ABSTRACT
Background
Many different movement disorders have similar “jerk‐like” phenomenology and can be misconstrued as myoclonus. Different types of myoclonus also share similar phenomenological characteristics that can be difficult to distinguish solely based on clinical exam. However, they have distinctive physiologic characteristics that can help refine categorization of jerk‐like movements.
Objectives
In this review, we briefly summarize the clinical, physiologic, and pathophysiologic characteristics of different types of myoclonus. The methodology and technical considerations for the electrophysiologic assessment of jerk‐like movements are reviewed. A simplistic pragmatic approach for the classification of myoclonus and other jerk‐like movements based on objective electrophysiologic characteristics is proposed.
Conclusions
Clinical neurophysiology is an underutilized tool in the diagnosis and treatment of movement disorders. Various jerk‐like movements have distinguishing physiologic characteristics, differentiated in the milliseconds range, which is beyond human capacity. We argue that the categorization of movement disorders as myoclonus can be refined based on objective physiology that can have important prognostic and therapeutic implications.
Keywords: myoclonus; startle, jerk‐like movements, electrophysiology
The clinical approach to the diagnosis of movement disorders begins with the initial clinical classification of the movements observed based on phenomenology into a discrete number of categories: tremor, myoclonus, chorea, ballism, athetosis, dystonia, spasms, tics, motor stereotypies, and functional. The correct categorization of the movement disorder, or syndrome identification, is a critical first step in arriving at the correct etiological diagnosis. In this article, we address the methodology for improving the clinical characterization of myoclonus, including the pitfalls and challenges. Myoclonus is defined as a syndrome clinically characterized by sudden, brief “jerk‐like” movements. It can be further classified either as positive, when associated with an active muscle contraction, or negative, when associated with a brief pause in ongoing muscle contraction. 1 , 2 , 3
Should any jerk‐like movement be called “myoclonus”? Tic disorders, chorea, ballism, dystonia, and functional movements can all have some jerk‐like phenomenology. 3 , 4 , 5 Tremors, in particular when the frequency is high, can sometimes look like repetitive jerk‐like movements and, oppositely, rhythmic myoclonic jerks can be misconstrued as tremor. 3 Startle syndrome commonly characterized by generalized jerk‐like movements is another important movement disorder that shares similarities in terms of clinical characteristics. 6 , 7 Many peripheral movement disorders such as clonus and fasciculations have jerk‐like phenomenology. In addition, myoclonus may coexist with other movement disorders and more than 1 type of jerk‐like movement disorder can coexist in an individual. So many different movement disorders have similar jerk‐like phenomenology and can be misconstrued as myoclonus based solely on clinical assessment.
Although many different types of jerks might look superficially the same, the physiologic characteristics of various types of jerk‐like movements noted previously are distinct. They allow for a clear, objective, and unequivocal diagnosis. We argue that categorization of a movement disorder as “myoclonus” can be further refined by electrophysiological characterization. 7 , 8 Using objective physiology allows for correct, reliable, and reproducible characterization of jerk‐like movements as defined by their physiologic signatures and takes away the errors associated with misclassifying various jerk‐like movement disorders solely based on clinical phenomenology. Objective physiology can further help localize the central or peripheral origin of a jerk‐like movement within the nervous system that can have important prognostic and therapeutic implications. 8 , 9 , 10 , 11
Clinical neurophysiology is an underutilized tool in the diagnosis and treatment of movement disorders. 12 We are not arguing against the importance of a good clinical exam; however, correct identification and appropriate categorization of jerk‐like movements rely on spatial and temporal discrimination of its characteristics in the millisecond range, which is far beyond human capacity. 2 , 5 , 13 , 14 , 15 , 16 , 17 , 18 , 19
Methods
Based on a relevant review of the literature, we summarize the clinical characteristics, phenomenology, physiologic characteristics, and pathophysiology of the common myoclonus and other jerk‐like movement disorder syndromes (summarized in Table 1). We briefly review the methodology and technical considerations for the recording of various jerk‐like movements. Based on these salient clinical and objective physiologic characteristics, we propose a pragmatic diagnostic algorithm for their evaluation and classification.
TABLE 1.
Summary of comparative clinical and physiologic characteristics of common jerk‐like movements
| Category of ‘Jerk‐like’ Movement | Cortical Myoclonus | Reticular Myoclonus | Propriospinal Myoclonus | Startle Reflex |
|---|---|---|---|---|
| Phenomenology | Spontaneous jerks more commonly involving the face and distal upper extremities | Spontaneous jerks involving the entire body that can also be evoked by somatosensory stimuli to distal extremities | Arrhythmic, usually flexor, brief jerks involving the trunk, hips, knees in a fairly uniform pattern | Bilaterally synchronous flexor response to a startling stimulus |
| Reflex physiology | Cortical onset short lasting EMG bursts (simultaneous agonist‐antagonist discharge) with a cortical EEG correlate on back averaging | Craniocaudal EMG discharge beginning at the lower medulla | Craniocaudal EMG discharge usually beginning at the level of the abdominal muscles; restricted to spinal cord propriospinal pathways | Early eyelid blink followed most consistently by SCM with subsequent cranio‐caudal propagation |
| Afferent stimulus | Spontaneous; but can be triggered by tactile or other somatosensory stimuli more commonly to distal upper extremities | Spontaneous; can be induced by somatosensory stimuli, touch, or muscle stretch of distal extremities | Spontaneous, but can be induced by tactile or auditory stimuli | Auditory; can also be elicited by visual, somatic, and vestibular stimulations |
| Pattern of muscle activation; flexors vs extensors | Mainly involving distal upper extremities and face (which have a large cortical representation) | Both flexors and extensors | Mainly flexors | Mainly flexors (but extensors also noted to be activated) |
| Site of origin | Cerebral cortex | Medullary reticular formation (likely nucleus reticularis gigantocellularis) | Limited to spinal cord (note that most cases are functional) | Caudal pontine reticular formation (nucleus reticularis pontis caudalis) |
| Velocity of conduction of bulbo‐spinal efferent volley | ~100 m/sec | > 50 m/sec | 5–15 m/sec | ~30 m/sec |
| EMG burst duration | <50 milliseconds | <50 milliseconds | ~150–450 milliseconds (can be longer) | >70 milliseconds |
EEG, electroencephalogram; EMG, electromyogram; SCM, sternocleidomastoid muscle.
Results
Myoclonus Syndromes
Cortical Myoclonus
Clinical Characteristics. The clinical characteristics include spontaneous jerks commonly involving the face and distal extremities that are usually increased with action. Can be triggered by tactile or other somatosensory stimuli to the distal extremities. 1
Physiologic Characteristics. The physiologic characteristics include very short duration bursts (usually <30 milliseconds) with simultaneous agonist–antagonist activity. The conduction velocity of the efferent volley is ~100 m/sec with cranio‐caudal propagation. Cortical spikes can be detected on electroencephalogram (EEG) preceding the jerks noted on electromyogram (EMG). There is an exaggeration of the P25/N33 components of the median nerve‐evoked sensory‐evoked potential (SEP). 15 Physiologic characteristics of cortical myoclonus are illustrated in Figure 1.
FIG. 1.
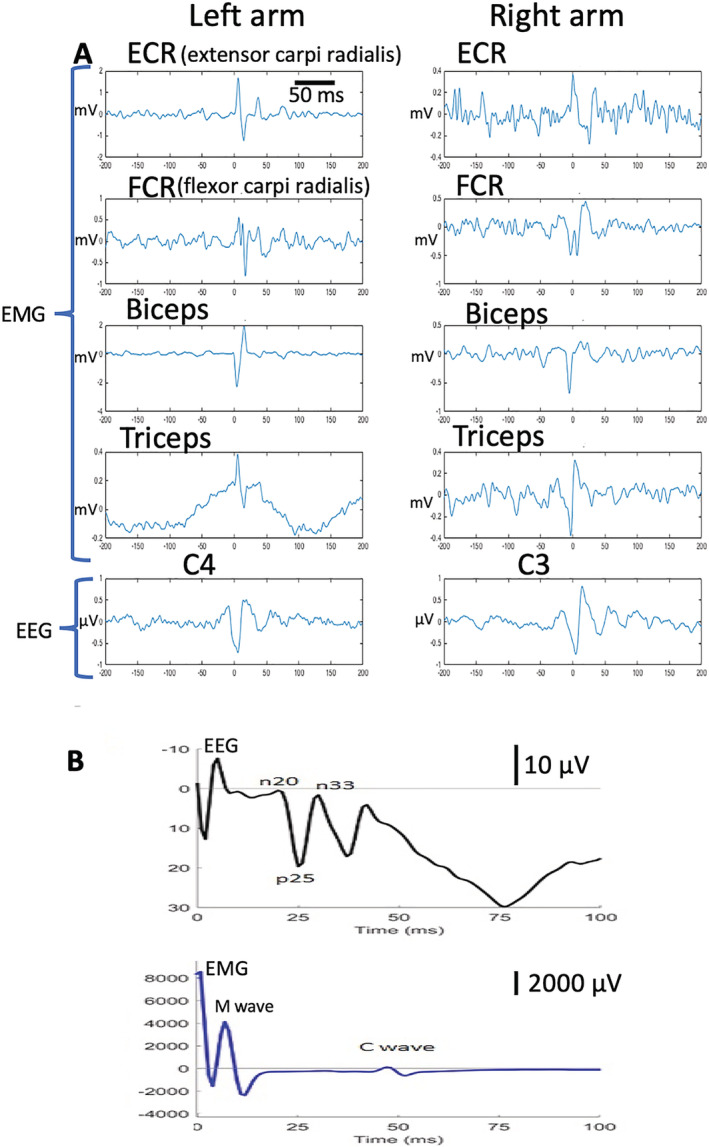
Cortical myoclonus physiology. (A) EMG traces showing short duration (<30 milliseconds) simultaneous agonist‐antagonist bursts with cortical EEG spike correlate. (B) Median nerve sensory‐evoked potential showing M wave followed by C‐reflex with corresponding EEG trace with giant p25 and n33 sensory‐evoked potential components. EEG, electroencephalogram; EMG, electromyogram.
Pathophysiology. The pathophysiology commonly involves body regions with the largest cortical homuncular representation suggestive of site of origin being the sensorimotor cortex with the velocity of conduction suggestive of transmission along the corticospinal tracts. Physiologic characteristics of enhanced SEP and exaggerated long loop reflexes suggest hyperexcitability of the sensorimotor cortex. The cerebellum is also implicated in the generation and propagation of cortical myoclonus based on aberrancies noted in the cerebello‐thalamo‐cortical pathways and the presence of cortical myoclonus in certain ataxia syndromes. 9 , 15
Brainstem Myoclonus
Clinical Characteristics. The clinical characteristics include axial predominant generalized jerk‐like movements beginning with the ascending order of cranial nerve innervated muscle activation and the descending order of limb and axial muscle activation. They can be spontaneous and can also be induced by somatosensory stimuli, especially the muscle stretch of distal upper extremities. 5 , 18
Physiologic Characteristics. The physiologic characteristics include short duration bursts (usually <50 milliseconds) beginning in the lower cranial nerve innervated muscles with subsequent cranio‐caudal propagation. The conduction velocity of the efferent volley is ~50 to 70 m/sec. Stimulus sensitive myoclonus may also be seen. 18 , 20
Pathophysiology. The order of muscle discharge is suggestive of origin in the caudal medullary reticular formation (nucleus reticularis gigantocellularis). The conduction velocity of the efferent volley is suggestive of the reticulospinal tract as its pathway. No cortical discharge is seen preceding the muscle jerks, although there may be an associated EEG spike. EEG spikes most commonly are noted after the EMG of cranial nerve innervated muscles; however, no clear EMG correlation noted with back averaging. 18 Most commonly etiology for reticular myoclonus is anoxic damage to the medullary reticular formation. 18 , 20
Myoclonus–Dystonia
Clinical Characteristics. The myoclonus–dystonia syndrome has been most systematically studied in patients with DYT11 mutations, where the myoclonus is observed in the majority of patients and is variably associated with dystonia. Patients with DYT11 show asymmetric rest and action myoclonus with predominant proximal limb and axial distribution. 21 , 22
Physiological Characteristics. EMG burst duration is in the 50 to 100 millisecond range with longer duration bursts likely related to dystonia. 22 Studies using trancranial magnetic stimulation (TMS), back averaging, SEPs, and C‐reflex failed to demonstrate hyperexcitability of the sensorimotor cortex in patients with DYT11, thus suggesting a subcortical generator. 21 , 22
Pathophysiology. Abnormal activity in the cerebello‐thalamic and the striato‐pallido‐thalamo‐cortical circuits probably plays a central role in DYT11 pathophysiology; however, whether these mechanisms underpins myoclonus rather than dystonia is unclear. 23 Finally, the presence in some patients of a startle response and the consistent presence of abnormal brainstem excitability as tested by blink reflex, suggests that stimulus‐evoked myoclonus is possible in DYT11, and this particular manifestation is suggestive of a likely brainstem generator. 21 , 22 , 23
Spinal Myoclonus
Clinical Characteristics. The clinical characteristics can involve one or a few contiguous spinal myotomes and is referred to as spinal segmental myoclonus. It can also present as generalized axial predominant jerks or involves multiple contiguous body segments and is referred to as propriospinal myoclonus. No cranial nerve innervated muscules are involved. Generalized jerks commonly originate in the abdominal musculature or other muscles innervated at the level of the thoracic spinal cord. 14 , 24 , 25
Physiologic Characteristics. The physiologic characteristics of segmental myoclonus include burst duration ranges between 50 and 500 milliseconds without caudo‐rostral propagation. 26 , 27
The physiologic characteristics of propriospinal myoclonus include long burst durations typically ranging from ~150 to 450 milliseconds (can be even longer). Conduction velocity of the efferent volley ranges from 5 to 15 m/sec limited to the spinal cord without any cranial nerve innervated muscle activation. Generalized jerks usually originate at the level of the thoracic spinal cord with subsequent cranio‐caudal propagation limited to the spinal cord. The pattern of muscle activation can be inconsistent. Stimulus‐evoked jerks can be of variable latency and at times longer than voluntary reaction times. Bereitschaftspotential or event‐related desynchronization of the beta band may be seen prior to EMG bursts on jerk‐locked back averaging of the EEG in many cases, suggestive of a functional syndrome. 14 , 24 , 25
Pathophysiology. Spinal segmental myoclonus can be localized to the regions affecting a spinal segment. Most cases of propriospinal myoclonus are deemed to be of functional origin; however, some cases of organic/symptomatic propriospinal myoclonus have been described. 14 , 16 , 28
Startle Syndromes
Startle syndromes, although originating in the brainstem, deserve a separate categorization as they are a specific aberrancy of normal human reflexes. 6
Startle and Exaggerated Startle Reflex (Hyperekplexia)
Clinical Characteristics. The clinical characteristics include bilaterally synchronous and predominant flexor jerk‐like movements induced by a sudden, unexpected stimulus. Fairly symmetrical activation is noted beginning in the muscles originating in the lower brainstem with subsequent cranio‐caudal propagation. Exaggerated startle reflex is characterized by nonhabituating and excessive motor response secondary to auditory and at times somesthetic and visual stimuli. It is characterized by a blink; contortion of the face; flexion of the neck, trunk, and abduction; and flexion of the arm. Exaggerated startling can result in falls and injuries. 6 , 7 , 13
Physiologic Characteristics. The physiologic characteristics include early blinking referred to as the stimulus induced blink reflex followed most consistently by activation of the sternocleidomastoid muscle (SCM) with subsequent cranio‐caudal propagation. EMG burst durations are ~70 milliseconds with the conduction velocity of the efferent volley being ~30 m/sec. Predominantly involves a flexor muscle response; however, extensor muscle involvement has been reported. Exaggerated startle reflex is characterized by excessive and more widespread muscle activation and excessive EMG burst amplitude with a lower threshold for response and impaired habituation. 19 , 29 , 30 , 31 Physiologic characteristics of startle reflex physiology are illustrated in Figure 2.
FIG. 2.
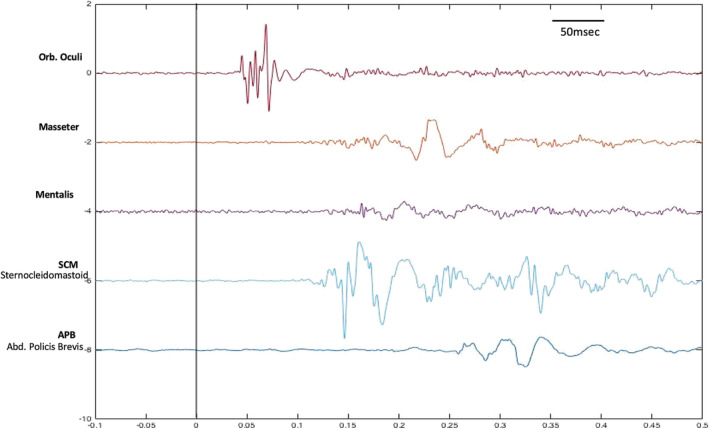
Startle reflex physiology. Surface poly‐electromyogram single trace of 5 muscles showing acoustically induced blink reflex at a latency of 35 milliseconds followed by an sternocleidomastoid muscle discharge with subsequent cranio‐caudal propagation. Abd, abductor; Orb, orbicularis.
Pathophysiology. Startle is a normal reflex deemed to originate in the caudal pontine reticular formation (nucleus reticularis pontis caudalis). Exaggerated startle reflex can be secondary to hereditary hyperekplexia caused by a variety of genes affecting the presynaptic or postsynaptic glycine receptors. These receptors are involved in the modulation of ligand gated chloride channels involved in brainstem and spinal cord reflex responses. Abnormalities in this receptor complex results in brainstem and spinal cord disinhibition in hereditary hyperekplexia. Cases resulting from sporadic mutations and idiopathic hyperekplexia have also been reported. 7 , 19 , 31 , 32 Differences in startle reflex abnormalities in parkinsonian disorders also provide some useful pathophysiologic insights. Startle reflexes are characteristically enhanced in patients with multisystem atrophy, whereas they are reduced in patients with progressive supranuclear palsy. The reflex responses are intermediate and not significantly different compared with healthy controls in patients with Parkinson's disease or dementia with Lewy bodies. Degeneration of the nucleus reticularis pontis caudalis in progressive supranuclear palsy and disinhibition of this nucleus in multisystem atrophy could explain the differences in reflex response. 33
Methodological Considerations for Physiologic Evaluation of Myoclonus Syndromes
The following electrophysiologic techniques can help with the characterization of myoclonic movements: poly EMG, SEP and “C” (cortical) reflexes, EEG correlates of myoclonus, and startle study. The selection of the study depends on the movement characteristics, but generally it is good to start with a poly EMG recording.
Poly EMG
Electrical activity of the muscles involved in the jerk are recorded with surface EMG. The correct selection of the muscles to record is critical and depends on the clinical pattern observed during examination. The more muscles recorded, the better the temporal‐spatial resolution, but this will increase the study time duration and time required for analysis of the data, a critical factor to be considered in a clinical setting. 1 , 17 Recordings should be obtained from a pair of agonist–antagonist muscles because their simultaneous activation is a helpful indicator of a centrally driven activity vs a peripheral injury that may also produce short lasting (in the myoclonic range) muscle bursts. 1 In our experience, the general distribution of the movement is also helpful; for generalized jerks involving proximal and more symmetric activation, it is important to record from different levels of spinal cord and cranial nerves to ascertain the temporal and spatial pattern of spread of an efferent discharge volley. We suggest recording from the following muscles: (1) orbicularis oculi (VII cranial nerve)/mentalis (VII cranial nerve), (2) masseter (V cranial nerve), (3) SCM (XI cranial nerve), (4) a pair of upper limb agonist/antagonist muscles (biceps/triceps or flexor carpi radialis/extensor carpi radialis), (5) abductor polices brevis (APB), (6) thoracic paraspinal muscles, and (7) Lumbar paraspinal muscles.
To avoid movement artefacts associated with brisk movements, it is advisable to scratch off skin debris using skin prep before placing the surface electrodes. Recommended filter settings for recording include a high pass of 20 Hz and low pass of 250 to 300 Hz, which will capture most of the frequency content of the EMG activity. The sampling rate has to be at least 2 times the highest frequency recorded (to avoid aliasing and make for a faithful recording) 1 . In general, for the data visualization and analysis, it may be helpful to rectify (make everything upward) the data to improve the signal to noise ratio.21,11,17
SEP and C Reflex
These studies will show an electrophysiologic correlate for cortical hyperexcitability and cortical reflex myoclonus. 1 They can be recorded together. Surface EMG electrodes are placed on the thenar muscles of the stimulated hand muscle with simultaneous EEG recordings obtained from CP3 or CP4 electrodes (contralateral to the stimulated hand referenced to the linked earlobe). Electrical stimulation is delivered to the median nerve at the wrist level; a square wave pulse of 0.2 to 0.5 milliseconds in duration at a frequency of 1 to 2 Hz at an intensity equivalent to 10% to 15% above the motor threshold. 3 It is important to monitor that the thenar muscles are at rest during the recording, as “C” reflexes can be evoked in healthy subjects if the activated muscles are stimulated in a tonically contracted state. 1 , 17 An SEP will be considered giant if the averaged amplitude of P25/N33 is greater than 10 uV 4 (normally around 1 uV). A “C” reflex observed in resting state at a latency of 45 to 50 milliseconds is considered abnormal and is an electrophysiologic correlate of cortical reflex myoclonus, suggestive of disinhibited motor cortical activity. 1 , 17 A normal H or F response can also be seen prior to the C‐reflex with a latency of around 30 milliseconds. 20 , 34
EEG Correlates of Myoclonus
Simultaneous Recording of EEG and EMG: EMG recordings are performed with a similar methodology as described for the poly EMG recordings. For EEG recordings, it is preferable to record from multiple electrodes placed on the frontal, supplementary motor area, sensorimotor cortex, and midline (Cz, C1, and C2 referenced to the linked earlobe). Conventional filtering parameters can be used for the EEG. EEG potentials preceding the EMG myoclonic activity, with a delay compatible with a corticospinal conduction (~20 milliseconds in myoclonus affecting hand muscles) support the cortical origin of the myoclonus. 1 , 17
Back Averaging : The idea of back averaging is to segment the EMG‐EEG data in equally sized windows using markers placed on the myoclonic bursts as fiducials. Then an average of all the segments is done. The signal that is in phase will survive the average, the activity that is out of phase (noise) will cancel out. This is a more sensible way of looking for a cortical correlate of the myoclonus. 1 , 17
Bereitschaftspotential Recording (BP) : One of the potentials that can be looked for with the back‐averaging technique are the BPs. BP recordings are useful for the diagnosis of jerk‐like movements of functional etiology. The BP is a slow rising negativity coming from the supplementary motor area and premotor cortex, noted about 2 seconds before voluntary or functional (psychogenic) movements. 5
Initial recordings are obtained at “rest” while the involuntary movements are occurring. In our experience, close to 50 movements are needed, the reason being that in general at least 20 to 25 movements are needed to be able to see a BP on the EEG after back averaging. Suggested EEG filtering parameters include a high‐pass filter to be as low as possible (we recommend using 0.01 Hz or, even direct current) and the low pass at 50 Hz. BP being a very slow wave, gradually rising over 2 seconds or even slower will be filtered out if the conventional EEG filter parameters are used. 35 , 36 If enough good and consistent recordings are obtained after back averaging the whole data, the data can be spilt into 2 sets to look for consistency. It is also recommended to record the subject doing voluntary movements and use that data as a positive control for the technique. 14 , 28 , 36
For back averaging, a marker is placed on the EMG at the beginning of each muscle burst, then the data are segmented in windows around that marker and then averaged. For focal jerks the placement of EMG marker is fairly straight forward. For multifocal and generalized jerks, the poly EMG recordings are evaluated to ascertain the most consistent and earliest muscle EMG activity and back averaging performed by segmenting the EEG/EMG window with a marker placed at the onset of the EMG burst. 1 , 10 It can be technically challenging and difficult to obtain BP if there are repetitive jerks occurring at high frequency.
Using a methodology similar to jerk‐locked back averaging, event‐related desynchronization and event‐related synchronization can also be evaluated. These are essentially changes in the power of certain EEG band frequencies after performing a time‐frequency transformation of the epoch of the data around the jerk‐like movement and comparing these changes to those noted at baseline. Event‐related desynchronization of the beta (13–30 Hz) and mu rhythms (8–12 Hz), characterized by a reduction in the power up to 20% to 30%, can be noted over the sensorimotor cortices (C3/C4) about 1.5 to 2 seconds preceding the movement. Event‐related synchronization of beta (13–30 Hz) rhythms is also noted in the first second after a voluntary movement. 37
Cortico‐Muscular Coherence: When the myoclonic activity has a very high frequency as observed in some forms of progressive myoclonic epilepsies, looking for the EEG correlation in the time domain can become difficult. One way of dealing with those cases is to run a coherence analysis between the EEG and the EMG signal. This technique is described in research background, but in the experience of the authors it is difficult to apply this in clinic because there is always some normal cortico‐muscular coherence because of muscle contraction. The cutoff for which level of coherence is abnormal is not well stablished. 38 , 39
Startle Response Recording
Startle is a reflex response to a potentially threatening stimulus and originates from the caudal pontine reticular formation with subsequent rostro‐caudal propagation. 29 , 30 For recording the startle reflex, surface EMG electrodes are placed on the muscles innervated by different cranial nerves and also the distal muscles to visualize the spatial and temporal characteristics of the EMG discharge. 29 The recording parameters are the same as described for poly EMG. We recommend recording from the following muscles: (1) orbicularis oculi (VII cranial nerve), (2) mentalis (VII cranial nerve), (3) masseter (V cranial nerve), (4) SCM (XI cranial nerve), (5) a pair of upper limb agonist/antagonist muscles (biceps/triceps or flexor carpi radialis/extensor carpi radialis) based on the clinical characteristics of discharge, (6) APB, (7) cervical paraspinal muscles, and (8) thoracic/lumbar paraspinal muscles.
The rationale for recording 2 cranial nerve VII innervated muscles, namely orbicularis oculi and mentalis, being that in acoustic‐evoked startle there is a first response of the orbicularis oculi that is thought to be an auditory blink reflex that is independent from the startle response. The auditory blink reflex also does not habituate as the startle response, normally. Recordings obtained from the APB are to note a very late response in this muscle around 100 milliseconds, which is another characteristic of the startle response. The prolonged latency suggestive of relatively slower conduction velocity of the startle volley, originating in the caudal pons and arguing against the startle volley being conducted via the fast conducting cortico‐spinal tracts as noted for cortical myoclonus where the APB discharge latency is ~20 milliseconds.
For recording the acoustic startle reflex, a tone burst of 125 dB intensity, 1500 Hz frequency, and 50 milliseconds duration is provided through headphones. This parameters are within the National Institute for Occupational Safety and Health (NIOSH) recommended exposure limits (https://www.cdc.gov/niosh/topics/noise/reducenoiseexposure/regsguidance.html). Ten stimuli are randomly given every 1 to 2 minutes. It is important to ensure that the stimuli delivered are unpredictable.
For the sensory‐evoked startle, an electrical stimulus of 0.1 millisecond duration can be given in the supraorbital notch 2 with an intensity about 3 times the sensory threshold. The recording setup is the same as for acoustic startle. For tactile‐induced jerks using a similar methodology of recording, a tactile stimulus can be delivered by tapping over the surface EMG to mark the stimulus on the poly EMG trace. 29 , 30 , 32
Pragmatic Physiologic Approach for Diagnosis of Jerk‐Like Movements
Jerk‐like movements can be broadly classified based on phenomenology into spontaneous, action induced, or stimulus induced. For spontaneous and action‐induced jerks, the next step is to record from the muscles identified based on the clinical exam. For stimulus‐induced jerks, the appropriate stimulus can be provided as noted in the methodology and the onset latency of the jerk‐like activity can be noted. Spontaneous jerks/induced jerks can be further subclassified as being focal/multifocal or generalized involving the axial musculature. The EMG recording should be based on the pattern of jerks. For focal jerks, limited recording of the involved muscles can be performed with surface EMG, with recordings to be obtained from an agonist–antagonist pair. For generalized jerks that commonly involve the axial musculature, poly EMG recordings should be performed as suggested in the methodology. Based solely on the surface EMG recordings, the jerk‐like movements can be classified into 3 broad categories based on the duration of the EMG discharge: (1) <50 milliseconds, (2) 50 to 100 milliseconds, and (3) >100 milliseconds. A summary of the suggested pragmatic approach for the diagnosis of myoclonus/jerk‐like movements is illustrated in Figure 3.
FIG. 3.
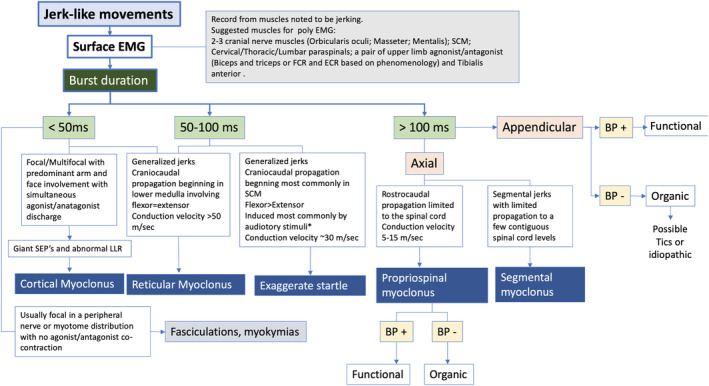
Schematic illustration for physiologic diagnostic approach for “jerk‐like” movements. Based on surface EMG recordings, jerk‐like movements can be categorized into 3 major categories based on burst duration: (1) <50 milliseconds, (2) 50 to 100 milliseconds, and (3) >100 milliseconds. BP, Bereitschaftspotential recording; ECR, extensor carpi radialis; EMG, electromyogram; FCR, flexor carpi radialis; LLR, long latency reflex; SCM, sternocleidomastoid muscle; SEP, sensory‐evoked potential.
EMG Burst Duration <50 Milliseconds
Characteristic of cortical myoclonus or myoclonus of brainstem origin. The distribution, pattern of spread, andconductionvelocity can help with further differentiation.
Focal/multifocal jerks predominantly involving the face and arms with simultaneous agonist–antagonist bursts are most likely consistent with a cortical myoclonus. Giant SEPs and C responses may be present, especially when there is a stimulus‐sensitive myoclonus.
For generalized jerks with truncal (flexors + extensors) involvement, a further analysis of the spatial and temporal characteristics of the discharge can be performed from the poly EMG recordings. Onset of the EMG discharge noted in the lower cranial nerves innervated muscles, with subsequent cranio‐caudal propagation involving the axial musculature, is most likely suggestive of a brainstem/reticular myoclonus. An analysis of the velocity of conduction of the efferent volley can then be performed to further affirm the diagnosis. A simplistic way to ascertain the velocity of conduction can be done utilizing the cranial nerve and spinal cord innervated paraspinal muscles. The relative distances between the muscles can be measured and divided by the time duration between the subjacent paraspinal muscles discharges noted on the poly EMG to arrive at the velocity of conduction. If the conduction velocity is >50 m/sec (can be up to 70 m/sec), it is suggestive of brainstem/reticular myoclonus. No giant SEPs are noted (although not suggested to be performed for most cases). Cortical myoclonus has a faster conduction velocity, usually >100 m/sec, and also the temporal onset is in the higher cranial nerves with caudal propagation. 1 , 5 , 40
Peripheral nerve injury–related fasciculation and myokymia may produce short duration muscle bursts with duration <50 milliseconds. These can be differentiated from cortical myoclonus by performing simultaneous recordings from agonist–antagonist pairs. 40 Peripheral nerve injury–related discharges are characterized by independent firing of the muscles. Cortical myoclonus on the other hand has characteristic simultaneous agonist–antagonist activation. Needle EMG can be used for the evaluation of fasciculations and myokymia, if ambiguities remain.
EMG Burst Duration 50 to 100 Milliseconds
Characteristic of a startle reflex. Duration range can be seen in the myoclonus of brainstem origin and rarely spinal myoclonus. The distribution, pattern of spread, and conduction velocity can help with further differentiation.
Generalized axial and flexor predominant jerks induced most commonly by auditory stimulus are most consistent with a startle syndrome. The spatial‐temporal characteristics of the poly EMG trace can provide further reaffirmation. Startle reflex is most commonly characterized by an initial short‐latency, auditory‐induced eyelid blink followed most consistently by SCM muscle discharge followed by subsequent cranio‐caudal propagation. The conduction velocity based on poly EMG recording is ~30 m/sec, notably slower than brainstem/reticular myoclonus and faster than a spinal myoclonus ~5 to 15 m/sec. A diagnosis of exaggerated startle/hyperekplexia can be made if there is no habituation to repetitive stimulation as per the methodology noted previously. 5 , 13 , 30
EMG Burst Duration >100 Milliseconds
Characteristic of voluntary movements, tics, or functional movement disorders. Could be seen in organic spinal/propriospinal myoclonus.
Focal/multifocal jerks with burst durations >100 milliseconds can be back averaged to cortical EEG to look for the presence/absence of a BP. The presence of BP is suggestive of a likely functional jerk disorder or voluntary movement. Event‐related desynchronization of the beta and mu rhythms in relation to the jerks could also further supplement and improve the diagnostic gain compared with BP alone for the diagnosis of functional jerks. 41 Focal or multifocal jerks preceded by a premonitory sensory urge, sometimes with a short‐duration BP, can be seen in tic disorders. 42
Generalized jerks, which are usually noted to originate at the level of the thoracic spinal cord with subsequent cranio‐caudal propagation limited to the spinal cord, are suggestive of a propriospinal myoclonus. The conduction velocity based on poly EMG is slower, usually 5 to 15 m/sec. The muscle most consistently noted to be activated based on poly EMG can be back averaged to cortical EEG for the presence/absence of BP, which can further help characterize the jerks as being functional or organic propriospinal myoclonus, respectively. The consistency of the pattern of discharge noted per poly EMG is also an important variable to note—more inconsistencies and variabilities in the pattern and spread of discharge noted in functional disorders. 5 , 14 , 16 , 28 , 35
Knowledge Gaps
Jerk‐like movements are also associated with certain dystonic syndromes. Based solely on phenomenology, some terms used interchangeably to refer to these syndromes are “myoclonic dystonia or dystonic myoclonus” and “myoclonus‐dystonia syndrome.” The myoclonus dystonia syndrome has been most systematically studied in patients with DYT11 mutations, where the myoclonus more commonly occurs independent of the dystonia. The physiologic characteristics of myoclonus noted in these patients are most consistent with those noted in patients with brainstem myoclonus. 21 , 22 , 43 Limited systematic studies are available to characterize the physiology of myoclonus noted in other forms of dystonia. Another entity that has been described as dystonic myoclonus where dystonia and myoclonus occur in combination resulting in longer duration of EMG bursts. 10
“Subcortical myoclonus” is another term that is used to describe myoclonus associated with certain disorders. It has been used to describe myoclonus in corticobasal syndrome (CBS), where the physiologic characteristics of myoclonus are most consistent with those noted in cortical myoclonus without a clear and consistent cortical correlate (no giant SEPs or EEG correlate). 9 , 38 , 43 However, whether myoclonus in CBS is cortical or subcortical remains unresolved. Arguments favoring a cortical origin are based on magnetoencephalogram (MEG) back averaging, latency of exaggerated long‐latency cutaneous reflexes with synchronous distal involvement, and paired pulse TMS studies suggestive of cortical disinhibition. 9 , 44 , 45
Myoclonus noted in orthostatic myoclonus is also deemed to be of subcortical origin; however, the site of its origin within the neuraxis largely remains unknown. 46 Orthostatic myoclonus (OM) was originally recognized in the bilateral leg muscles of older patients with a variety of cerebral lesions while standing. 47 A subsequent, retrospective study of a large number of OM patients confirmed some of the findings in the original report but revealed that, although the lower extremity myoclonus occurs in the leg muscles of affected patients while standing, this is only while they are bearing their entire body weight. 46 For example, having them lean forward onto a chair, the leg myoclonus utterly extinguishes but rarely, if ever, emerges in the upper limbs. 46 The myoclonic bursts of OM are typically 50 milliseconds in duration, and ostensibly never longer than 100 milliseconds. 46 They are of very low amplitude and resemble trembling, which is how patients usually describe them, but are often not visible. The myoclonus is incessant, occurring irregularly at approximately 5 to 7 Hz, with interposed tonic muscle activity lasting hundreds of milliseconds in affected muscles, which are overwhelmingly the tibialis anteriors > gastrocnemii, occurring often synchronously in homologous muscles. 46 , 48 These homologous, synchronous bursts may alternate semirhythmically between the tibialis anteriors and gastrocnemii for several hundred milliseconds. 46 The quadriceps are rarely involved and paraspinals appear to be unaffected. 46 As originally noted, 47 cerebral disorders are invariably present, with microvascular leukoencephalopathy being most common, followed by Parkinson's disease and atypical parkinsonism. Although systematic EEG back averaging has not been performed on OM patients, many have undergone cortical SEPs and assessment for long‐latency reflexes at rest, which have been invariably unremarkable. 48 In total, the aforementioned characteristics, coupled with the absence of involvement of facial muscles, rare cases unilateral OM secondary to contralateral large vessel frontal lobe strokes, 48 and in particular the striking lower extremity distribution of the myoclonus, suggest that the generator of OM may be within the vertex of the frontal lobes. 46 , 47 , 48
The overlap noted in the physiologic characteristics of subcortical myoclonus as currently described in the literature does not merit any distinctive classification of this entity. It is more meaningful to describe the myoclonus based on the physiologic characteristics. The similarities noted with the more established forms of myoclonic disorders can provide useful insights into their pathophysiology.
The diagnostic sensitivity and specificity of many of the electrophysiologic measures described are not known. The absence of certain neurophysiologic features in certain jerk‐like movements do not necessarily rule out the diagnosis. As an example, the absence of cortical spikes and giant SEP do not necessarily rule out cortical myoclonus that could be secondary to technical limitations and limitations of scalp EEG. 49 On a similar vein, cortical myoclonus may not be stimulus sensitive at times, and as such the C‐reflexes and long latency reflexes may also be normal. Therefore, the results of the electrophysiologic assessment need to be interpreted in their totality and should serve as complementary to a carful history and physical examination.
Conclusion
Many different movement disorders with a ‘jerk‐like’ phenomenology share similar clinical characteristics. 4 , 13 Based solely on clinical characteristics they can be mislabeled and misclassified as myoclonus. 5 , 14 However, different types of myoclonus and reflex disorders have distinctive physiologic characteristics. 10 , 15 Refining the characterization of these disorders using objective physiology makes this classification more meaningful. It also helps ascertain the site of origin within the motor system. We propose a simplistic diagnostic approach for the objective physiologic diagnosis of myoclonic disorders, functional movement disorders with a jerk‐like phenomenology, and startle/startle‐like reflexes. As per the proposed algorithm, most jerk‐like movement disorders can be recognized based on surface EMG recordings. Based solely on the duration of EMG discharge and pattern of spread to the different muscles, we can easily make the correct diagnosis. These techniques can be easily incorporated into clinical practice as a diagnostic test, and the methodology of recording can be adapted to suit most neurophysiology laboratories. Correct characterization of myoclonus can have important pathophysiologic and therapeutic implications. One of the biggest challenges for the diagnosis and treatment of functional movement disorders remains getting the patients to accept the diagnosis. 24 , 28 Having an objective physiologic test for diagnosis can help facilitate the acceptance of the diagnosis, which can have implications on prognosis and health care expenditures. 24 , 50
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
S.H.I.M.: 1A, 1B, 1C, 3A, 3B
F.V.: 1A, 1B, 1C, 3A, 3B
G.L.: 1B, 3B
V.J.A.: 3B
M.H.: 1B, 3B
Disclosures
Ethical Compliance Statement: The authors confirm that approval by an institutional review board and patient consent was not required for this work. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: The authors declare there are no conflicts of interest relevant to this work. M.H. was supported by National Institute of Neurological Disorders and Stroke (NINDS) intramural program.
Financial Disclosures for the Previous 12 Months: S.H.I.M. serves as a contractor for New Touch Digital Inc. and has received fees for his time for consultation. M.H. holds patents for an immunotoxin for the treatment of focal movement disorders and the H‐coil for magnetic stimulation; in relation to the latter, he has received license fee payments from the National Institutes of Health (from Brainsway). He is on the Medical Advisory Boards of CALA Health and Brainsway. He has research grants from Allergan for studies of methods to inject botulinum toxins, Medtronic, Inc. for a study of DBS for dystonia, and CALA Health for studies of a device to suppress tremor.
S.H.I. M. and F.V.‐U. have contributed equally to this manuscript and are cofirst authors for this article.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Shibasaki H. Electrophysiological studies of myoclonus. Muscle Nerve 2000;23:321–335. [DOI] [PubMed] [Google Scholar]
- 2. Caviness JN, Brown P. Myoclonus: current concepts and recent advances. Lancet Neurol 2004;3:598–607. [DOI] [PubMed] [Google Scholar]
- 3. Toro C, Pascual‐Leone A, Deuschl G, Tate E, Pranzatelli MR, Hallett M. Cortical tremor. A common manifestation of cortical myoclonus. Neurology 1993;43:2346–2353. [DOI] [PubMed] [Google Scholar]
- 4. Caviness JN. Myoclonus. Parkinsonism Relat Disord 2007;13(suppl 3):S375–S384. [DOI] [PubMed] [Google Scholar]
- 5. Merchant SH, Vial F, Leodori G, Fahn S, Pullman SL, Hallett M. A novel exaggerated "spino‐bulbo‐spinal like" reflex of lower brainstem origin. Parkinsonism Relat Disord 2018;61:34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bakker MJ, van Dijk JG, van den Maagdenberg AM, Tijssen MA. Startle syndromes. Lancet Neurol 2006;5:513–524. [DOI] [PubMed] [Google Scholar]
- 7. Dreissen YE, Bakker MJ, Koelman JH, Tijssen MA. Exaggerated startle reactions. Clin Neurophysiol 2012;123:34–44. [DOI] [PubMed] [Google Scholar]
- 8. Shibasaki H. Physiology of negative myoclonus. Adv Neurol 2002;89:103–113. [PubMed] [Google Scholar]
- 9. Mima T, Nagamine T, Ikeda A, Yazawa S, Kimura J, Shibasaki H. Pathogenesis of cortical myoclonus studied by magnetoencephalography. Ann Neurol 1998;43:598–607. [DOI] [PubMed] [Google Scholar]
- 10. Shibasaki H. Neurophysiological classification of myoclonus. Neurophysiol Clin 2006;36:267–269. [DOI] [PubMed] [Google Scholar]
- 11. Tassinari CA, Rubboli G, Shibasaki H. Neurophysiology of positive and negative myoclonus. Electroencephalogr Clin Neurophysiol 1998;107:181–195. [DOI] [PubMed] [Google Scholar]
- 12. Zutt R, Elting JW, van Zijl JC, et al. Electrophysiologic testing aids diagnosis and subtyping of myoclonus. Neurology 2018;90:e647–e657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andermann F, Keene DL, Andermann E, Quesney LF. Startle disease or hyperekplexia: further delineation of the syndrome. Brain 1980;103:985–997. [DOI] [PubMed] [Google Scholar]
- 14. Erro R, Bhatia KP, Edwards MJ, Farmer SF, Cordivari C. Clinical diagnosis of propriospinal myoclonus is unreliable: an electrophysiologic study. Mov Disord 2013;28:1868–1873. [DOI] [PubMed] [Google Scholar]
- 15. Avanzini G, Shibasaki H, Rubboli G, et al. Neurophysiology of myoclonus and progressive myoclonus epilepsies. Epileptic Disord 2016;18:11–27. [DOI] [PubMed] [Google Scholar]
- 16. Esposito M, Erro R, Edwards MJ, et al. The pathophysiology of symptomatic propriospinal myoclonus. Mov Disord 2014;29:1097–1099. [DOI] [PubMed] [Google Scholar]
- 17. Shibasaki H, Hallett M. Electrophysiological studies of myoclonus. Muscle Nerve 2005;31:157–174. [DOI] [PubMed] [Google Scholar]
- 18. Hallett M, Chadwick D, Adam J, Marsden CD. Reticular reflex myoclonus: a physiological type of human post‐hypoxic myoclonus. J Neurol Neurosurg Psychiatry 1977;40:253‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown P. Neurophysiology of the startle syndrome and hyperekplexia. Adv Neurol 2002;89:153–159. [PubMed] [Google Scholar]
- 20. Rektor I, Kadanka Z, Bednarik J. Reflex reticular myoclonus: relationship to some brainstem pathophysiological mechanisms. Acta Neurol Scand 1991;83:221–225. [DOI] [PubMed] [Google Scholar]
- 21. Popa T, Milani P, Richard A, et al. The neurophysiological features of myoclonus‐dystonia and differentiation from other dystonias. JAMA Neurol 2014;71:612–619. [DOI] [PubMed] [Google Scholar]
- 22. Roze E, Apartis E, Clot F, et al. Myoclonus‐dystonia: clinical and electrophysiologic pattern related to SGCE mutations. Neurology 2008;70:1010–1016. [DOI] [PubMed] [Google Scholar]
- 23. Welter ML, Grabli D, Karachi C, et al. Pallidal activity in myoclonus dystonia correlates with motor signs. Mov Disord 2015;30:992–996. [DOI] [PubMed] [Google Scholar]
- 24. Erro R, Edwards MJ, Bhatia KP, Esposito M, Farmer SF, Cordivari C. Psychogenic axial myoclonus: clinical features and long‐term outcome. Parkinsonism Relat Disord 2014;20:596–599. [DOI] [PubMed] [Google Scholar]
- 25. Esposito M, Edwards MJ, Bhatia KP, Brown P, Cordivari C. Idiopathic spinal myoclonus: a clinical and neurophysiological assessment of a movement disorder of uncertain origin. Mov Disord 2009;24:2344–2349. [DOI] [PubMed] [Google Scholar]
- 26. Ahn JE, Yoo D, Jung KY, Kim JM, Jeon B, Lee MC. Spinal myoclonus responding to continuous intrathecal morphine pump. J Mov Disord 2017;10:158–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wong SSC, Qiu Q, Cheung CW. Segmental spinal myoclonus complicating lumbar transforaminal epidural steroid injection. Reg Anesth Pain Med 2018;43:554–556. [DOI] [PubMed] [Google Scholar]
- 28. van der Salm SM, Erro R, Cordivari C, et al. Propriospinal myoclonus: clinical reappraisal and review of literature. Neurology 2014;83:1862–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown P. Physiology of startle phenomena. Adv Neurol 1995;67:273–287. [PubMed] [Google Scholar]
- 30. Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. New observations on the normal auditory startle reflex in man. Brain 1991;114 ( Pt 4):1891–1902. [DOI] [PubMed] [Google Scholar]
- 31. Matsumoto J, Fuhr P, Nigro M, Hallett M. Physiological abnormalities in hereditary hyperekplexia. Ann Neurol 1992;32:41–50. [DOI] [PubMed] [Google Scholar]
- 32. Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. The hyperekplexias and their relationship to the normal startle reflex. Brain 1991;114 ( Pt 4):1903–1928. [DOI] [PubMed] [Google Scholar]
- 33. Kofler M, Muller J, Wenning GK, et al. The auditory startle reaction in parkinsonian disorders. Mov Disord 2001;16:62–71. [DOI] [PubMed] [Google Scholar]
- 34. Gilmore RL. Sensory evoked potentials in clinical practice. Semin Neurol 1990;10:185–195. [DOI] [PubMed] [Google Scholar]
- 35. Meppelink AM, Little S, Oswal A, et al. Event related desynchronisation predicts functional propriospinal myoclonus. Parkinsonism Relat Disord 2016;31:116–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Terada K, Ikeda A, Van Ness PC, et al. Presence of Bereitschaftspotential preceding psychogenic myoclonus: clinical application of jerk‐locked back averaging. J Neurol Neurosurg Psychiatry 1995;58:745–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pfurtscheller G, Lopes da Silva FH. Event‐related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 1999;110:1842–1857. [DOI] [PubMed] [Google Scholar]
- 38. Grosse P, Kuhn A, Cordivari C, Brown P. Coherence analysis in the myoclonus of corticobasal degeneration. Mov Disord 2003;18:1345–1350. [DOI] [PubMed] [Google Scholar]
- 39. Caviness JN, Adler CH, Sabbagh MN, Connor DJ, Hernandez JL, Lagerlund TD. Abnormal corticomuscular coherence is associated with the small amplitude cortical myoclonus in Parkinson's disease. Mov Disord 2003;18:1157–1162. [DOI] [PubMed] [Google Scholar]
- 40. Tunc S, Bruggemann N, Baaske MK, et al. Facial twitches in ADCY5‐associated disease—myokymia or myoclonus? An electromyography study. Parkinsonism Relat Disord 2017;40:73–75. [DOI] [PubMed] [Google Scholar]
- 41. Beudel M, Zutt R, Meppelink AM, et al. Improving neurophysiological biomarkers for functional myoclonic movements. Parkinsonism Relat Disord 2018;51:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Karp BI, Porter S, Toro C, Hallett M. Simple motor tics may be preceded by a premotor potential. J Neurol Neurosurg Psychiatry 1996;61:103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thompson PD, Day BL, Rothwell JC, Brown P, Britton TC, Marsden CD. The myoclonus in corticobasal degeneration. Evidence for two forms of cortical reflex myoclonus. Brain 1994;117 (Pt 5):1197–1207. [DOI] [PubMed] [Google Scholar]
- 44. Chen R, Ashby P, Lang AE. Stimulus‐sensitive myoclonus in akinetic‐rigid syndromes. Brain 1992;115 ( Pt 6):1875–1888. [DOI] [PubMed] [Google Scholar]
- 45. Pal PK, Gunraj CA, Li JY, Lang AE, Chen R. Reduced intracortical and interhemispheric inhibitions in corticobasal syndrome. J Clin Neurophysiol 2008;25:304–312. [DOI] [PubMed] [Google Scholar]
- 46. van Gerpen JA. A retrospective study of the clinical and electrophysiological characteristics of 32 patients with orthostatic myoclonus. Parkinsonism Relat Disord 2014;20:889–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Glass GA, Ahlskog JE, Matsumoto JY. Orthostatic myoclonus: a contributor to gait decline in selected elderly. Neurology 2007;68:1826–1830. [DOI] [PubMed] [Google Scholar]
- 48. Hassan A, van Gerpen JA. Orthostatic tremor and orthostatic myoclonus: weight‐bearing hyperkinetic disorders: a systematic review, new insights, and unresolved questions. Tremor Other Hyperkinet Mov (N Y) 2016;6:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pillai J, Sperling MR. Interictal EEG and the diagnosis of epilepsy. Epilepsia 2006;47(suppl 1):14–22. [DOI] [PubMed] [Google Scholar]
- 50. Carson A, Hallett M, Stone J. Assessment of patients with functional neurologic disorders. Handb Clin Neurol 2016;139:169–188. [DOI] [PubMed] [Google Scholar]


