. On initial medical review, subtle dysarthria, ataxia, and tremor of the upper limbs was noticed. Vertical supranuclear gaze palsy was only noted some time later. There was no family history, and the patient was born of a nonconsanguineous marriage. After a diagnostic delay of 2 years, skin fibroblast analysis was performed, with features consistent with NPC, which was then confirmed with genetic testing, with a bi‐allelic mutation in the NPC1 gene (genotype not available). Brain MRI and EEG were normal. Examination showed a vertical supranuclear gaze palsy. There was mild limb and gait ataxia. There was no spontaneous or stimulus‐sensitive limb myoclonus. There was no acoustic startle. A pathological HRR was present (Video S1, segment 3). A multichannel EMG recording demonstrated a single response with the earliest onset of EMG response seen in the SCM, with a latency of 20 milliseconds, followed by a near simultaneous onset across muscles (Fig. 3).
FIG 3.
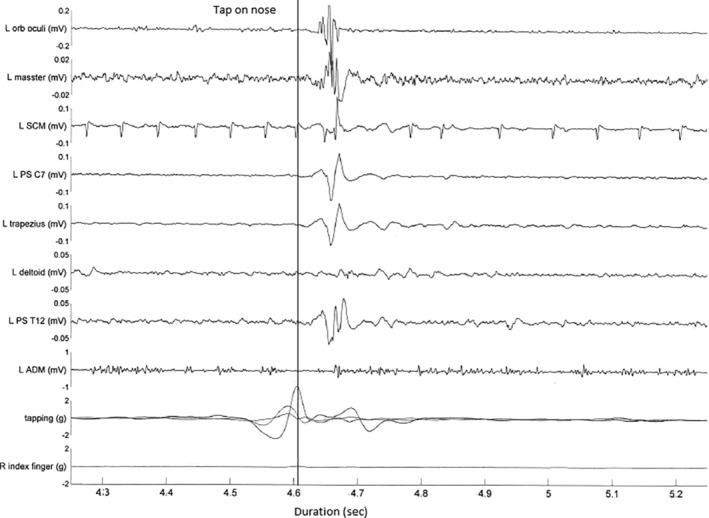
Patient 3: multichannel surface electromyogram recording after a tap to the nose. Bottom channel represents accelerometer attached to finger. Note the sweep duration 100 milliseconds per division. Initial movement represents finger withdrawal, with tap occurring at the peak of the curve (as marked). The earliest onset of electromyogram response is seen in trapezius at a latency 20 milliseconds followed by spread to the rostral and caudal muscles. ADM, abductor digiti minimi; L, left; Orb, orbicularis; PS, paraspinals; R, right; SCM, sternocleidomastoid.
Control Series
A total of 3 healthy individuals aged 30 to 36 years (2 women) were studied clinically and neurophysiologically. There was no clinical response in 2, and subtle head retraction that habituated after 2 to 3 taps was in the third. Neurophysiological recordings did not show an EMG response other than a habituating blink reflex (Fig. S1).
Discussion
The HRR was first described by Foerster in 1921 in those with postencephalitic parkinsonism or severe arteriosclerotic disease. 1 Wartenburg 4 then described it primarily in association with amyotrophic lateral sclerosis and considered it to be an exaggerated reflex with origin from the corticospinal tracts. Since that time, there have been several small series in patients with stiff‐person spectrum and hyperekplexia syndromes. 1 , 5 Somewhat interestingly, Sandyk and colleagues 6 found it to be positive in a high proportion of patients with parkinsonism compared with controls, although this finding has not been confirmed.
The HRR is elicited by sharply tapping the region of the face. The response is best seen after stimulus to the midline, 7 and particularly to the nose or upper lip. 1 A positive response ranges from simple facial muscular contraction to dramatic withdrawals. A similar neurophysiological response (the TCR), without clear clinical correlate, is seen in some healthy individuals via surface EMG sampling of the involved muscles and is composed of early and late responses. The TCR may be considered analogous to the protective withdrawal reflex seen in the lower limbs. The TCR, however, is of longer latency and habituates (particularly the early response), suggesting that the clinical presence of a pathological HRR may be an exaggeration of a physiological reflex. 2 , 3 Neurophysiological recordings of the pathological HRR, in contrast, have shown synchronized activation of craniocervical musculature with a short latency and lack of habituation, similar to that seen in our patients, suggesting a brainstem origin. 5 The pathological HRR is therefore currently generally considered a polysynaptic cutaneomuscular brainstem reflex and possibly represents an exaggerated TCR.
NPC is a genetic disorder related to mutation in 2 genes, NPC1 and NPC2, and is characterized by the accumulation of lysosphingomyelin in various tissues, including the nervous system. Initial manifestations commonly include vertical supranuclear gaze palsy, ataxia, parkinsonism, neuropsychiatric disturbance, seizures, haematologic abnormalities, and hepatosplenomegaly, among other features. There is often a lag in diagnosis, however, because of its rarity and, apart from the supranuclear gaze palsy and gelastic cataplexy, lack of specific signs. With the increasing availability of targeted treatment, such as miglustat and now cyclodextrin, 8 earlier diagnosis is assuming greater importance with regard to the potential treatment and prevention of irreversible damage.
The phenomenology and electrophysiological findings seen in our 3 patients with biochemically and genetically confirmed NPC are typical of a pathological HRR. Absence in controls supports its presence as a diagnostic aid. The authors regularly check for the HRR in a speciality movement disorder clinic and have not found it consistently present in other disorders. Myoclonus is only rarely seen in NPC and is particularly rare during the early stages. 9 Furthermore, there were no historical clues to stimulus‐sensitive myoclonus or hyperekplexia in our patients, necessitating the deliberate testing for the HRR to elicit its presence. A pathological HRR may be present without other examination signs of exaggerated startle or reflex myoclonus, as in our patients, demonstrating that a pathological HRR may therefore provide a diagnostic clue that the pathological HRR, similar to vertical supranuclear gaze palsy, might be relatively specific for NPC. Further studies in a larger series of patients with both suspected and confirmed NPC, in both early and more advanced disease, are required to confirm or refute this suggestion.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
A.J.M.: 1A, 1B, 1C, 3A
H.M.‐B.: 1A, 1B, 1C, 3B
M.T.: 1A, 1B, 1C, 3B
V.S.C.F.: 1C, 3A, 3B
Disclosures
Ethical Compliance Statement: The patient has provided written consent, which can be obtained on request by the editor. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: There are no funding sources to declare for this article. There are no conflicts of interest to declare for this article.
Financial Disclosures for the previous 12 months: V.S.C.F. receives a salary from NSW Health; has received unrestricted research grants from the Michael J. Fox Foundation, Abbvie, and Merz; is on advisory boards and/or has received travel grants from Abbvie, Allergan, Cavion, Ipsen, Merz, Praxis, Seqirus, Stada, Teva, and UCB; and receives royalties from Health Press Ltd. A.J.M., H.M.‐B., and M.T. have no funding sources for the past 12 months to declare.
Supporting information
Figure S1 Control sample. Rastered trace. First trial from each control. Multichannel surface electromyogram recording after a tap to the nose. Bottom channel represents accelerometer attached to finger. Initial movement represents finger withdrawal, with tap occurring at the peak of the curve (as marked). No response seen.
Video S1 Segment 1, patient 1: vertical supranuclear gaze palsy. Positive pathological head retraction reflex to tap on the nose. Segment 2, patient 2: vertical supranuclear gaze palsy. Positive pathological head retraction reflex to tap on the nose. Segment 3: positive pathological head retraction reflex. Not shown is vertical supranuclear gaze palsy and mild limb ataxia.
Acknowledgments
We wish to thank the patients.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Berger C, Meinck HM. Head retraction reflex in stiff‐man syndrome and related disorders. Mov Disord 2003;18(8):906–911. [DOI] [PubMed] [Google Scholar]
- 2. Ertekin C, Celebisoy N, Uludağ B. Trigemino‐cervical reflexes in normal subjects. J Neurol Sci 1996;143(1–2):84–90. [DOI] [PubMed] [Google Scholar]
- 3. Di Lazzaro V, Guney F, Akpinar Z, et al. Trigemino‐cervical reflexes: clinical applications and neuroradiological correlations. Suppl Clin Neurophysiol 2006;58:110–119. [DOI] [PubMed] [Google Scholar]
- 4. Wartenberg R. Head retraction reflex. Calif Med 1949;70(5):382. [PMC free article] [PubMed] [Google Scholar]
- 5. Khasani S, Becker K, Meinck HM. Hyperekplexia and stiff‐man syndrome: abnormal brainstem reflexes suggest a physiological relationship. J Neurol Neurosurg Psychiatry 2004;75(9):1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sandyk R, Fleming J, Brennan MJ. The head retraction reflex—its specificity in Parkinson's disease. Clin Neurol Neurosurg 1982;84(3):159–162. [DOI] [PubMed] [Google Scholar]
- 7. Serrao M, Cortese F, Andersen OK, et al. Modular organization of the head retraction responses elicited by electrical painful stimulation of the facial skin in humans. Clin Neurophysiol 2015;126(12):2306–2313. [DOI] [PubMed] [Google Scholar]
- 8. Ory DS, Ottinger EA, Farhat NY et al. Intrathecal 2‐hydroxypropyl‐ß‐cyclodextrin decreases neurological disease progression in Niemann‐Pick disease, type C1: a non‐randomised, open‐label, phase 1‐2 trial. Lancet 2017;390(10104):1758–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wijburg FA, Sedel F, Pineda M, et al. Development of a suspicion index to aid diagnosis of Niemann‐Pick disease type C. Neurology 2012;78(20):1560–1567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Control sample. Rastered trace. First trial from each control. Multichannel surface electromyogram recording after a tap to the nose. Bottom channel represents accelerometer attached to finger. Initial movement represents finger withdrawal, with tap occurring at the peak of the curve (as marked). No response seen.
Video S1 Segment 1, patient 1: vertical supranuclear gaze palsy. Positive pathological head retraction reflex to tap on the nose. Segment 2, patient 2: vertical supranuclear gaze palsy. Positive pathological head retraction reflex to tap on the nose. Segment 3: positive pathological head retraction reflex. Not shown is vertical supranuclear gaze palsy and mild limb ataxia.


