Abstract
Accumulating evidence supports the role of sleep in synaptic plasticity and memory consolidation. One line of investigation, the synaptic homeostasis hypothesis, has emphasized the increase in synaptic strength during waking, and compensatory downsizing of (presumably less frequently used) synapses during sleep. Conversely, other studies have reported downsizing and loss of dendritic spines following sleep deprivation. We wanted to determine the effect of sleep deprivation on dendritic spines of hippocampal CA1 neurons using genetic methods for fluorescent labeling of dendritic spines. Male Vglut2-Cre mice were injected with an AAV-DIO-ChR2-mCherry reporter in CA1 hippocampus. Gentle handling was used to sleep deprive mice for 5 hours, from lights on (7 AM) to 12 noon. Control and sleep-deprived mice were euthanized at 12 noon and processed for quantification of dendritic spines. We used confocal microscope imaging and 3D analysis to quantify thin, mushroom, and stubby spines from CA1 dendrites, distinguishing between branch segments. We observed significantly greater density of spines in CA1 of sleep deprived mice, driven primarily by greater numbers of thin spines, and significantly larger spine volume and head diameter. Branch and region specific analysis revealed that spine volume was greater in primary dendrites of apical and basal segments, along with proximal segments on both apical and basal dendrites, and spine density was increased in secondary branches and distal segments on apical dendrites following sleep deprivation. Our three-dimensional quantification suggests sleep contributes to region - and branch-specific synaptic downscaling in the hippocampus, supporting the theory of broad but selective synaptic downscaling during sleep.
Keywords: memory consolidation, dendritic spines, sleep, hippocampus, RRID:Addgene_47636, RRID: IMSR_JAX:016963
Graphical Abstract
1. Introduction:
The function of sleep has been a subject of debate for centuries. One of the theories that has received considerable attention in recent years is that sleep is necessary to promote memory consolidation. Human studies indicate that learning followed by a period of sleep is retained better than learning followed by an equal period that does not entail sleep (Payne, Chambers, & Kensinger, 2012; Stickgold, 2005; Stickgold, Hobson, Fosse, & Fosse, 2001; Wagner, Hallschmid, Rasch, & Born, 2006). Animal studies in various learning paradigms, including contextual fear conditioning, have yielded similar results (Graves, Heller, Pack, & Abel, 2003; Hagewoud, Bultsma, Barf, Koolhaas, & Meerlo, 2011; Hagewoud et al., 2010; Prince et al., 2014; Rosier et al., 2018; Shi et al., 2011). In contextual fear conditioning the environmental context appears to be encoded in the CA1 field of the hippocampus (Ji & Maren, 2008). This encoding is thought to be dependent upon increases and decreases of the strength of specific synapses, which are reflected by the size and number of the post-synaptic elements, dendritic spines. Thus, one would expect consolidation of contextual memories during sleep to be accompanied by alterations in CA1 dendritic spines.
The synaptic homeostasis hypothesis proposes that increases in synaptic strength of excitatory synapses during wakefulness are counterbalanced by overall reduction in strength of synapses in the following sleep period to achieve homeostasis (de Vivo et al., 2017; Tononi & Cirelli, 2006). To achieve memory consolidation, this process would presumably involve selective downsizing of weak synapses, reflecting infrequent Hebbian time-dependent firing of the pre- and post-synaptic elements. Work by Cirelli and colleagues found electron microscopic evidence of increases in axon-spine interface and spine head volume selective for small and intermediate synapses in the motor cortex during prolonged wake, and reduction during subsequent sleep (de Vivo et al., 2017). Recently, increased axon-spine interface and synapse density was observed in the hippocampus following sleep deprivation using electron microscopy (Spano et al., 2019).
On the other hand, work by Havekes and colleagues has suggested that synaptic spines of hippocampal CA1 neurons are diminished in size and number during prolonged wakefulness (i.e., sleep deprivation) (Havekes et al., 2016). While the studies by Cirelli and colleagues were conducted with electron microscopy, and therefore were limited to sampling synapses in a small part of the motor cortex, the study by Havekes and coworkers used Golgi staining to quantify the numbers of dendritic spines over a larger area of the hippocampus. Thus, the difference in their results could be due to looking at different structures, but it could also be due to differences in the techniques used to visualize synaptic elements. In particular, the Golgi method stains only a small percentage of cells, but the reasons why some cells fill with dense staining while most do not is not understood. Hence, the cells sampled by Golgi staining may not be representative of the entire population. Furthermore, the complexity of inputs to dendritic spines may contribute to discrepancies in reported effects of sleep deprivation. In the hippocampus, inputs to dendritic arborizations of CA1 pyramidal neurons vary in a branch- and segment-specific manner (Megias, Emri, Freund, & Gulyas, 2001; Spruston & McBain, 2006). Thus, dendritic spines on these neurons may be differentially regulated during sleep based on the type and origin of synaptic inputs.
We examined the CA1 field of the hippocampus to see if we could determine the overall changes that may occur in dendritic spines during a period of sleep deprivation compared to an equivalent period of natural sleep. To gain an unbiased view of CA1 pyramidal cell dendrites, we used an adeno-associated viral vector (AAV) expressing red fluorescent protein (mCherry) to fill CA1 pyramidal cell bodies and dendrites so that we could quantify numbers of dendritic spines of different types and in different parts of the dendritic tree.
We used confocal imaging and 3D analysis to determine how sleep deprivation broadly affects dendritic spine density and size on specific dendritic segments of CA1 hippocampal pyramidal cells.
2. Materials and Methods:
2.1. Animals
All mice used in experiments were male and animals were maintained on a 12:12 h light-dark cycle with ad libitum access to water and food. All animal procedures met National Institutes of Health standards, as set forth in the Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee (IACUC).
We used male Vglut2-IRES-Cre mice RRID: IMSR_JAX:016963 that were produced by Dr. Brad Lowell (mice with this construct are also available from Jackson Laboratories, Bar Harbor, ME). These were backcrossed with C57BL6 mice obtained from Jackson Labs for at least six generations.
2.2. Viral Vector Injection and Sleep Deprivation
Adult (3–4 months old) male Vglut2-Cre mice (5 control, 5 sleep deprived mice) were stereotaxically microinjected with 23 nanoliters of AAV8::EF1a::DIO::ChR2(H134R)::mCherry Virus (Vector Labs, cat# VB2090), RRID:Addgene_47636. The injections targeted the CA1 region of the dorsal hippocampus (stereotaxic coordinates AP: −2.3, ML:1.75, DV: 1.0) using a glass micropipette and an air pressure injection system. After 5 weeks, animals were divided randomly into two cohorts of five mice each (5 control, 5 sleep deprived mice).
Gentle handling was used to sleep deprive mice for 5 hours, from lights on (7 AM) to 12 PM. Singly housed mice undergoing sleep deprivation were transported to a procedure room at lights on (7AM) and the experimenter manually observed each mouse and gently rocked the home cage when mice showed any sign of falling asleep. Control mice were left to sleep undisturbed in their home cages. Both control and sleep deprived mice were sacrificed at 12 PM and processed for quantification of dendritic spines.
2.3. Tissue Processing
Mice were deeply anesthetized and perfused with 0.1M PBS containing 10% formalin. Brains were removed and cryoprotected in 30% sucrose in 0.1M PBS (pH 7.4), then frozen sectioned into coronal 40 µm sections containing the hippocampus. Sections were mounted on gelatin coated slides to quantify dendritic spine density from images captured using confocal microscopy.
2.4. Confocal Imaging
A Zeiss LSM 5 confocal microscope system interfaced with Zen imaging software was used to acquire 3D image stacks of dendritic branches from CA1 pyramidal neurons in sections from control and sleep deprived mice. Images of 40 μm-thick sections were acquired, with z-step 0.25 μm, using a 60x oil immersion objective (numerical aperture 1.4; pixel size, 0.23 × 0.23 μm) as described in our previous study (Gisabella et al., 2016). For dendritic spine quantification, confocal microscopy images were analyzed using Neurolucida 360 software with Autospine to measure spine density in apical and basal dendrites of hippocampus CA1 pyramidal cells and their secondary branches using an approach previously described (Dickstein et al., 2016; Rodriguez, Ehlenberger, Dickstein, Hof, & Wearne, 2008). Dendritic spines were sampled from proximal and distal dendrites, in both primary and secondary branches by an investigator who was blinded to the treatment group of the animals. Unbiased systematic random sampling was used for sampling neurons for confocal imaging. Slides were coded for blind analysis, and sampling boxes were placed in the CA1 area. The length from the border of CA2 to the medial end of CA1 was measured for each section, and sampling boxes were placed on either side of the mid-point between these two ends of CA, halfway between the lateral border of CA1/CA2 and the medial end of CA1. Two sampling boxes (450 μm x 300 μm) were placed in the stratum radiatum, and two equivalent sampling boxes in stratum oriens, each 300 μm left and right of the midline, positioned immediately above and below the pyramidal cell layer. We then imaged dendrites located in each box in which we could follow the full extent of the dendrites to the cell body. We sampled dendrites from the first 5–6 neurons encountered, starting from the left side of the sampling box, per each sampling box in each region, per section, 4–5 sections per animal. This resulted in 3 to 4 apical proximal segments, 3 to 4 apical distal segments, 3 to 4 basal proximal segments, and 2 to 3 basal distal segments per neuron. Spine density, shape, and volume was quantified using Neurolucida 360 with semi-automated analysis from 3-dimensional confocal image stacks in an unbiased manner. We examined each neuron by looking at its apical and basal dendrites in 10 micron increments from the cell body, quantifying numbers of mushroom, thin, and stubby spines in each 10 micron segment, and the spine head diameter and volume of each spine.
2.5. Dendritic Spine Quantification
For dendritic spine quantification confocal microscopy images were analyzed using Neurolucida 360 software with Autospine to measure spine density as described in our previous study (Gisabella et al., 2016). Dendritic spines were grouped into proximal and distal dendrites, in both primary and secondary branches and in stratum oriens (basal dendrites) vs stratum radiatum (apical dendrites). Spines were grouped into thin spines, stubby spines, and mushroom spines automatically by the Neurolucida 360 software on the basis of the spine head to neck diameter ratio, mushroom head size, and filopodia length according to previously established criteria (Rodriguez et al., 2008). Spine density, shape, and volume was quantified using Neurolucida 360 with semi-automated analysis from 3-dimensional confocal image stacks in an unbiased manner.
2.6. Statistical Analysis
For all statistical tests, the significance threshold was p ≤ 0.05. Unpaired Student’s t-test was used to compare population estimates for control and sleep deprived groups for each outcome measure. All p-values reflect Bonferroni correction for multiple comparisons. All graphs reflect the mean for each group with n=5 control and n=5 Sleep deprived animal), and error bars represent 95% confidence intervals.
3. Results:
3.1. Overall Greater Dendritic Spine Density and Size Following Sleep Deprivation
We observed a significantly greater overall dendritic spine density when calculated per branch length (p,0.04, Fig. 1a) and when calculated as the number of spines per branch surface area (p<0.002, Fig. 1b) in the mice that were sleep deprived compared to the littermates that were permitted to sleep undisturbed. In addition, both spine volume (p<0.008, Fig 1e) and spine head diameter (p<0.05, Fig 1f), were significantly greater following 5 hours of sleep deprivation. While there was a general trend in these directions across all types of spines in all segments of the dendritic tree that we examined, the subgroup analysis revealed that these overall changes were largely driven by statistically significant changes in specific spine types and on specific parts of the dendritic tree.
Figure 1: Sleep-Deprived Mice Display Higher Density and Volume of Dendritic Spines.
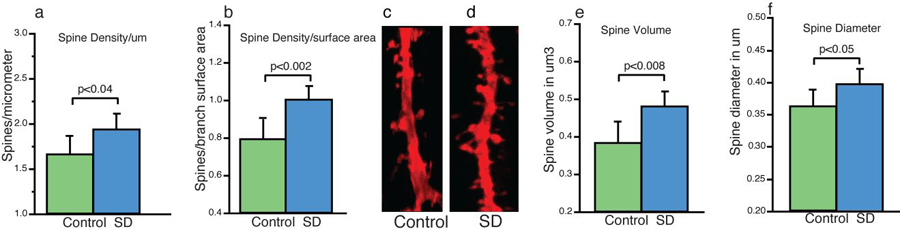
Overall analysis of dendritic spine measures of CA1 pyramidal cells in sleep-deprived mice compared to control mice revealed that at a broad level, dendritic spine density is greater in sleep deprived mice when calculated as spines per micrometer of branch segment length (a). This difference was stronger when calculated in the three dimensional measure of spines per branch segment surface area (b). Representative confocal micrograph of a = 10 μm branch segment from a sleep deprived mouse (c), with more spines than a comparable branch segment from a control (control) mouse (d). Measures of spine volume (e) and spine head diameter (F) were also greater in sleep deprived mice compared to control mice. Data represent an average of 120–160 dendritic segments per animal, 600–800 segments per group. Error bars represent standard deviation.
3.2. Differential Spine Changes in Primary vs Secondary Branches
We analyzed dendritic spine measures following sleep deprivation in primary dendrites compared to secondary branches. For both apical and basal dendrites, volumes and head diameters of all three types of dendritic spines were significantly greater in sleep deprived compared to sleeping animals in primary dendrites, but not on secondary branches (Fig. 2b&c; Table 1). On the other hand, dendritic spine density was significantly greater mainly in secondary branches (Fig. 2a). However, these differences were not uniform across different spine types or distance from the cell body.
Figure 2: Dendritic Spines are Differentially Affected by Sleep Deprivation in Apical Primary Branches Compared to Secondary Branches.
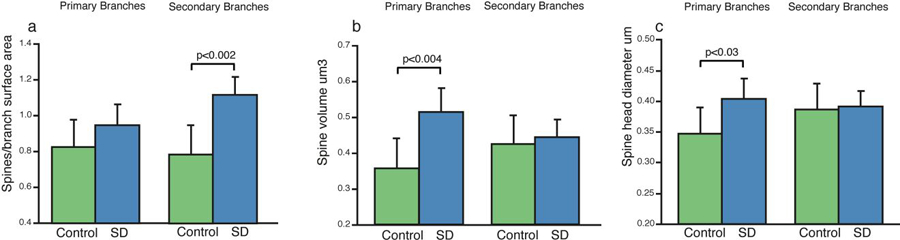
Analysis of dendritic spine measures divided into primary vs secondary branch segments revealed that greater spine density in sleep deprived mice is selective to secondary branches (a), whereas increases in spine volume (b) and spine head diameter (c) are selective to primary branches. Data represent an average of 60–80 dendritic segments per animal, 300–400 segments per group. Error bars represent standard deviation.
Table 1:
Summary Table of Branch and Segment Specific Measures in Control and Sleep Deprived Mice
| Apical prox 10 | Apical distal 10 | Apical prox 20 | Apical distal 20 | Basal prox 10 | Basal distal 10 | Basal prox 20 | Basal dist 20 | |
|---|---|---|---|---|---|---|---|---|
| Thin # of spines/μm Control | 0.85 +/− 0.15 | 0.71 +/− 0.12 | 0.75 +/− 0.20 | 0.52 +/− 0.11 | 0.91 +/− 0.23 | 0.90 +/− 0.12 | 0.86 +/− 0.10 | 0.92 +/− 0.11 |
| Thin # of spines/μm SD | 1.12 +/− 0.10 | 0.96 +/− 0.09 | 1.36 +/− 0.15 | 0.90 +/− 0.04 | 1.02 +/− 0.13 | 0.86 +/− 0.08 | 0.93 +/− 0.12 | 0.98 +/− 0.13 |
| Thin total spine volume Control | 0.31 +/− 0.05 | 0.34 +/− 0.07 | 0.39 +/− 0.04 | 0.41 +/− 0.07 | 0.30 +/− 0.05 | 0.39 +/− 0.05 | 0.37 +/− 0.08 | 0.41 +/− 0.10 |
| Thin total spine volume SD | 0.39 +/− 0.03 | 0.36 +/− 0.09 | 0.37 +/− 0.06 | 0.39 +/− 0.09 | 0.40 +/− 0.03 | 0.41 +/− 0.06 | 0.38 +/− 0.09 | 0.39 +/− 0.08 |
| Thin head diameter Control | 0.10 +/− 0.02 | 0.13 +/− 0.04 | 0.10 +/− 0.06 | 0.12 +/− 0.03 | 0.11 +/− 0.04 | 0.13 +/− 0.09 | 0.11 +/− 0.05 | 0.13 +/− 0.06 |
| Thin head diameter SD | 0.17 +/− 0.03 | 0.16 +/− 0.06 | 0.14 +/− 0.09 | 0.18 +/− 0.06 | 0.18 +/− 0.03 | 0.14 +/− 0.10 | 0.16 +/− 0.09 | 0.17 +/− 0.03 |
| Stubby # of spines/μm Control | 0.43 +/− 0.11 | 0.39 +/− 0.13 | 0.31 +/− 0.09 | 0.28 +/− 0.12 | 0.35+/− 0.15 | 0.47+/− 0.15 | 0.35 +/− 0.08 | 0.45 +/− 0.14 |
| Stubby # of spines/μm SD | 0.42 +/− 0.08 | 0.44 +/− 0.11 | 0.37 +/− 0.07 | 0.36 +/− 0.07 | 0.40 +/− 0.11 | 0.44 +/− 0.14 | 0.43 +/− 0.09 | 0.41 +/− 0.12 |
| Stubby total spine volume Control | 0.46+/− 0.03 | 0.43+/− 0.03 | 0.48+/− 0.04 | 0.50+/− 0.07 | 0.44+/− 0.04 | 0.47+/− 0.08 | 0.49+/− 0.03 | 0.50+/− 0.06 |
| Stubby total spine volume SD | 0.55+/− 0.03 | 0.51+/− 0.04 | 0.52+/− 0.04 | 0.52+/− 0.07 | 0.53+/− 0.03 | 0.49+/− 0.08 | 0.51+/− 0.03 | 0.51+/− 0.06 |
| Stubby spine head diameter Control | 0.57 +/− 0.03 | 0.54 +/− 0.05 | 0.57 +/− 0.06 | 0.54 +/− 0.05 | 0.52 +/− 0.04 | 0.59 +/− 0.10 | 0.53 +/− 0.09 | 0.59 +/− 0.04 |
| Stubby spine head diameter SD | 0.62 +/− 0.05 | 0.65 +/− 0.03 | 0.61 +/− 0.07 | 0.57 +/− 0.08 | 0.59 +/− 0.02 | 0.60 +/− 0.06 | 0.57 +/− 0.04 | 0.62 +/− 0.05 |
| Mushroom # of spines/μm Control | 0.48 +/− 0.06 | 0.37 +/− 0.12 | 0.43 +/− 0.02 | 0.40 +/− 0.08 | 0.32 +/− 0.11 | 0.47 +/− 0.07 | 0.33 +/− 0.02 | 0.47 +/− 0.09 |
| Mushroom # of spines/μm SD | 0.48 +/− 0.04 | 0.51 +/− 0.07 | 0.39 +/− 0.02 | 0.47 +/− 0.07 | 0.40 +/− 0.07 | 0.36 +/− 0.08 | 0.41 +/− 0.01 | 0.49 +/− 0.07 |
| Mushroom total spine volume Control | 0.35+/− 0.04 | 0.37+/− 0.06 | 0.39+/− 0.03 | 0.35+/− 0.08 | 0.38+/− 0.05 | 0.37+/− 0.09 | 0.35+/− 0.04 | 0.39+/− 0.10 |
| Mushroom total spine volume SD | 0.44+/− 0.03 | 0.41+/− 0.07 | 0.40+/− 0.09 | 0.43+/− 0.10 | 0.54+/− 0.03 | 0.43+/− 0.07 | 0.42+/− 0.06 | 0.43+/− 0.08 |
| Mushroom spine head diameter Control | 0.44 +/− 0.05 | 0.48 +/− 0.08 | 0.45 +/− 0.04 | 0.52 +/− 0.09 | 0.47 +/− 0.03 | 0.46 +/− 0.07 | 0.42 +/− 0.08 | 0.46 +/− 0.10 |
| Mushroom spine head diameter SD | 0.54 +/− 0.03 | 0.51 +/− 0.06 | 0.48 +/− 0.05 | 0.50 +/− 0.10 | 0.58 +/− 0.04 | 0.49 +/− 0.04 | 0.47 +/− 0.09 | 0.50 +/− 0.07 |
Values represent mean and SEM of the number of spines per micrometer. Statistically significant differences are indicated in BOLD = p<0.05. Data represent an average of 120–160 dendritic segments per animal, 600–800 segments per group.
3.3. Sleep Deprivation Spine Density Increase is Selective for Thin Spines
In order to identify which morphological subtypes of spines are affected by sleep vs. sleep deprivation, we analyzed the dendritic spine density selectively for thin, stubby, and mushroom spines. This approach revealed that the greater dendritic spine density following sleep deprivation was primarily due to significantly more thin spines on apical dendrites, as the densities of mushroom and stubby spines were not significantly different (Fig. 3), and among basal dendrites only the numbers of mushroom spines on proximal secondary branches were greater in the sleep deprived animals.
Figure 3: Greater Density of Dendritic Spines Following Sleep Deprivation is Selective for Thin Spines.
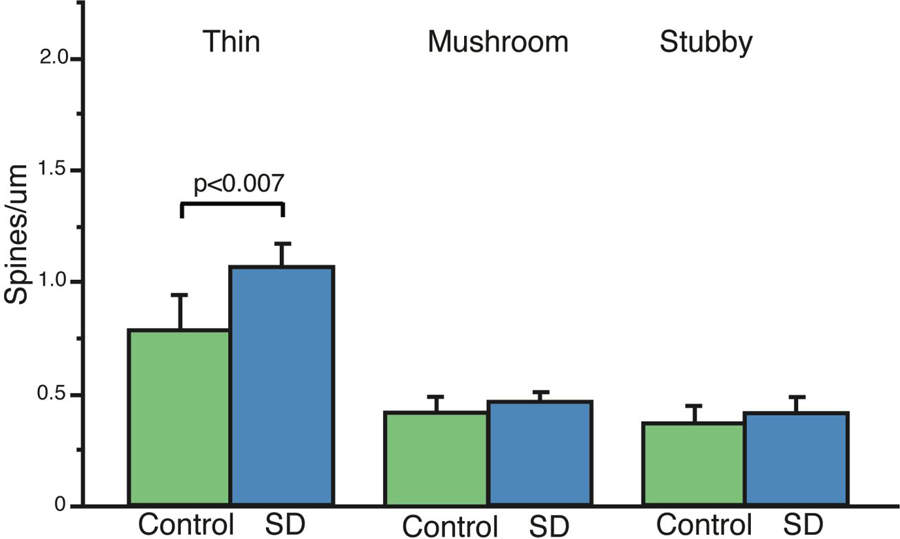
Comparison of spine density measures in morphological spine subtypes revealed that the greater density of dendritic spines in sleep deprived mice is selective for thin spines (a) No overall significant differences were observed for mushroom or stubby spines (a), although (see Table 1) the density of mushroom spines was selectively increased in proximal secondary basal dendrites. Data represent an average of 120–160 dendritic segments per animal, 600–800 segments per group. Error bars represent standard deviation.
3.4. Sleep Deprivation Differentially Affects Spines in Proximal vs Distal Branches
We divided our dendritic branch data into proximal (0–150 μm from the pyramidal cell body) vs. distal (150–300 μm from the pyramidal cell body) branch segments. Dendritic spine density was greater selectively in distal segments and secondary branches of apical (but not basal) dendrites after sleep deprivation (p<0.002, Fig. 4a), whereas dendritic spine volume and head diameter were selectively greater in proximal segments of both apical and basal dendrites (Fig. 4b&c). The only exception, as noted above, was mushroom spines which were greater in number in proximal portions of secondary basal dendrites in sleep deprived animals.
Figure 4: Differential Changes in Dendritic Spines in Apical Proximal vs Distal Dendritic Segments.
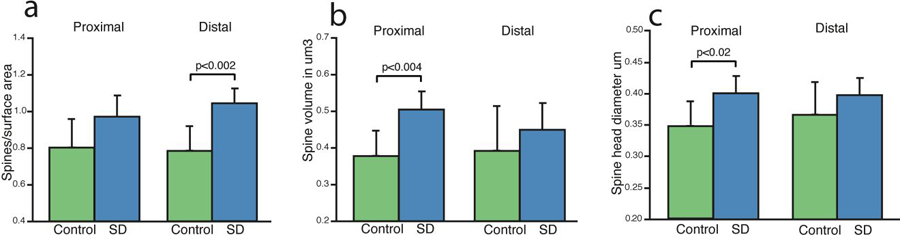
Analysis of dendritic spine measures divided into proximal vs distal branch segments shows greater spine density selective for distal branch segments (a), and selective increases in spine volume (b) and spine head diameter (c) in proximal segments. Data represent an average of 60–80 dendritic segments per animal, 300–400 segments per group. Error bars represent standard deviation.
3.5. Dendritic spines are Differentially Affected by Sleep Deprivation in Basal Dendrites in Stratum Oriens vs Apical Dendrites in Stratum Radiatum
Lastly, we divided our data by apical vs basal dendrites to analyze hippocampal layer specific effects of sleep deprivation on dendritic spines. We observed significantly greater dendritic spine density following sleep deprivation selectively in apical dendrites in stratum radiatum (p<0.002; Fig 5a). In comparison, dendritic spine volume and head diameter were selectively greater in basal dendrites in stratum oriens (Fig. 5b&c).
Figure 5: Basal Stratum Oriens Dendritic Spines are Differentially Affected by Sleep Deprivation Compared to Apical Stratum Radiatum Spines.
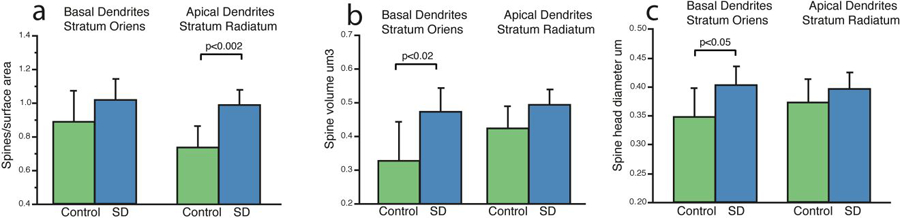
We divided dendritic spine measures into basal stratum oriens dendritic segments compared to apical stratum radiatum branch segments. Greater spine density was selective for apical segments from stratum radiatum (a), whereas increases in spine volume (b) and spine head diameter (c) were selective for basal segments from stratum oriens. Data represent an average of 60–80 dendritic segments per animal, 300–400 segments per group. Error bars represent standard deviation.
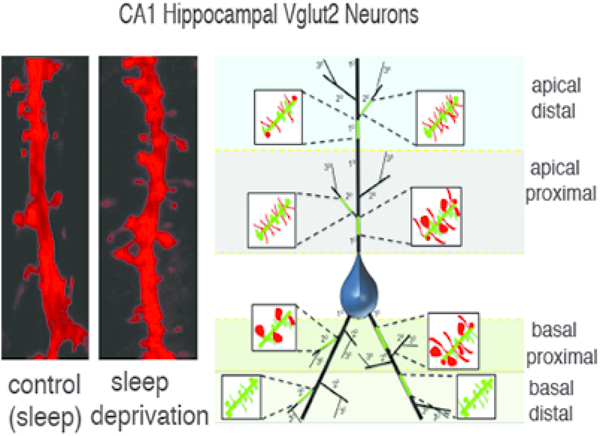
For a complete summary of branch specific measures of spine density, spine volume, and spine head diameter, see table 1 and Figure 6.
Figure 6: Summary Diagram of Dendritic Spine Changes Following Sleep Deprivation.
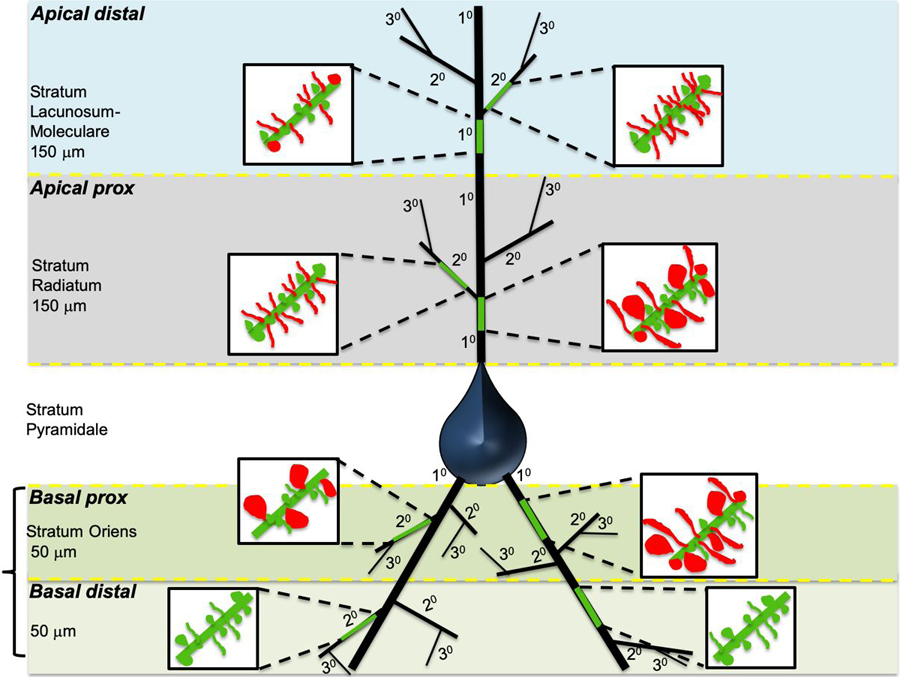
Schematic diagram depicting a pyramidal neuron from CA1, with the sampled branches highlighted in green for apical distal, apical proximal, basal proximal, and basal distal branches respectively. Primary branches indicated as 10 and secondary branches as 20. Magnified inserts depict changes in dendritic spine density and volume for each branch.
4. Discussion:
We report that sleep deprivation results in greater branch and segment specific spine volume and density in CA1 of sleep-deprived mice (Figure 6; Table 1). As we did not measure spines at the end of the dark period (before either the sleep or sleep deprivation periods), we do not know if sleep deprivation caused an increase or if sleep allowed a decrease in spine measures, although the synaptic homeostasis hypothesis would suggest that both may have been occurring. If so, our results suggest that in the hippocampus, sleep contributes to synaptic downscaling primarily for thin spines in apical secondary and distal branch segments, along with reduction of spine size in primary and proximal segments on both apical basal dendrites. Taken together, our data support the theory of broad synaptic downscaling, particularly of weak synaptic connections onto thin spines during sleep. Despite a contrary report that dendritic spines decrease following sleep deprivation in CA1, our data suggest that synaptic downscaling during sleep does apply to this region as well, however in a complex branch and segment specific manner.
4.1. Technical Considerations
Discrepancies with the previous study by Havekes et al., (2016), who found general loss of dendritic spines in CA1 neurons after sleep deprivation, may result from methodological considerations. In their primary analysis, they quantified dendritic spines using Golgi labeling and two-dimensional quantification, whereas we used viral vector labeling for Vglut2+ neurons (all CA1 pyramidal cells are Vglut2+). Golgi labeling is notoriously capricious, as only a very small percentage of all neurons are labeled, but it is not known why these neurons are labeled and others are not; hence it is possible that the Golgi labeling may favor a subset of CA1 neurons that differ from most in terms of spine integrity. In addition, because Golgi staining produces dense, solid labeling, it is impossible to see the smaller spines on the back or front of the dendrite in a microscopic section, so that the resulting 2D quantification results in underestimates of spines, and it may be difficult to distinguish spines in close proximity to each other, particularly if they are overlapping in the z-plane (Attardo, Fitzgerald, & Schnitzer, 2015; Mancuso, Chen, Li, Xue, & Wong, 2013). It is particularly difficult to visualize the smallest thin spines when they are in the z-plane, which may result in further underestimation. Viral vector labeling of spines with a Vglut2 selective construct however, allowing for confocal microscopy imaging with 3D quantification, overcomes these limitations. Havekes and coworkers also used DiI labeling of dendritic spines, but due to limitations of the diffusion of DiI, mainly analyzed secondary and tertiary branches of the apical dendrites. We found no changes in proximal apical secondary dendrites, and did not examine tertiary dendrites at all (because in our 40μm thick sections, dendrites this far from the cell body in the z-dimension would be truncated).
Thus, our evidence for branch- and segment-specific changes in spine density and spine volume suggests a complex regulation of dendritic spines on CA1 pyramidal neurons during sleep, discussed in further detail below. This complexity may contribute to differential results depending on sampling strategies and labeling specificity. Future studies using unbiased labeling of complete dendritic trees in thicker sections to capture higher order dendritic branches will clearly be of interest.
In our study, the control group of animals were left to sleep undisturbed in their home cage environment. We did not verify sleep amount and sleep quality with electro-encephalograph (EEG) recordings, in order to avoid potential effects of surgical implantation of electrodes on both groups. We refer to this group as control rather than normal sleep or sleep, as we did not characterize sleep states of these animals with EEG recordings. We assume that the control group of animals each slept normally, and on average, the control group reflects minor, normal variations in sleep quality.
An additional limitation in our study is that we did not measure spines at the end of the dark period, thus we do not know if sleep deprivation caused an increase or if sleep allowed a decrease in spine measures. A recent study on the effect of sleep deprivation on the mouse hippocampus using electron microscopy analysis however, which included this control group, indicates that sleep reduces spine density and size (Spano et al., 2019). Taken together with evidence for synaptic downscaling during sleep in other brain regions (de Vivo et al., 2017; Tononi & Cirelli, 2006), we interpret our data in the context of the synaptic homeostasis hypothesis of downscaling during sleep.
4.2. Differential Effects on Proximal vs Distal Dendritic Segments
The branch and segment selective dendritic spine differences in volume and density suggests specific inputs to CA1 pyramidal neurons may be differentially regulated during sleep. Our observation that spine density was greater following sleep deprivation in distal segments whereas spine volume was greater in proximal segments (Fig. 4) may point to differential regulation of inhibitory vs excitatory synapses during sleep. Specifically, spine volume differences in proximal branches may reflect regulation of inhibitory synapses, as these synapses are predominantly concentrated on the soma and proximal segments, whereas distal branches are more likely to reflect regulation of excitatory synapses, which are highly concentrated in distal segments (Megias et al., 2001). In addition, it is also possible that proximal vs distal segment differences reflect, in part, differences in thresholds of long-term plasticity (LTP) induction. Recent work suggests that proximal vs distal CA1 dendritic segments have different thresholds for LTP induction, with proximal segments displaying long-lasting late phase LTP with subthreshold stimulation in comparison to distal segments (Sajikumar & Korte, 2011). Thus, spines sampled from proximal segments may be more likely to be strengthened through long-lasting LTP during wakefulness, and thus not as easily eliminated during sleep but instead are reduced in size. Finally, the inputs to CA1 cells are stratified, with the inputs from the septum mainly to the basal dendrites and proximal part of the apical dendrites, the terminals from the Schaffer collaterals mainly to the midportion of the apical and to the basal dendrites, and the perforant pathway inputs from the entorhinal cortex mainly on the distal dendrites (Johnston & Amaral, 2004). Thus, changes in the fate of synapses on different parts of the dendritic tree may reflect the origins of those inputs. It would be interesting in future work to label synaptic terminals from the medial septum, CA3, and entorhinal cortex anterogradely, to determine the sources of input to different types of dendritic spines.
4.3. Selective Sleep Deprivation Effect on Density of Thin Spines
The observed differences in apical spine density were specific to thin spines (Fig. 3), suggesting immature spines formed from recent learning are downscaled during sleep. This result is in accordance with observations from electron microscopy studies by Cirelli and colleagues (de Vivo et al., 2017; Spano et al., 2019). Furthermore, dendritic spine density differences were selective for secondary branches (Fig. 2) and distal segments (Fig. 4). Taken together, our evidence suggests that thin spines, particularly those concentrated in dendritic segments that provide environmental contextual information, are short-term spines formed during wakefulness in response to sensory stimuli that are downscaled during sleep, and this process may be conserved across brain regions.
4.4. Sleep Deprivation Differentially Effects Apical and Basal Dendrites
Differential innervation of apical vs basal dendrites may also contribute to variable effects of sleep deprivation on dendritic spines. Distal apical dendrites receive more sensory information from polymodal cortical systems which relay to the hippocampus through the entorhinal cortex (Amaral & Witter, 1989; Steward, 1976), thus they may be more involved in forming thin spines in response to sensory inputs during wakefulness that are downscaled during sleep. Inputs to distal CA1 apical dendrites may also contribute to regulating the size of proximal dendrites. For example, sensory information to CA1 distal synapses from the perforant path has been proposed to function as a saliency signal to strengthen proximal synapses by stimulating LTP (Dudman, Tsay, & Siegelbaum, 2007). Downscaling of the density of CA1 apical distal spines during sleep may thus contribute to decreases in spine volume of apical proximal spines, in agreement with our observation of larger changes in the volume of proximal spines vs greater changes in density of distal spines following sleep deprivation.
Differential innervation to selective segments of CA1 dendrites may also contribute to the branch- and segment-specific changes. Apical dendrites of CA1 pyramidal neurons receive synaptic inputs from CA3 Schaffer collaterals onto mid and proximal apical segments, whereas distal apical segments primarily receive input from the perforant pathway (Spruston & McBain, 2006). Similarly, basal dendrites receive large amounts of input from Schaffer collaterals and septal neurons (Spruston & McBain, 2006). The increased density of dendritic spines following sleep deprivation we observed in apical dendrites (Fig. 5) and distal segments (Fig. 4) may correspond primarily to sensory inputs from the perforant path, whereas the increased spine volumes we observed in proximal segments (Fig. 4) and basal dendrites (Fig. 5) are more likely to reflect inputs from CA3 Schaffer collaterals or the medial septum.
The perforant path is a large source of multimodal sensory input into CA1 from cortical areas through the entorhinal cortex (Doller & Weight, 1982; Witter, Griffioen, Jorritsma-Byham, & Krijnen, 1988). This pathway is essential for processing spatial memory and novelty (Parron, Poucet, & Save, 2006; Vago, Bevan, & Kesner, 2007; Vago & Kesner, 2008). During the normal waking period of a mouse, this pathway may be involved in formation of synapses from novel environmental inputs and spatial cues, resulting in multiple thin spines that are downscaled during sleep. Lesion studies suggest that cortical inputs to CA1 neurons through the perforant path are necessary for consolidation of spatial memories (Remondes & Schuman, 2004). In comparison, the CA3 Schaffer collateral pathway is involved in formation of emotional memory (Daumas, Ceccom, Halley, Frances, & Lassalle, 2009; Remaud et al., 2014). This pathway may not have been sufficiently stimulated during wakefulness in our experiments (where the animals were singly housed in a standard cage) to allow for detection of changes following sleep deprivation. Mice may show more pronounced changes in spine measures in the proximal and basal segments in response to sleep deprivation if they undergo emotional memory formation. Thus, future studies focusing on the functionally specific changes in dendritic spines during prolonged wake or sleep following emotionally charged behavioral training such as fear conditioning will be of great interest.
We sought to determine the effect of sleep deprivation on dendritic spines of hippocampal CA1 neurons. Male Vglut2-Cre mice were injected with an AAV-DIO-ChR2-mCherry reporter in CA1 hippocampus. Gentle handling was used to sleep deprive mice for 5 hours. We used confocal microscope imaging and 3D analysis to quantify thin, mushroom, and stubby spines from CA1 dendrites, distinguishing between branch segments. We observed significantly greater density of spines in CA1 of sleep deprived mice, driven primarily by greater numbers of thin spines, and significantly larger spine volume and head diameter. Branch and region specific analysis revealed that spine volume was greater in primary dendrites of apical and basal segments, along with proximal segments on both apical and basal dendrites, and spine density was increased in secondary branches and distal segments on apical dendrites following sleep deprivation. Our three-dimensional quantification suggests sleep contributes to region- and branch-specific synaptic downscaling in the hippocampus, supporting the theory of broad but selective synaptic downscaling during sleep.
Acknowledgements:
This work was funded by NIH 4T32HL007901-19, NIH MH117460 and HL095491. The authors declare no competing financial interests.
Footnotes
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of Interest Statement: The authors have no competing financial interests to disclose.
References:
- Amaral DG, & Witter MP (1989). The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience, 31(3), 571–591. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2687721 [DOI] [PubMed] [Google Scholar]
- Attardo A, Fitzgerald JE, & Schnitzer MJ (2015). Impermanence of dendritic spines in live adult CA1 hippocampus. Nature, 523(7562), 592–596. doi: 10.1038/nature14467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumas S, Ceccom J, Halley H, Frances B, & Lassalle JM (2009). Activation of metabotropic glutamate receptor type 2/3 supports the involvement of the hippocampal mossy fiber pathway on contextual fear memory consolidation. Learning & memory, 16(8), 504–507. doi: 10.1101/lm.1418309 [DOI] [PubMed] [Google Scholar]
- de Vivo L, Bellesi M, Marshall W, Bushong EA, Ellisman MH, Tononi G, & Cirelli C (2017). Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science, 355(6324), 507–510. doi: 10.1126/science.aah5982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DL, Dickstein DR, Janssen WG, Hof PR, Glaser JR, Rodriguez A, … Tappan SJ (2016). Automatic Dendritic Spine Quantification from Confocal Data with Neurolucida 360. Curr Protoc Neurosci, 77, 1 27 21–21 27 21. doi: 10.1002/cpns.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doller HJ, & Weight FF (1982). Perforant pathway activation of hippocampal CA1 stratum pyramidale neurons: electrophysiological evidence for a direct pathway. Brain Res, 237(1), 1–13. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7074352 [DOI] [PubMed] [Google Scholar]
- Dudman JT, Tsay D, & Siegelbaum SA (2007). A role for synaptic inputs at distal dendrites: instructive signals for hippocampal long-term plasticity. Neuron, 56(5), 866–879. doi: 10.1016/j.neuron.2007.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisabella B, Farah S, Peng X, Burgos-Robles A, Lim SH, & Goosens KA (2016). Growth hormone biases amygdala network activation after fear learning. Transl Psychiatry, 6(11), e960. doi: 10.1038/tp.2016.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves LA, Heller EA, Pack AI, & Abel T (2003). Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learning & memory, 10(3), 168–176. doi: 10.1101/lm.48803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagewoud R, Bultsma LJ, Barf RP, Koolhaas JM, & Meerlo P (2011). Sleep deprivation impairs contextual fear conditioning and attenuates subsequent behavioural, endocrine and neuronal responses. J Sleep Res, 20(2), 259–266. doi: 10.1111/j.1365-2869.2010.00895.x [DOI] [PubMed] [Google Scholar]
- Hagewoud R, Havekes R, Novati A, Keijser JN, Van der Zee EA, & Meerlo P (2010). Sleep deprivation impairs spatial working memory and reduces hippocampal AMPA receptor phosphorylation. J Sleep Res, 19(2), 280–288. doi: 10.1111/j.1365-2869.2009.00799.x [DOI] [PubMed] [Google Scholar]
- Havekes R, Park AJ, Tudor JC, Luczak VG, Hansen RT, Ferri SL, … Abel T (2016). Sleep deprivation causes memory deficits by negatively impacting neuronal connectivity in hippocampal area CA1. Elife, 5. doi: 10.7554/eLife.13424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, & Maren S (2008). Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learning & memory, 15(4), 244–251. doi: 10.1101/lm.794808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, & Amaral DG (2004). Hippocampus In Shepherd GM (Ed.), The Synaptic Organization of the Brain (5th ed.): Oxford University Press. [Google Scholar]
- Mancuso JJ, Chen Y, Li X, Xue Z, & Wong ST (2013). Methods of dendritic spine detection: from Golgi to high-resolution optical imaging. Neuroscience, 251, 129–140. doi: 10.1016/j.neuroscience.2012.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megias M, Emri Z, Freund TF, & Gulyas AI (2001). Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience, 102(3), 527–540. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11226691 [DOI] [PubMed] [Google Scholar]
- Parron C, Poucet B, & Save E (2006). Cooperation between the hippocampus and the entorhinal cortex in spatial memory: a disconnection study. Behav Brain Res, 170(1), 99–109. doi: 10.1016/j.bbr.2006.02.006 [DOI] [PubMed] [Google Scholar]
- Payne JD, Chambers AM, & Kensinger EA (2012). Sleep promotes lasting changes in selective memory for emotional scenes. Front Integr Neurosci, 6, 108. doi: 10.3389/fnint.2012.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince TM, Wimmer M, Choi J, Havekes R, Aton S, & Abel T (2014). Sleep deprivation during a specific 3-hour time window post-training impairs hippocampal synaptic plasticity and memory. Neurobiology of learning and memory, 109, 122–130. doi: 10.1016/j.nlm.2013.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaud J, Ceccom J, Carponcy J, Dugue L, Menchon G, Pech S, … Dahan L (2014). Anisomycin injection in area CA3 of the hippocampus impairs both short-term and long-term memories of contextual fear. Learning & memory, 21(6), 311–315. doi: 10.1101/lm.033969.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remondes M, & Schuman EM (2004). Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature, 431(7009), 699–703. doi: 10.1038/nature02965 [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, & Wearne SL (2008). Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS One, 3(4), e1997. doi: 10.1371/journal.pone.0001997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosier M, Le Barillier L, Meunier D, El Yacoubi M, Malleret G, & Salin PA (2018). Post-learning paradoxical sleep deprivation impairs reorganization of limbic and cortical networks associated with consolidation of remote contextual fear memory in mice. Sleep, 41(12). doi: 10.1093/sleep/zsy188 [DOI] [PubMed] [Google Scholar]
- Sajikumar S, & Korte M (2011). Different compartments of apical CA1 dendrites have different plasticity thresholds for expressing synaptic tagging and capture. Learning & memory, 18(5), 327–331. doi: 10.1101/lm.2095811 [DOI] [PubMed] [Google Scholar]
- Shi HS, Luo YX, Xue YX, Wu P, Zhu WL, Ding ZB, & Lu L (2011). Effects of sleep deprivation on retrieval and reconsolidation of morphine reward memory in rats. Pharmacol Biochem Behav, 98(2), 299–303. doi: 10.1016/j.pbb.2011.01.006 [DOI] [PubMed] [Google Scholar]
- Spano GM, Banningh SW, Marshall W, De Vivo L, Bellesi M, Loschky SS, … Cirelli C (2019). Short sleep deprivation increases synapse density and axon-spine interface in the hippocampal CA1 region of adolescent mice. Journal of Neuroscience, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N, & McBain CJ (2006). Structural and Functional Properties of Hippocampal Neurons In Andersen P, Morris R, Amaral DG, Bliss TV, & O’Keefe J (Eds.), The Hippocampus Book (pp. 133–201): Oxford Scholarship Online. [Google Scholar]
- Steward O (1976). Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol, 167(3), 285–314. doi: 10.1002/cne.901670303 [DOI] [PubMed] [Google Scholar]
- Stickgold R (2005). Sleep-dependent memory consolidation. Nature, 437(7063), 1272–1278. doi: 10.1038/nature04286 [DOI] [PubMed] [Google Scholar]
- Stickgold R, Hobson JA, Fosse R, & Fosse M (2001). Sleep, learning, and dreams: off-line memory reprocessing. Science, 294(5544), 1052–1057. doi: 10.1126/science.1063530 [DOI] [PubMed] [Google Scholar]
- Tononi G, & Cirelli C (2006). Sleep function and synaptic homeostasis. Sleep Med Rev, 10(1), 49–62. doi: 10.1016/j.smrv.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Vago DR, Bevan A, & Kesner RP (2007). The role of the direct perforant path input to the CA1 subregion of the dorsal hippocampus in memory retention and retrieval. Hippocampus, 17(10), 977–987. doi: 10.1002/hipo.20329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vago DR, & Kesner RP (2008). Disruption of the direct perforant path input to the CA1 subregion of the dorsal hippocampus interferes with spatial working memory and novelty detection. Behav Brain Res, 189(2), 273–283. doi: 10.1016/j.bbr.2008.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Hallschmid M, Rasch B, & Born J (2006). Brief sleep after learning keeps emotional memories alive for years. Biol Psychiatry, 60(7), 788–790. doi: 10.1016/j.biopsych.2006.03.061 [DOI] [PubMed] [Google Scholar]
- Witter MP, Griffioen AW, Jorritsma-Byham B, & Krijnen JL (1988). Entorhinal projections to the hippocampal CA1 region in the rat: an underestimated pathway. Neurosci Lett, 85(2), 193–198. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3374835 [DOI] [PubMed] [Google Scholar]


