Abstract
Objectives:
The main objective of the study was to determine the prevalence of venous thromboembolism events in patients infected with severe acute respiratory syndrome coronavirus 2 requiring venovenous extracorporeal membrane oxygenation. The secondary objective was to compare venous thromboembolism events and coagulation variables in patients requiring venovenous extracorporeal membrane oxygenation according to the pathogen.
Design:
Retrospective observational analysis at a single center.
Setting:
Tertiary referral university teaching hospital.
Patients:
Patients with severe acute respiratory syndrome coronavirus 2-related severe acute respiratory distress syndrome requiring venovenous extracorporeal membrane oxygenation therapy with an injected CT scan performed after extracorporeal membrane oxygenation retrieval.
Interventions:
None.
Measurements and Main Results:
We included 13 severe acute respiratory syndrome coronavirus 2 patients requiring venovenous extracorporeal membrane oxygenation. All of these patients experienced venous thromboembolism: 10 patients (76.9%) had isolated cannula-associated deep vein thrombosis, two patients (15.4%) had isolated pulmonary embolism, and one patient (7.7%) had both cannula-associated deep vein thrombosis and pulmonary embolism. Eleven patients (84.6%) had cannula-associated deep vein thrombosis. A jugular associated cannula-associated deep vein thrombosis was identified in seven patients (53.8%), a femoral associated cannula-associated deep vein thrombosis was identified in 10 patients (76.9%), and six patients (46.2%) had both femoral and jugular cannula-associated deep vein thrombosis. A pulmonary embolism was found in three patients (23.1%). No patient had central venous catheter-related deep vein thrombosis. One patient had thrombotic occlusion of the centrifugal pump, and one had oxygenator thrombosis requiring circuit replacement. Three patients (23.1%) had significant bleeding. Three patients (23.1%) had laboratory-confirmed heparin-induced thrombocytopenia, and all of them developed cannula-associated deep vein thrombosis. These three patients had femoral cannula-associated deep vein thrombosis, and two had an oxygenator or pump thrombosis. The mean activated partial thromboplastin time ratio was higher in the severe acute respiratory syndrome coronavirus 2 group than in the influenza group and the community-acquired pneumonia group (1.91 vs 1.48 vs 1.53; p = 0.001), which was also found in regard to the percentage of patients with an activated partial thromboplastin time ratio greater than 1.8 (47.8% vs 20% vs 20.9%; p = 0.003) and the mean prothrombin ratio (86.3 vs 61.6 vs 67.1; p = 0.003). There was no difference in baseline characteristics or venous thromboembolism events.
Conclusions:
We report a 100% occurrence of venous thromboembolism in critically ill patients supported by venovenous extracorporeal membrane oxygenation for severe acute respiratory syndrome coronavirus 2-related acute respiratory distress syndrome using CT scan imaging despite a high target and close monitoring of anticoagulation.
Keywords: acute respiratory distress syndrome, cannula, deep vein thrombosis, extracorporeal membrane oxygenation, severe acute respiratory syndrome coronavirus 2
Venovenous extracorporeal membrane oxygenation (ECMO) is a validated therapeutic option for very severe acute respiratory distress syndrome (ARDS) that does not respond to a lung protective ventilation strategy in the prone position (1).
The global coronavirus disease pandemic has led to numerous admissions to the ICU for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with very high mortality among patients requiring invasive mechanical ventilation (2).
SARS-CoV-2 induces coagulation disorders resulting from a hyperinflammatory state (“cytokine storm”) (3), leading to a high incidence of thromboembolism events, especially in intensive care patients (4), despite the use of anticoagulant therapy (5, 6). Therefore, close monitoring of anticoagulation with a higher target than usual, especially in patients with risk factors for thrombosis and/or elevated d-dimer, is suggested (4). During venovenous ECMO, the occurrence of venous thromboembolism has been studied, with an incidence of up to 85% of cases (7). In SARS-CoV-2 patients requiring venovenous ECMO, the prevalence of venous thromboembolism events has only been investigated using ultrasonography.
Thus, we hypothesized that SARS-CoV-2-related ARDS patients requiring venovenous ECMO are at very high risk of venous thromboembolism. The main objective of the study was to determine the prevalence of venous thromboembolism events in SARS-CoV-2 patients requiring venovenous ECMO using a CT scan for diagnosis. The secondary objective was to compare venous thromboembolism events in patients supported by venovenous ECMO according to the pathogen involved.
MATERIALS AND METHODS
We conducted a retrospective study in a medical ICU of a teaching university hospital in Marseille, France. All patients admitted for SARS-CoV-2-related ARDS requiring venovenous ECMO therapy with an injected CT scan performed after ECMO weaning were included. According to French ethics laws and our Institutional Review Board, the requirement for informed consent was waived.
A thoraco-abdominopelvic CT scan with iodinated contrast injection is routinely performed after venovenous ECMO retrieval in our center.
Anticoagulation practice was protocolized and was not modified throughout the study period. Unfractionated heparin (UFH) was used for anticoagulation. Due to prothrombotic evidence in severe SARS-CoV-2 patients, a therapeutic UFH dose was administered using anti-factor Xa for monitoring with a range between 0.3 and 0.6 international units (IU)/mL. Heparin-induced thrombocytopenia (HIT) was systematically investigated in cases of thrombocytopenia less than 100 g/L or a reduction of greater than 50% from the patient’s baseline platelet count using heparin-platelet factor 4 antibody testing associated with the platelet aggregation test. If positive, UFH was stopped and substituted for argatroban.
The intensity of anticoagulation was assessed using anti-factor Xa and the activated partial thromboplastin time (APTT) ratio. The mean anti-factor Xa was calculated as the mean anti-factor Xa value of all anti-factor Xa measured during the venovenous ECMO period. The same calculation was performed to assess the platelet count, fibrinogen level, APTT ratio, and prothrombin ratio. Furthermore, we calculated the percentage of platelet counts lower than 100 g/L, defined as the number of platelet counts lower than 100 g/L divided by the total number of platelet counts performed times 100. The same operation was performed for fibrinogen level (threshold ≥ 6 g/L) and APTT ratio (threshold > 1.8) (8). The APTT ratio and prothrombin ratio were determined using a normal control plasma value for the denominator.
For comparison with SARS-CoV-2 patients, we extracted data from a group with 10 influenza patients and a group with 24 bacterial community-acquired pneumonia (CAP) patients from a database of a previous study on venous thromboembolism events after venovenous ECMO (8). All of these patients underwent femoro-jugular cannulation.
First, we reported the prevalence of venous thromboembolism in patients with SARS-CoV-2-related ARDS. Second, we compared venous thromboembolism events and coagulation variables during venovenous ECMO in SARS-CoV-2 patients and non-SARS-CoV-2-related ARDS patients, including influenza patients and bacterial CAP patients.
Descriptive statistics included percentages for categorical variables and the median (interquartile range) for continuous variables. Comparisons between the three categories (SARS-CoV-2 group, influenza group, and bacterial CAP group) for continuous variables were performed using the Kruskal-Wallis test with a post hoc method for multiple comparisons (step-up Simes method to calculate adjusted p value). Comparisons between the three categories (SARS-CoV-2 group, influenza group, and CAP group) for categorical variables were performed using the Pearson chi-square test for trend.
RESULTS
Between March 18, 2020, and May 5, 2020, 14 patients were admitted for SARS-CoV-2-related ARDS requiring ECMO support. One patient required venoarterial ECMO for acute cor pulmonale due to massive pulmonary embolism, and the 13 remaining patients required venovenous ECMO for refractory hypoxemia. All of these patients were weaned off ECMO support. All venovenous ECMO patients were included in the analysis.
All patients were in shock requiring norepinephrine at a dose greater than 1 µg/kg/min during the venovenous ECMO period. Femoro-jugular cannulation was performed for all patients. CT scans were performed a median of 1 day (0–3 d) after decannulation occurred. On May 5, seven patients (53.1%) were weaned from mechanical ventilation and the median duration of mechanical ventilation was 24 days (18–29.5 d).
One-hundred percent of SARS-CoV-2 patients supported by venovenous ECMO experienced venous thromboembolism: 10 patients had isolated cannula-associated deep vein thrombosis (CaDVT), two patients had isolated pulmonary embolism, and one patient had both CaDVT and pulmonary embolism. The venous thromboembolism characteristics of the 13 patients supported by venovenous ECMO are presented in Table 1. Eleven patients (84.6%) had CaDVT. A pulmonary embolism was found in three patients (23.1%) including one patient with femoral and jugular CaDVT and two with an isolated pulmonary embolism (Table 1). No patient had central venous catheter-related deep vein thrombosis. One patient had thrombotic occlusion of the centrifugal pump, and one had oxygenator thrombosis. Five patients (38.5%) had hemolysis, and four required ECMO circuit replacement. Three patients (23.1%) had significant bleeding (one epistaxis, one subarachnoid hemorrhage, and one gynecological bleeding), and one required RBC transfusion. The mean APTT ratios were 1.91, 1.50, and 1.76, and anti-factor Xa levels were 0.41, 0.39, and 0.72 IU/mL.
Table 1.
Characteristics of Venous Thromboembolism and Anticoagulation in Severe Acute Respiratory Syndrome Coronavirus 2 Patients
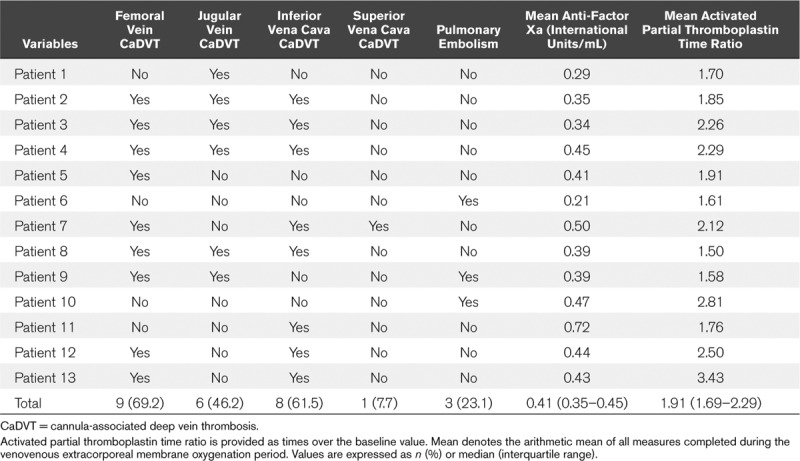
One patient had a moderate antithrombin III deficiency requiring human antithrombin III concentrate administration. The median level of antithrombin III in the cohort was 81% (66.5–91.9%). A HIT test was performed in eight patients (61.5%) and three patients (23.1%) had a laboratory-confirmed HIT. These three patients had femoral CaDVT, and two had oxygenator or pump thrombosis.
The comparison between groups according to the pathogen is presented in Table 2. The mean APTT ratio, the percentage of APTT ratio greater than 1.8 and the mean prothrombin ratio were higher in the SARS-CoV-2 group than in the influenza group and CAP group. There was no difference considering baseline characteristics or venous thromboembolism events.
DISCUSSION
We found that venous thromboembolism was systematically found in SARS-CoV-2-related ARDS patients requiring venovenous ECMO. One-hundred percent of SARS-CoV-2 patients supported by venovenous ECMO experienced venous thromboembolism: 10 patients (76.9%) had isolated CaDVT, two patients (15.4%) had isolated pulmonary embolism, and one patient (7.7%) had both CaDVT and pulmonary embolism. Prothrombotic activity in SARS-CoV-2 patients is increased and leads to a higher venous thromboembolism rate in ICU patients despite venous thromboembolism prophylaxis (5, 6). In our cohort, 100% of the patients developed venous thromboembolism despite relatively high and close monitoring of anticoagulation during ECMO support. Abnormal coagulation tests are common in SARS-CoV-2 patients due to the presence of lupus anticoagulant and coagulation factor deficiency (9), which may induce a spontaneous prolonged APTT and interfere with UFH monitoring. Therein, therapeutic anticoagulation was monitored using anti-factor Xa in our cohort and confirmed therapeutic use of UFH without reduction in thromboembolism events. However, three patients (23.1%) developed an HIT, suggesting a high incidence of HIT in SARS-CoV-2 patients, but the finding was not statistically significant (Table 2); this finding awaits confirmation in a larger series.
Table 2.
Comparison of Coronavirus Disease 2019 Patients With Influenza Pneumonia Patients and Bacterial Community-Acquired Pneumonia Patients
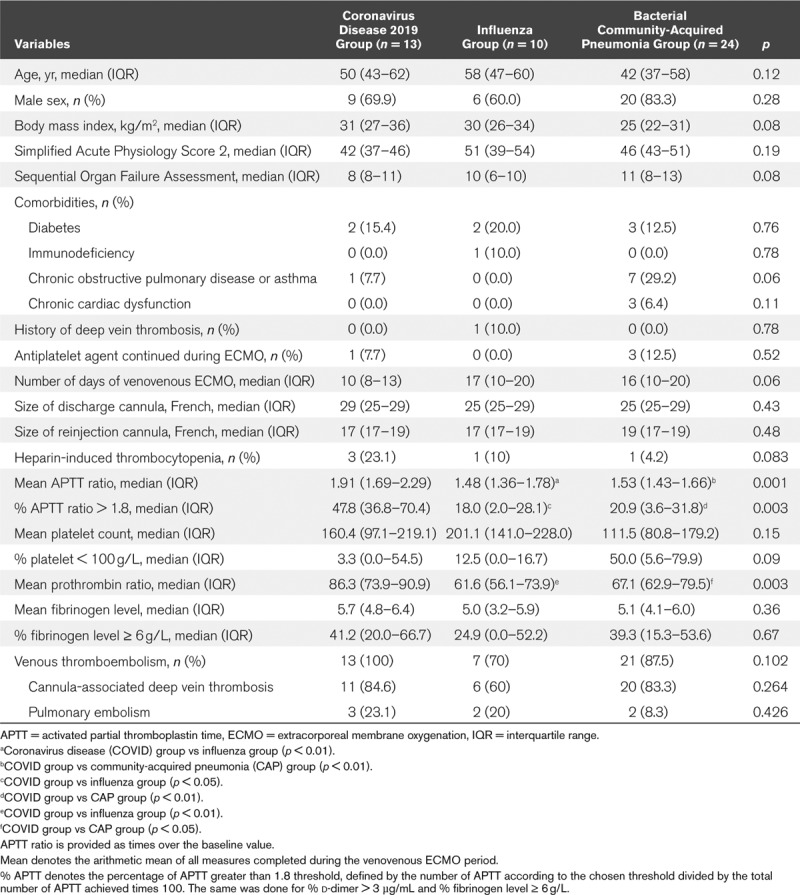
The incidence of CaDVT varies between 18.1% and 85.4% according to diagnostic methods (7, 10, 11), and recently we have reported a prevalence of 71.4% when using exclusively a CT scan for diagnosis (8) which is consistent with the present results. When compared with the influenza group, we found a higher prevalence of CaDVT in the SARS-CoV-2 group, but the difference was not statistically significant, probably due to a lack of power (84.6% vs 60%, respectively). Two recent cohorts of 12 SARS-CoV-2 patients requiring venovenous ECMO reported three cases of oxygenator thrombosis, and in the second study, a cannula thrombosis in two patients and a CaDVT in five patients using ultrasonography for diagnosis (4, 11). In our study, oxygenator or pump thrombosis occurred only in patients with HIT. We found a higher prevalence of thromboembolism events in our study, which may be explained by the use of CT scans for diagnosis because of the higher performance of CT scans for the identification of pelvic, superior and inferior vena cava, and pulmonary artery thrombosis.
Furthermore, three patients had significant bleeding highlighting the precarious balance between thrombotic risk and hemorrhagic risk in this population of patients.
The nonsignificant findings are likely due to the small sample size of this study and its retrospective nature with inherent risk of bias. CT scan delay may have caused thrombus migration and nonevaluation of distal veins of the limbs to be missed, which could have underestimated the prevalence of venous thromboembolism. Larger series are needed to confirm these findings.
CONCLUSIONS
We report a 100% occurrence of venous thromboembolism in critically ill patients supported by venovenous ECMO for SARS-CoV-2-related ARDS using CT scan imaging despite a high target and close monitoring of anticoagulation. Our results emphasize that coagulation and thrombosis are at the interplay between SARS-CoV-2 and ECMO. Although venovenous ECMO is potentially lifesaving, clinicians must be aware of such complications that require very high attention for thrombosis prevention and diagnosis in SARS-CoV-2 patients.
ACKNOWLEDGMENTS
This article was edited for proper English language, grammar, punctuation, spelling, and overall style by American Journal Expert.
Footnotes
Dr. Guervilly received funding from Xenios Fresenius Medical Care. Dr. Papazian’s institution received funding from Sedana (grant for a study), and he received funding from Lowenstein and Hamilton. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Abrams D, Ferguson ND, Brochard L, et al. ECMO for ARDS: From salvage to standard of care? Lancet Respir Med 2019; 7:108–110 [DOI] [PubMed] [Google Scholar]
- 2.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323:2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGonagle D, O’Donnell JS, Sharif K, et al. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30121-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helms J, Tacquard C, Severac F, et al. ; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med 2020; 46:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikdeli B, Madhavan MV, Jimenez D, et al. ; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-Up: JACC state-of-the-art review. J Am Coll Cardiol 2020; 75:2950–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llitjos J-F, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020 doi: 10.1111/jth.14869. Apr 22. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menaker J, Tabatabai A, Rector R, et al. Incidence of cannula-associated deep vein thrombosis after veno-venous extracorporeal membrane oxygenation. ASAIO J 2017; 63:588–591 [DOI] [PubMed] [Google Scholar]
- 8.Parzy G, Daviet F, Persico N, et al. Prevalence and risk factors for thrombotic complications following venovenous extracorporeal membrane oxygenation: A CT scan study. Crit Care Med 2020; 48:192–199 [DOI] [PubMed] [Google Scholar]
- 9.Bowles L, Platton S, Yartey N, et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMc2013656. May 5. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper E, Burns J, Retter A, et al. Prevalence of venous thrombosis following venovenous extracorporeal membrane oxygenation in patients with severe respiratory failure. Crit Care Med 2015; 43:e581–e584 [DOI] [PubMed] [Google Scholar]
- 11.Beyls C, Huette P, Arab OA, et al. Extracorporeal membrane oxygenation for COVID-19-associated severe acute respiratory distress syndrome and risk of thrombosis. Br J Anaesth. 2020 doi: 10.1016/j.bja.2020.04.079. May 4. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


