Abstract
Secretion of cell contents through extracellular vesicles (EVs), such as exosomes and microvesicles, is a fundamental cell behavior. Compared with their normal counterparts, cancer cells are different in the amount and composition of EVs they secrete as a result of intrinsic and extrinsic (microenvironmental) alterations. Although EVs were originally recognized as a means to remove undesired cell components, recent studies show their critical role in mediating intercellular interaction through cargo transport. In cancer, EVs can be transferred between different cancer cell subpopulations and between cancer and normal cells localized inside and outside of the tumor. By regulating various aspects of cellular functions, EVs contribute to tumor heterogeneity and plasticity, vascular remodeling, cancer–niche coevolution, immunomodulation, and establishment of premetastatic niche, all of which are important to the process of metastasis. Recent discoveries on EV-mediated mechanisms lead to a new understanding of the multifaceted changes in tumor and nontumor tissues before and after cancer metastasis, paving the way for new therapeutic strategies.
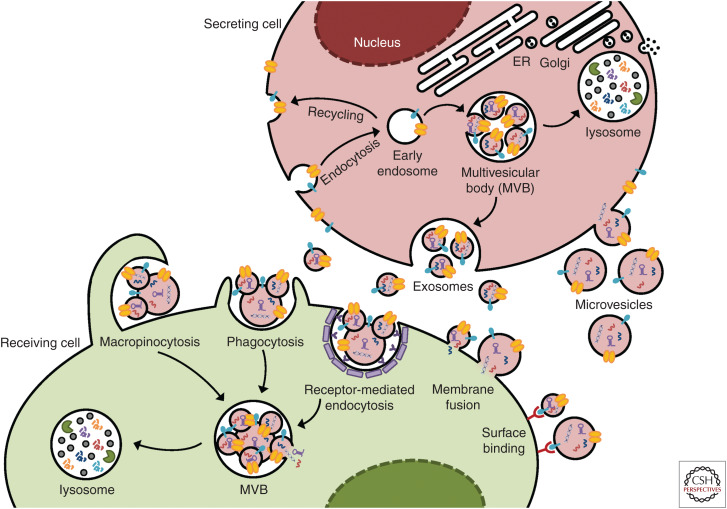
Metastasis is a multistep task that engages multiple cell types at various organs. This requires accurate and dynamic intercellular communication to orchestrate different cellular behaviors. In addition to the long-studied role of cancer- and host-derived cytokines in this process, cell-secreted membrane-enclosed structures, known as extracellular vesicles (EVs), have been recognized in the past decade for their unique function in mediating short-range and long-range crosstalk between different cell types at various stages of metastasis (Zhang and Wang 2015; Becker et al. 2016; Tkach and Théry 2016). EVs represent a heterogeneous population of secreted vesicles that include exosomes, microvesicles, and those secreted by certain types of cells or under certain conditions such as large oncosomes produced by some cancer cells (Minciacchi et al. 2015) and apoptotic bodies generated during cell apoptosis. Exosomes (not to be confused with exosome complex, an RNase-containing multiprotein intracellular complex responsible for RNA degradation), often with a characteristic small size of 30–100 nm, originate from the endosomal system, forming in the lumen of an intermediate endocytic compartment called multivesicular bodies (MVBs) through membrane invagination and secreted on fusion of MVBs with the plasma membrane. In contrast, microvesicles as well as large oncosomes and apoptotic bodies are shed from the plasma membrane regions that bulge outward, with a typical size ranging from 50 to 500 nm, but sometimes can reach 1–10 µm (especially for the case of large oncosomes). Advanced analytical tools, such as asymmetric flow field-flow fractionation, enable further categorization of EVs and discovery of an abundant population of cell-secreted nonmembranous nanoparticles (∼35 nm) named “exomeres” (Zhang et al. 2018). The effect of EVs on a target cell may be triggered by molecular interactions at the interfaces of EVs and cell membrane or start with fusion of EV membrane to cell membrane to release EV contents, or may require internalization of EVs through endocytosis, phagocytosis, or macropinocytosis and subsequent relocalization of EV cargo molecules into their functioning compartments inside of the recipient cell (Fig. 1).
Figure 1.
Biogenesis and receipt of extracellular vesicles (EVs). Exosomes are generated from an early endosome, which undergoes subsequent membrane inward invagination and captures a variety of cytosolic components including DNA, RNA, and proteins to form exosomes within the lumen. The resultant multivesicular bodies (MVBs) may fuse with the plasma membrane to release the exosomes, or turn into a lysosome for content degradation. In contrast, microvesicles are generated through outward budding of the plasma membrane. On receipt by a target cell, the possible fates of EVs include binding to cell surface to trigger intracellular signaling, fusing to the plasma membrane to release EV contents, and internalization through endocytosis, phagocytosis, or macropinocytosis, to eventually release EV contents into the cytoplasm.
Although exosomes and microvesicles are generated through distinct routes and therefore may display different biophysical properties, molecular composition, targeting specificity, and physiological functions, it is difficult to completely differentiate the two subtypes of EVs because of their overlapping size and similar appearance. Exosomes are often enriched from cell culture supernatants and biological fluids by differential ultracentrifugation with a final spin at 110,000g for 70 min to pellet exosomes. However, this procedure may also retain larger vesicles such as microvesicles, even though some of them could be pelleted by a 10,000–20,000g spin before exosome collection. Further purification can be achieved by buoyant density centrifugation to separate vesicles by their differences in density, or by immunoisolation using exosome surface markers such as the tetraspanins CD81 and CD63. However, it has been noted that some exosome subpopulations lack these markers, and that some microvesicles also carry tetraspanins captured from the plasma membrane (Kowal et al. 2016). As a result, many studies experiment on a mixed group of EVs containing both exosomes and microvesicles. This review follows the definition of vesicles in cited research articles for consistency.
Secretion of EVs is an evolutionarily conserved biological process found from bacteria to humans (Deatherage and Cookson 2012; van Niel et al. 2018). Unlike hormones and neurotransmitters released by specialized cells via secretory vesicles, EVs can be secreted by all cells in a higher organism (van Niel et al. 2018). EVs were recognized in the 1980s as a means to transfer functional enzymes (Trams et al. 1981) or to remove unwanted proteins during reticulocyte maturation (Harding et al. 1983; Pan and Johnstone 1983; Johnstone et al. 1987). Subsequent study discovers a role of B-cell–derived EVs in antigen presentation and T-cell stimulation (Raposo et al. 1996). Seminal works published in the 2000s clearly show that EVs function as vehicles allowing cells to exchange their components such as DNA, RNA, proteins, and lipids, leading to an explosion of interest in EV-mediated functions in multiple disciplines including cancer biology (Théry et al. 2002; Valadi et al. 2007; Skog et al. 2008; El Andaloussi et al. 2013; Villarroya-Beltri et al. 2014; Jeppesen et al. 2019). EVs derived from cancer and noncancer cells play roles during all stages of cancer including tumor initiation, progression, and evolution (autonomous or in response to therapy), as well as preparation and development of metastases. They mediate an important layer of the complex interplay between cancer and host through modulating the immune system, vasculature, parenchymal, and stromal cells inside and outside the tumor, and cells comprising a premetastatic niche (Kaplan et al. 2006; Psaila and Lyden 2009).
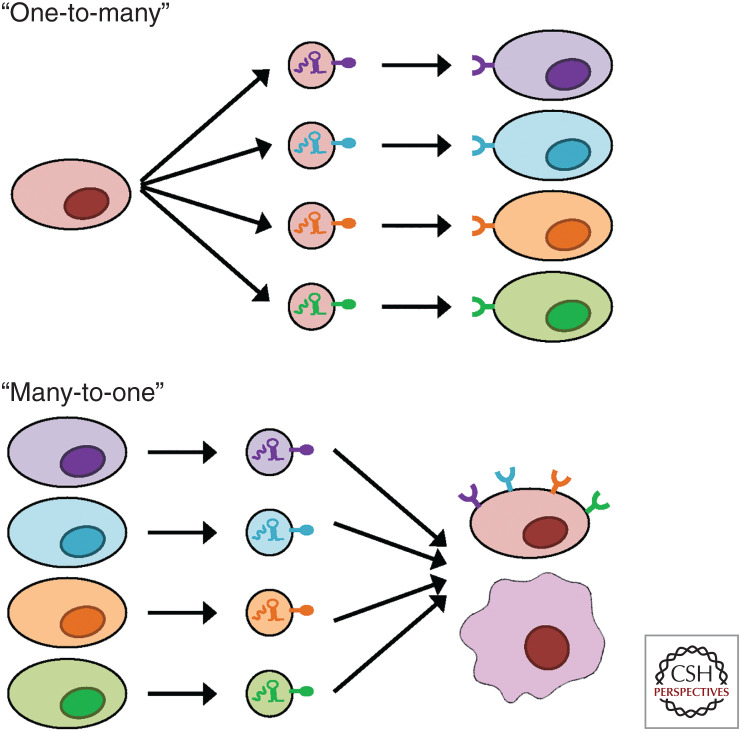
As an increasingly accepted basic mode of intercellular communication, EVs can trigger autocrine, paracrine, and endocrine signaling without the need of direct cell-to-cell contact. Compared with the secretion of singular or complexed biomolecules such as cytokines and hormones, EVs are unique in their capacity to simultaneously transfer a broad collection of bioactive molecules of different categories to more comprehensively represent the identity and physiological state of their producing cells. On their receipt by target cells, which could be based on recognition of certain molecules on EVs by the target cell surface, the various cargo molecules in EVs may exert additive, synergistic, or multifaceted effects to achieve robust and precise regulation of target cell behaviors. The “bulk delivery” mode of EVs is associated with their highly heterogeneous nature, as different groups of cargo molecules can be selectively packed into differently addressed EVs for targeted delivery to specific recipient cells (Chin and Wang 2016). This would allow one-to-many as well as many-to-one signaling between cells, while maintaining the specificity of the messages to be sent to different recipients (Fig. 2).
Figure 2.
The one-to-many and many-to-one intercellular communication mediated by extracellular vesicles (EVs). A secreting cell can generate different subpopulations of EVs with different contents, which target their corresponding groups of target cells through recognition of the EVs by the recipient cell surface (the one-to-many communication). On the other hand, EVs secreted by different types of cells and carrying different contents can target the same recipient cell through receptor-dependent or -independent interaction (the many-to-one communication). In all cases, the bulk delivery mode of EVs allows simultaneous transfer of groups of signaling molecules for precise communication.
Here, I try to summarize the profound impact of EV-mediated intercellular communication on multiple aspects underlying cancer metastasis with an emphasis on recent discoveries.
Altered EV Secretion Pattern in Cancer
Compared with their normal counterparts, some cancer cells secrete higher amounts of exosomes (Riches et al. 2014), and cancer patients show higher levels of circulating exosomes compared with healthy individuals (Taylor and Gercel-Taylor 2008; Rabinowits et al. 2009; Melo et al. 2014). Both oncogenic signaling intrinsic to cancer cells and the unique conditions in a tumor microenvironment could contribute to enhanced EV secretion. Cancer cells frequently show overexpression of proteins critical for EV biogenesis. For example, small GTPases Rab27a and Rab27b, which control different steps in exosome secretion (Ostrowski et al. 2010), are overexpressed in breast cancer cells with Rab27b's level associated with lymph node metastasis and poorer survival (Bobrie et al. 2012; Zhang et al. 2012). Inhibition of Rab27a in mammary carcinoma cells reduces exosome secretion, and decreases lung metastases and systemic accumulation of tumor-promoting neutrophils (Bobrie et al. 2012). Interestingly, expression of Rab27b is positively correlated with mesenchymal markers indicating epithelial–mesenchymal transition (EMT) (Zhang et al. 2012), and cancer cells undergone EMT indeed show increased EV secretion (Garnier et al. 2012). Neutral sphyngomyelinase 2 (nSMase2), an enzyme involved in ceramide biosynthesis, is overexpressed in cancer cells and is required for exosome secretion as well as tumor angiogenesis and metastasis (Kosaka et al. 2013). Cancer cells with increased heparanase expression or exposed to exogenous heparanase show increased exosome secretion and higher exosomal levels of syndecan-1, vascular endothelial growth factor (VEGF), and hepatocyte growth factor (HGF) to promote metastasis (Thompson et al. 2013).
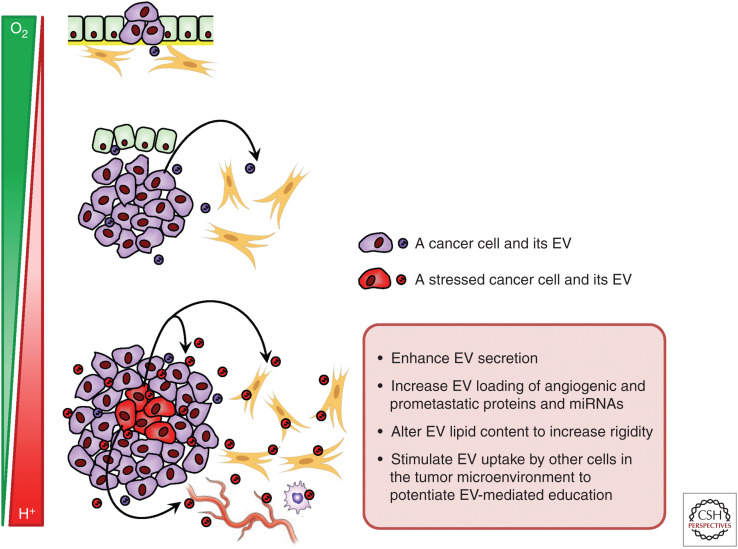
Common metabolic stresses in the tumor microenvironment, such as hypoxia and acidity, have been shown to enhance EV secretion and may also alter the content of EVs. In breast cancer cells, hypoxia induces exosome release and increases the level of hypoxically regulated miR-210 in exosomes (King et al. 2012). The hypoxia-inducible factors (HIFs) induce the expression of Rab22a, which colocalizes with budding microvesicles, to stimulate shedding of microvesicles and their consequent prometastatic effects (Wang et al. 2014). An acidic tumor microenvironment, partially owing to enhanced glycolysis of cancer cells, increases both the release and uptake of exosomes compared with a neutral pH, possibly because of enhanced rigidity and sphingomyelin/ganglioside GM3 content in exosomes released at low pH (Parolini et al. 2009). Cancer-derived exosomes released at low pH also contain high levels of caveolin-1, which has been associated with tumor progression and metastasis in late-stage cancers. Since the intracellular levels of many proteins, RNA, and metabolites are regulated by the dynamic environmental cues such as levels of oxygen and nutrients, it is expected that the content of EVs may show corresponding alterations to reflect the metabolic state of EV-producing cells in a solid tumor. This adds another layer of complexity to the dynamic and heterogeneous composition of a tumor (Fig. 3).
Figure 3.
Effect of metabolic stresses on extracellular vesicle (EV)-mediated communication in a solid tumor. As a tumor grows in size, metabolic stresses, such as hypoxia and acidity, are frequently experienced in some tumor areas, and can influence EV secretion, composition, and uptake in the tumor microenvironment to promote angiogenesis and metastasis. The spatiotemporal metabolic patterns in a tumor thus contribute to EV heterogeneity.
Oncogenic cell transformation also alters the composition of EVs, contributing to the functional differences between cancer- and normal-cell–derived EVs. Many studies have shown that the microRNA (miRNA) profiles of EVs are distinct from their corresponding cellular profiles, and that some miRNAs retained inside normal cells can become highly secreted in malignant cells, suggesting cancer-specific mechanisms for selective miRNA sorting into EVs (Pigati et al. 2010; Cha et al. 2015; Fong et al. 2015). EVs derived from cancer cells are more heterogeneous in appearances including size and density. Different miRNAs can be sorted into different subpopulations of cancer EVs (Palma et al. 2012). Exosomes derived from metastatic breast cancer cells show an enrichment in miRNAs compared with those from nonmetastatic breast cancer cells (Melo et al. 2014). EVs from breast cancer cells and the blood of patients, but not from normal controls, contain the RNA induced silencing complex (RISC)-loading proteins Dicer, TRBP, and AGO2 through CD43-dependent protein transport, and therefore display cell-independent capacity to process pre-miRNAs into mature miRNAs (Melo et al. 2014). Colorectal cancer cells carrying mutant KRAS show a different specificity in exosomal RNA secretion compared with cells with wild-type KRAS, with increased miR-100 and Rab13 mRNA and decreased miR-10b secretion suggesting KRAS-dependent RNA export (Cha et al. 2015; Hinger et al. 2018). This may contribute to the maintenance of a desired intracellular RNA level in the corresponding cellular context; for instance, miR-100 functions as a suppressor of multiple metastatic traits and its expression is decreased in metastatic cancers (Cha et al. 2015). Exosomes from mutant KRAS cells also contain unique oncogenic proteins such as KRAS (mutant), epidermal growth factor receptor (EGFR), and SRC, which can be transferred to cancer cells with wild-type KRAS to promote their malignancy (Demory Beckler et al. 2013). Transformation of epithelial cells with oncogenic HRAS stimulates generation of exosomes enriched in several proteases and integrins implicated in modifications of the tumor microenvironment and metastatic progression (Tauro et al. 2013). These findings clearly show the distinct composition and function of cancer-derived EVs, and support the potential use of increased amount of circulating, tumor-derived EVs and their characteristic cargo as biomarkers for cancer diagnosis and prognosis.
Transfer of EVs between Subpopulations of Cancer Cells
All solid tumors show intratumoral heterogeneity to a certain degree. This includes the divergent genetic and epigenetic profiles of cancer cells as a result of their genomic instability and continuous cell selection and evolution, as well as the heterogeneous composition of a tumor microenvironment, for example, the abundances of noncancer cell populations, blood vessels, various cytokines, nutrients and metabolic wastes, and therapeutic agents, etc. All these variable tumor-cell–intrinsic and –extrinsic factors could influence the secretion and content of EVs, as exemplified above. Thus, different subpopulations of cancer cells may communicate with each other through EV secretion and uptake as a paracrine mechanism to coordinate populational behaviors.
Through the effects of EVs, the signaling cancer cells could alter the phenotype of recipient cells in a way to simulate, at least partially, traits of the former. Cancer exosomes even enable nontumorigenic epithelial cells to form tumors (Melo et al. 2014). Exosomes from cancer cells with higher metastatic potentials are able to induce migration, invasion, EMT, and the metastatic capacity of less aggressive cancer cells, which has been associated with exosomal transfer of proteins (such as Hsp90α) and miRNAs (such as the miR-200 family and miR-10b) (McCready et al. 2010; O'Brien et al. 2013; Le et al. 2014; Singh et al. 2014; Harris et al. 2015). Glioblastoma cells expressing tumor-specific EGFRvIII secrete EVs that contain EGFRvIII mRNA and proteins, which can be transferred to cancer cells lacking this EGFR variant to promote aggressiveness (Al-Nedawi et al. 2008; Skog et al. 2008). Similarly, EVs from medulloblastoma cells with MYC amplification contain MYC DNA and RNA to enable horizontal gene transfer (Balaj et al. 2011). A majority of the studies show gain of a more aggressive phenotype by non- or less aggressive cancer cells on receipt of EVs from highly aggressive cancers. This may reflect a unique means for the tumor entity to evolve to a more aggressive stage, with only a subset of cancer cells that have acquired advantageous genetic alterations serving as leaders in evolution through horizontally spreading oncogenic signals to drive other cancer cells lacking such permanent alterations.
Another way for EVs derived from some cancer cells to directly influence themselves and other cancer cells is to serve as the “stepping stones” for directional cell movement. Detachment of cancer cells from the extracellular matrix (ECM) triggers rapid secretion of exosomes, which attach to the cell surfaces and mediate cellular adhesion and spreading (Koumangoye et al. 2011). Migrating cells secrete exosomes at invadopodia to facilitate cell invasion (Hoshino et al. 2013). Through integrin-dependent sorting, the ECM protein fibronectin is enriched in cancer exosomes to enable an autocrine/paracrine mechanism that promotes cancer cell adhesion and directionally persistent movement (Sung et al. 2015; Purushothaman et al. 2016). Together, these exemplified modes of EV-mediated interplay between different cancer cell subsets show a role of EV secretion in driving tumor cell evolution towards a more metastatic phenotype.
Mutual Adaptations between Cancer and Stromal Cells through Exchange of EVs
Stromal cells, especially fibroblasts, are frequent targets of cancer-secreted EVs. Exosomes from some cancer cells contain latent transforming growth factor (TGF)-β, which can trigger fibroblast differentiation into myofibroblasts and elevated FGF2 production (Webber et al. 2010). Exosomes from breast cancer cells induce differentiation of adipose tissue-derived mesenchymal stromal cells (MSCs) towards myofibroblasts, leading to increased secretion of SDF-1, VEGF, CCL5, and TGF-β to promote tumor growth, angiogenesis, and metastasis (Cho et al. 2012). Prostate cancer cell-derived large oncosomes carrying functional miRNA and AKT1 kinase can be detected in the circulation of metastatic prostate cancer patients, and can induce MYC activation and expression of α-SMA, IL-6, and MMP9 in normal prostate fibroblasts to promote tumor growth (Morello et al. 2013; Minciacchi et al. 2017). miR-105 encapsulated in EVs from metastatic breast cancer cells activates MYC signaling in cancer-associated fibroblasts (CAFs), conferring these cells the ability to detoxify metabolic wastes such as lactic acid and ammonium in the tumor microenvironment to support sustained tumor growth and progression (Yan et al. 2018).
Exosome transfer from stromal to cancer cells have also been well established. Cancer cells induce NOTCH-MYC signaling in stromal fibroblasts, leading to increased packing of unshielded RN7SL1 RNA in stromal exosomes (Nabet et al. 2017). This unique RNA component of stromal exosomes can then stimulate the pattern recognition receptor RIG-I to activate antiviral signaling in cancer cells, promoting cancer progression and resistance to therapy (Boelens et al. 2014). Fibroblast-secreted exosomes also promote the motility and metastasis of breast cancer cells through Wnt-planar cell polarity signaling (Luga et al. 2012). Prostate cancer-associated fibroblasts (CAFs) secrete exosomes that can suppress mitochondrial oxidative phosphorylation and induce hypoxia-like metabolic alterations in cancer cells. In addition, metabolites including amino acids, lipids, and tricarboxylic acid (TCA)-cycle intermediates have been found in CAF-derived exosomes, and can be used to fuel cancer cell metabolism to promote tumor growth (Zhao et al. 2016). Therefore, cancer- and stroma-derived EVs participate in the mutual adaptation and coevolution between cancer and stromal cells during tumor progression and metastasis.
Immunomodulation by Tumor-Derived EVs
Similar to the dual role of the immune system in preventing and supporting tumor progression, cancer-secreted EVs modulate various types of immune cells to activate or suppress their function. Tumors that are deficient in Hippo pathway secrete EVs containing a higher level of nucleic acids that induce a type I interferon response, leading to enhanced antitumor immunity and tumor destruction in immunocompetent mice (Moroishi et al. 2016). Exosomes derived from cancer cells, but not from dendritic cells or B cells, suppress natural killer (NK) cell function to promote tumor progression (Liu et al. 2006). Tumor-derived exosomes interact with B cells to trigger tumor-promoting humoral immunity; this can be blocked by subcapsular sinus macrophages in tumor-draining lymph nodes during lymphatic dissemination of exosomes (Pucci et al. 2016). Cancer patient-derived EVs containing Fas ligand induce apoptosis of activated T cells (Kim et al. 2005). CD39 and CD73 on cancer EVs suppress T cells through inducing adenosine production (Clayton et al. 2011). EVs produced by metastatic melanomas carry programmed death-ligand 1 (PD-L1), which can be increased by interferon-γ stimulation and in turn suppresses T-cell function to facilitate tumor growth. Thus, changes in the level of circulating exosomal PD-L1 during early stages of treatment are associated with response to anti-PD-1 therapy (Chen et al. 2018).
Myeloid cells are also influenced by tumor EVs. On uptake of cancer-derived EVs by bone marrow myeloid cells, EV-encapsulated prostaglandin E2 and TGF-β induce cell differentiation into myeloid-derived suppressor cells (MDSCs), which are enriched in primary tumors and lungs to promote tumor growth and metastasis (Xiang et al. 2009). In addition, tumor exosome-associated Hsp72 triggers Stat3 activation in MDSCs leading to immunosuppression (Chalmin et al. 2010). Melanoma exosomes home to sentinel lymph nodes to promote melanoma cell recruitment and metastasis (Hood et al. 2011). This is at least partially through exosomes’ effect to promote mixed M1 and M2 macrophage polarization (Bardi et al. 2018). Breast cancer-derived EVs contain high levels of protein palmitoylation, which can activate macrophages in axillary lymph nodes, lungs, and brain to induce inflammatory cytokines, such as IL-6 and TNF-α, potentially promoting metastasis (Chow et al. 2014). Exosomal transfer of miR-21 from neuroblastoma cells to monocytes and miR-155 from monocytes to neuroblastoma cells contribute to resistance to chemotherapy (Challagundla et al. 2015). Prostate cancer secretes exosomes containing the αvβ3 integrin, whose levels in the circulation are higher in prostate cancer patients compared with healthy controls (Krishn et al. 2019). These exosomes also carry the αvβ6 integrin, which can be transferred to peripheral blood mononuclear cells to mediate monocyte M2 polarization and promote prostate cancer progression (Lu et al. 2018). On the other hand, EVs secreted by activated macrophages induce the invasiveness of cancer cells by transferring miR-223, miR-21-5p, and miR-155-5p (Yang et al. 2011; Lan et al. 2019). Together, these findings establish the potential mechanistic links between EV-mediated immunomodulation and metastasis, and suggest the use of EVs as biomarkers and targets in cancer immunotherapy.
Cancer-Derived EVs Remodel Local and Distant Vasculature
Blood vessels play critical roles in metastasis as they not only supply circulating nutrients, cells, and factors to the tumor, but also allow dissemination of cancer cells and EVs. Most studies focus on the two nonconflicting effects of tumor EVs on blood vessels—angiogenesis and vascular leakiness—both contributing to metastasis. Tumor EVs contain higher levels of miR-9 to promote angiogenesis by activating the JAK-STAT pathway (Zhuang et al. 2012). EVs from glioblastomas are enriched in angiogenic proteins angiogenin and VEGF, and can stimulate endothelial tubule formation (Skog et al. 2008). Annexin II in breast cancer exosomes promotes angiogenesis and distant metastasis through macrophage-mediated secretion of IL-6 and TNF-α (Maji et al. 2017). Tetraspanin Tspan8/CO-029/D6.1A enriched in exosomes from pancreatic cancer cells induces systemic angiogenesis (Gesierich et al. 2006), possibly through Tspan8-mediated exosome recruitment of CD106 and CD49d to facilitate exosome uptake by endothelial cells and the subsequent induction of angiogenesis-related genes including von Willebrand factor as well as VEGF and its receptor (Nazarenko et al. 2010). EVs also mediate hypoxia-induced angiogenesis. Acute hypoxia significantly induces nSMase2 in vivo (Cogolludo et al. 2009), which enhances secretion of exosomal miR-210 to promote tumor angiogenesis and metastasis (Kosaka et al. 2013).
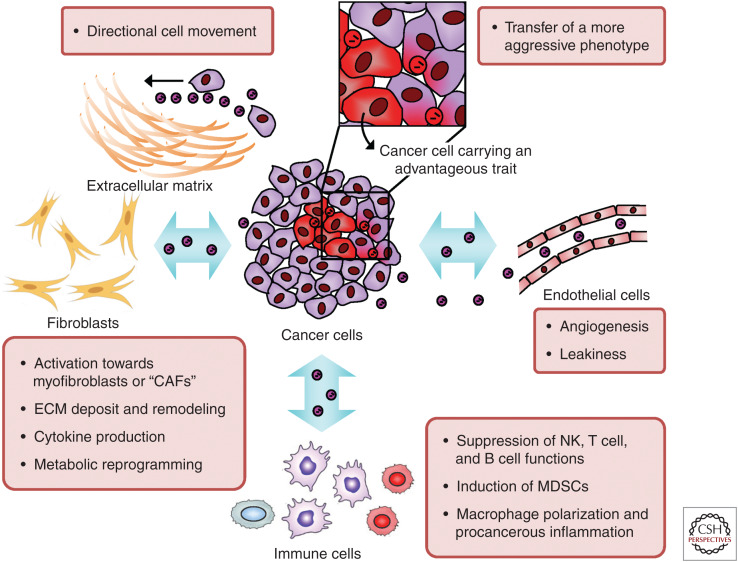
Tumor vasculatures are often immature and hyperpermeable (Weis and Cheresh 2011), and vascular destabilization is a critical characteristic of a premetastatic niche (Huang et al. 2009; Psaila and Lyden 2009). Melanoma-derived exosomes induce vascular leakiness and recruitment of bone marrow progenitor cells at premetastatic sites through induction of inflammation factors such as S100a8, S100a9, and tumor necrosis factor (TNF)-α (Peinado et al. 2012). Metastatic breast cancer cells secrete EV-associated miR-105 to down-regulate ZO-1 and tight junctions in endothelial monolayers at both the primary tumor site and premetastatic sites to increase vascular permeability. This potentially facilitates tumor cell intravasation and extravasation to enhance distant metastasis, and serum levels of miR-105 are associated with a subsequent metastatic event in early-stage breast cancer patients (Zhou et al. 2014). Brain-metastatic breast cancer cells secrete EV-associated miR-181c, which promotes the destruction of blood–brain barrier (BBB) by down-regulating PDPK1 to cause abnormal localization of actin, thereby facilitating brain metastasis (Tominaga et al. 2015). Therefore, cancer-derived EVs are extensively involved in vasculature remodeling both in the tumor and systemically. Figure 4 summarizes EV-mediated interactions between cancer and various niche cells in the primary tumor microenvironment.
Figure 4.
Cancer-derived extracellular vesicles (EVs) in the primary tumor microenvironment mediate tumor evolution and progression. Exchange of EVs within the tumor facilitates phenotypic transfer between different subpopulations of cancer cells to spread metastatic traits during tumor evolution. EVs carrying extracellular matrix (ECM) proteins can serve as the “stepping stones” for directional cell movement to promote cancer cell invasion. EVs secreted by cancer cells also influence other cell types including endothelial cells, immune cells, and fibroblasts, leading to coevolution of the tumor microenvironment and tumor progression. The various types of cells in the tumor microenvironment also secrete EVs to influence cancer cell behaviors.
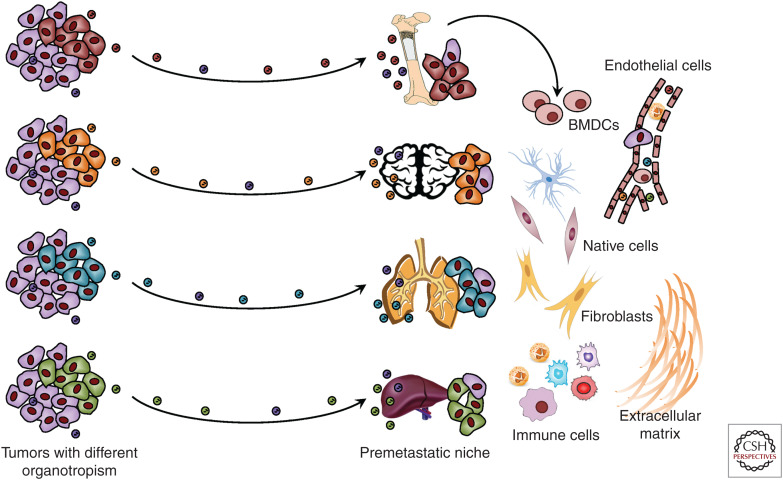
EVs Mediate Establishment of a Premetastatic Niche
A permissive metastatic environment, known as the premetastatic niche, is essential for the successful distant colonization of disseminated tumor cells. EVs from renal cell stem cells induce angiogenesis and promote the formation of lung premetastatic niche (Grange et al. 2011). Interestingly, cancer-derived exosomes inherit organotropism from their producing cancer cells, which is partially determined by the distinct integrin expression patterns on exosomal surface (Hoshino et al. 2015). Cancer exosomes from different origins preferentially target niche cells at different metastatic sites, including lung fibroblasts and epithelial cells, liver Kupffer cells, and brain endothelial cells, to promote organotropic metastasis; the exosomal integrin profiles can be used to predict organ-specific metastasis. The EV-conditioned premetastatic niche is determinant to organotropic metastasis, as treatment with exosomes from lung-tropic cancer cells redirects the metastasis of bone-tropic cancer cells to lungs (Hoshino et al. 2015). Systemic vascular leakiness, as discussed above, can further facilitate recruitment of circulating EVs and noncancer cells during premetastatic niche formation. Exosomes from metastatic melanomas contain oncoprotein MET, which permanently educates bone marrow progenitor cells towards a provasculogenic phenotype to enable their recruitment to lungs to form the premetastatic niche (Peinado et al. 2012). Pancreatic cancer-derived exosomes contain macrophage migration inhibitory factor, which induces TGF-β production on exosome uptake by Kupffer cells to stimulate hepatic stellate cells, leading to increased fibronectin production, recruitment of bone marrow-derived cells to the liver, and formation of a premetastatic niche (Costa-Silva et al. 2015).
In the bone marrow, cancer-derived EVs also reprogram mesenchymal stromal cells (BM-MSCs). miR-940 in prostate cancer-secreted exosomes promotes the osteogenic differentiation of MSCs to promote bone metastasis (Hashimoto et al. 2018). Neuroblastoma-derived EVs are captured by BM-MSCs and induce production of tumor-promoting factors including IL-6, IL-8, VEGF, and MCP-1, simulating an inflammation response (Nakata et al. 2017). Recent studies reveal a nuclear structure named spathasomes that deliver EV proteins and RNA to the nucleus of target cells, and blockade of nuclear translocation abolishes melanoma EV-induced transcriptomic changes in MSCs (Rappa et al. 2017; Santos et al. 2018). Studies are warranted to explore whether inhibition of the spathasome pathway results in suppression of the metastatic process. Exosomes from BM-MSCs, in turn, have been shown to induce quiescence in bone-metastatic breast cancer cells through miR-23b and miR-222/223 (Ono et al. 2014; Bliss et al. 2016).
Cells in premetastatic niche can be metabolically reprogrammed. Breast cancer cells secrete EV-associated miR-122, which reprograms lung fibroblasts and astrocytes to suppress glucose metabolism through down-regulation of pyruvate kinase. This can be detected before distant metastasis, and may save more extracellular glucose to fuel metastasized cancer cells (Fong et al. 2015). Cytotoxic chemotherapy has been shown to induce prometastatic effects, which could be partially mediated by EVs. Chemotherapy induces EV release by breast cancer cells, and chemotherapy-elicited EVs are enriched in annexin A6, which mediates endothelial cell activation and monocyte expansion in premetastatic lung niche to promote metastasis (Keklikoglou et al. 2019). Importantly, uptake of cancer-derived EVs by cells in premetastatic niche can be inhibited by cholesterol 25-hydroxylase (CH25H), and reserpine, an antihypertensive drug, may be used to pharmacologically block both premetastatic niche formation and metastasis (Ortiz et al. 2019). Melanoma-derived EVs suppress the expression of CH25H in recipient cells through down-regulation of type I interferon receptor, leading to increased uptake of cancer EVs and the consequent reprogramming in premetastatic lungs as well as metastasis (Ortiz et al. 2019). Last but not the least, EVs released by cells in a premetastatic niche can also influence metastasized cancer cells. In a metastatic brain niche, astrocytes secrete exosomes containing miR-17∼92, which silence PTEN expression in breast cancer cells metastasized to the brain. This, in turn, induces cancer cell secretion of CCL2 to recruit myeloid cells that can promote the proliferation and survival of metastasized cancer cells (Zhang et al. 2015). These findings initiate important research on factors regulating EV uptake and secretion by normal cells in premetastatic niches for novel therapeutic opportunities. Reported effects of tumor-derived EVs on premetastatic niche formation are summarized in Figure 5.
Figure 5.
Disseminated tumor-derived extracellular vesicles (EVs) promote formation of a premetastatic niche and distant metastasis. EVs can inherit the organotropism of their producing cancer cells and specifically target a distant organ to initiate premetastatic niche formation. Tumor-derived EVs recruit bone marrow-derived cells (BMDCs) to a premetastatic niche and influence other niche cells to create a permissive environment for cancer metastasis.
Clinical Implications
EVs are actively pursued as biomarkers, therapeutic targets, and drug delivery vehicles. Levels of total exosomes in the circulation are significantly higher in cancer patients compared with healthy controls and may increase along with cancer stage (Taylor and Gercel-Taylor 2008; Rabinowits et al. 2009; Melo et al. 2014). Because all cells in the body, in principle, can contribute to the load of EVs in blood, detection of cancer-specific EV cargo would provide improved specificity and sensitivity. Many studies including some discussed earlier have shown that the circulating levels of specific cancer EV-associated miRNAs or proteins can potentially differentiate cancer from noncancer controls, and between cancers with low or high metastatic potential. These blood-borne, EV-based biomarkers include but are not limited to: MET, TYRP2, VLA-4, and HSP70 associated with stage 3-4 melanoma (Peinado et al. 2012); miR-122 and miR-105 associated with breast cancer metastasis (Wu et al. 2012; Zhou et al. 2014); miR-181c associated with breast cancer metastasis to brain (Tominaga et al. 2015); miR-218 associated with breast cancer metastasis to bone (Liu et al. 2018); integrin β4 associated with lung metastasis (Hoshino et al. 2015); integrin αv associated with liver metastasis (Hoshino et al. 2015); MIF associated with pancreatic cancer metastasis to liver (Costa-Silva et al. 2015); and glypican-1 associated with early breast and pancreatic cancer as well as with pancreatic cancer burden (Melo et al. 2015). In addition, integrins α3 and β1 in urine exosomes are associated with prostate cancer metastasis (Bijnsdorp et al. 2013). A comparison between exosomes from isogenic bladder cancer cells reveals enrichment of EMT-associated proteins in EVs from metastatic cells compared with nonmetastatic cells (Jeppesen et al. 2014). Since it is unlikely that a universal EV biomarker can be identified for a certain type of cancer or metastasis, future efforts will focus on multiplex detection of a selected panel of EV RNA and proteins to achieve high sensitivity, specificity, and predictive capacity.
Pharmacologically blocking EV biogenesis is challenging because of the conserved physiological functions of EVs. However, a few strategies are pursued to hopefully target cancer-specific EV secretion. Heparanase, which is frequently up-regulated during tumor progression and can promote metastasis, is required for the high secretion and unique composition of cancer exosomes (Thompson et al. 2013). Syndecans, heparan sulphate proteoglycans shed by heparanase, control exosome formation through the ALIX endosomal sorting complexes required for transport (ESCRT) machinery (Baietti et al. 2012). Cancer-derived exosomes also depend on heparan sulfate proteoglycans expressed on the surface of recipient cells for their internalization (Christianson et al. 2013). Therefore, therapies targeting heparanase/syndecans, currently tested in clinical studies, may hold promise for blocking both the production and uptake of cancer EVs. In addition, inhibition of nSMase2 with GW4869 has been shown to suppress cancer exosome secretion and abolish exosome-mediated prometastatic effects (Kosaka et al. 2013). Blockade of normal cell uptake of cancer-derived EVs provides another possibility to prevent their systemic prometastatic effects. Recent success by using the antihypertensive drug reserpine shows a highly promising avenue towards this direction (Ortiz et al. 2019).
As naturally generated nanoparticles, EVs, especially exosomes, are engineered towards the use as a therapeutic tool because of their ability to overcome natural barriers such as BBB, the intrinsic cell targeting properties, and low-to-none immunogenicity when used autologously (Vader et al. 2016). Compared with synthetic nanoparticles, exosomes show enhanced retention in circulation, likely because of the CD47-mediated “don't eat me” signal protecting exosomes from phagocytosis (Kamerkar et al. 2017). Fibroblast-derived exosomes engineered to deliver therapeutic RNA targeting mutant KRAS efficiently suppress pancreatic tumor growth and metastasis (Kamerkar et al. 2017). Organ-specific targeting can be achieved by engineering exosomal surface proteins. Exosomes from dendritic cells engineered to express Lamp2b fused to a brain-specific peptide can be loaded with therapeutic RNA to specifically target brain cells (Alvarez-Erviti et al. 2011). Similar strategies may be used to achieve targeted delivery of combined therapeutics into cancer cells or normal cells in a premetastatic niche as a preventive or curative treatment for metastasis.
CONCLUDING REMARKS
Metastasis is a multistep process that involves many different types of normal cells. Recent studies have shown that cancer-derived EVs can play determinant roles in every step of metastasis and affect all kinds of involved cells in a direct or indirect manner. Normal cells, in turn, also influence cancer cells’ metastatic potential through EV secretion. Recognition of these EV-mediated functions in metastasis further emphasizes the importance of detecting, decoding, and targeting massages that are sent throughout the body before metastasis for new therapeutic opportunities. Exciting results have been seen in the recent development of EV-based biomarkers and therapeutics. However, the heterogeneous presence of EVs remains an obstacle in the field, and most current studies describe EV behaviors at a populational level. Maturing technologies enabling streamlined and standardized detection and isolation of different subgroups of EVs at single-particle level (Smith et al. 2015; Kibria et al. 2016; Lee et al. 2018; Welsh et al. 2018; Fraser et al. 2019), as well as marker development to differentiate EVs of different origins, will dramatically advance the field, ultimately leading to an improved management of metastasis.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (NIH)/National Cancer Institute (NCI) grants R01CA218140 and R01CA206911. The author has declared that no conflict of interest exists.
Footnotes
Editors: Jeffrey W. Pollard and Yibin Kang
Additional Perspectives on Metastasis: Mechanism to Therapy available at www.perspectivesinmedicine.org
REFERENCES
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. 2008. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 10: 619–624. 10.1038/ncb1725 [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. 2011. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29: 341–345. 10.1038/nbt.1807 [DOI] [PubMed] [Google Scholar]
- Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, et al. 2012. Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat Cell Biol 14: 677–685. 10.1038/ncb2502 [DOI] [Google Scholar]
- Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, Skog J. 2011. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun 2: 180 10.1038/ncomms1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardi GT, Smith MA, Hood JL. 2018. Melanoma exosomes promote mixed M1 and M2 macrophage polarization. Cytokine 105: 63–72. 10.1016/j.cyto.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. 2016. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell 30: 836–848. 10.1016/j.ccell.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijnsdorp IV, Geldof AA, Lavaei M, Piersma SR, van Moorselaar RJ, Jimenez CR. 2013. Exosomal ITGA3 interferes with non-cancerous prostate cell functions and is increased in urine exosomes of metastatic prostate cancer patients. J Extracell Vesicles 2: 22097 10.3402/jev.v2i0.22097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss SA, Sinha G, Sandiford OA, Williams LM, Engelberth DJ, Guiro K, Isenalumhe LL, Greco SJ, Ayer S, Bryan M, et al. 2016. Mesenchymal stem cell-derived exosomes stimulate cycling quiescence and early breast cancer dormancy in bone marrow. Cancer Res 76: 5832–5844. 10.1158/0008-5472.CAN-16-1092 [DOI] [PubMed] [Google Scholar]
- Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, Thery C. 2012. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res 72: 4920–4930. 10.1158/0008-5472.CAN-12-0925 [DOI] [PubMed] [Google Scholar]
- Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H, et al. 2014. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell 159: 499–513. 10.1016/j.cell.2014.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha DJ, Franklin JL, Dou Y, Liu Q, Higginbotham JN, Demory Beckler M, Weaver AM, Vickers K, Prasad N, Levy S, et al. 2015. KRAS-dependent sorting of miRNA to exosomes. eLife 4: e07197 10.7554/eLife.07197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challagundla KB, Wise PM, Neviani P, Chava H, Murtadha M, Xu T, Kennedy R, Ivan C, Zhang X, Vannini I, et al. 2015. Exosome-mediated transfer of microRNAs within the tumor microenvironment and neuroblastoma resistance to chemotherapy. J Natl Cancer Inst 107: djv135 10.1093/jnci/djv135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, et al. 2010. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest 120: 457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, et al. 2018. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560: 382–386. 10.1038/s41586-018-0392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin AR, Wang SE. 2016. Cancer-derived extracellular vesicles: the ‘soil conditioner’ in breast cancer metastasis? Cancer Metastasis Rev 35: 669–676. 10.1007/s10555-016-9639-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JA, Park H, Lim EH, Lee KW. 2012. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol 40: 130–138. [DOI] [PubMed] [Google Scholar]
- Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, Chin AR, Ren X, Gugiu BG, Meng Z, et al. 2014. Macrophage immunomodulation by breast cancer-derived exosomes requires toll-like receptor 2-mediated activation of NF-κB. Sci Rep 4: 5750 10.1038/srep05750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. 2013. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci 110: 17380–17385. 10.1073/pnas.1304266110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. 2011. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol 187: 676–683. 10.4049/jimmunol.1003884 [DOI] [PubMed] [Google Scholar]
- Cogolludo A, Moreno L, Frazziano G, Moral-Sanz J, Menendez C, Castañeda J, González C, Villamor E, Perez-Vizcaino F. 2009. Activation of neutral sphingomyelinase is involved in acute hypoxic pulmonary vasoconstriction. Cardiovasc Res 82: 296–302. 10.1093/cvr/cvn349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et al. 2015. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 17: 816–826. 10.1038/ncb3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatherage BL, Cookson BT. 2012. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun 80: 1948–1957. 10.1128/IAI.06014-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demory Beckler M, Higginbotham JN, Franklin JL, Ham AJ, Halvey PJ, Imasuen IE, Whitwell C, Li M, Liebler DC, Coffey RJ. 2013. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics 12: 343–355. 10.1074/mcp.M112.022806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Andaloussi S, Mäger I, Breakefield XO, Wood MJ. 2013. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 12: 347–357. 10.1038/nrd3978 [DOI] [PubMed] [Google Scholar]
- Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, Chow A, O'Connor ST, Li S, Chin AR, et al. 2015. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol 17: 183–194. 10.1038/ncb3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser K, Jo A, Giedt J, Vinegoni C, Yang KS, Peruzzi P, Chiocca EA, Breakefield XO, Lee H, Weissleder R. 2019. Characterization of single microvesicles in plasma from glioblastoma patients. Neuro Oncol 21: 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier D, Magnus N, Lee TH, Bentley V, Meehan B, Milsom C, Montermini L, Kislinger T, Rak J. 2012. Cancer cells induced to express mesenchymal phenotype release exosome-like extracellular vesicles carrying tissue factor. J Biol Chem 287: 43565–43572. 10.1074/jbc.M112.401760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesierich S, Berezovskiy I, Ryschich E, Zöller M. 2006. Systemic induction of the angiogenesis switch by the tetraspanin D6.1A/CO-029. Cancer Res 66: 7083–7094. 10.1158/0008-5472.CAN-06-0391 [DOI] [PubMed] [Google Scholar]
- Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. 2011. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res 71: 5346–5356. 10.1158/0008-5472.CAN-11-0241 [DOI] [PubMed] [Google Scholar]
- Harding C, Heuser J, Stahl P. 1983. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 97: 329–339. 10.1083/jcb.97.2.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DA, Patel SH, Gucek M, Hendrix A, Westbroek W, Taraska JW. 2015. Exosomes released from breast cancer carcinomas stimulate cell movement. PLoS ONE 10: e0117495 10.1371/journal.pone.0117495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Ochi H, Sunamura S, Kosaka N, Mabuchi Y, Fukuda T, Yao K, Kanda H, Ae K, Okawa A, et al. 2018. Cancer-secreted hsa-miR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A. Proc Natl Acad Sci 115: 2204–2209. 10.1073/pnas.1717363115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinger SA, Cha DJ, Franklin JL, Higginbotham JN, Dou Y, Ping J, Shu L, Prasad N, Levy S, Zhang B, et al. 2018. Diverse long RNAs are differentially sorted into extracellular vesicles secreted by colorectal cancer cells. Cell Rep 25: 715–725.e4. 10.1016/j.celrep.2018.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood JL, San RS, Wickline SA. 2011. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res 71: 3792–3801. 10.1158/0008-5472.CAN-10-4455 [DOI] [PubMed] [Google Scholar]
- Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, Tyska MJ, Weaver AM. 2013. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep 5: 1159–1168. 10.1016/j.celrep.2013.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. 2015. Tumour exosome integrins determine organotropic metastasis. Nature 527: 329–335. 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Song N, Ding Y, Yuan S, Li X, Cai H, Shi H, Luo Y. 2009. Pulmonary vascular destabilization in the premetastatic phase facilitates lung metastasis. Cancer Res 69: 7529–7537. 10.1158/0008-5472.CAN-08-4382 [DOI] [PubMed] [Google Scholar]
- Jeppesen DK, Nawrocki A, Jensen SG, Thorsen K, Whitehead B, Howard KA, Dyrskjøt L, Ørntoft TF, Larsen MR, Ostenfeld MS. 2014. Quantitative proteomics of fractionated membrane and lumen exosome proteins from isogenic metastatic and nonmetastatic bladder cancer cells reveal differential expression of EMT factors. Proteomics 14: 699–712. 10.1002/pmic.201300452 [DOI] [PubMed] [Google Scholar]
- Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, et al. 2019. Reassessment of exosome composition. Cell 177: 428–445.e18. 10.1016/j.cell.2019.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. 1987. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262: 9412–9420. [PubMed] [Google Scholar]
- Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R. 2017. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546: 498–503. 10.1038/nature22341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Rafii S, Lyden D. 2006. Preparing the “soil”: the premetastatic niche. Cancer Res 66: 11089–11093. 10.1158/0008-5472.CAN-06-2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keklikoglou I, Cianciaruso C, Güç E, Squadrito ML, Spring LM, Tazzyman S, Lambein L, Poissonnier A, Ferraro GB, Baer C, et al. 2019. Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat Cell Biol 21: 190–202. 10.1038/s41556-018-0256-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibria G, Ramos EK, Lee KE, Bedoyan S, Huang S, Samaeekia R, Athman JJ, Harding CV, Lötvall J, Harris L, et al. 2016. A rapid, automated surface protein profiling of single circulating exosomes in human blood. Sci Rep 6: 36502 10.1038/srep36502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. 2005. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res 11: 1010–1020. [PubMed] [Google Scholar]
- King HW, Michael MZ, Gleadle JM. 2012. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 12: 421 10.1186/1471-2407-12-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T. 2013. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem 288: 10849–10859. 10.1074/jbc.M112.446831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumangoye RB, Sakwe AM, Goodwin JS, Patel T, Ochieng J. 2011. Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PLoS ONE 6: e24234 10.1371/journal.pone.0024234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Théry C. 2016. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci 113: E968–E977. 10.1073/pnas.1521230113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishn SR, Singh A, Bowler N, Duffy AN, Friedman A, Fedele C, Kurtoglu S, Tripathi SK, Wang K, Hawkins A, et al. 2019. Prostate cancer sheds the αvβ3 integrin in vivo through exosomes. Matrix Biol 77: 41–57. 10.1016/j.matbio.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J, Sun L, Xu F, Liu L, Hu F, Song D, Hou Z, Wu W, Luo X, Wang J, et al. 2019. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res 79: 146–158. 10.1158/0008-5472.CAN-18-0014 [DOI] [PubMed] [Google Scholar]
- Le MT, Hamar P, Guo C, Basar E, Perdigão-Henriques R, Balaj L, Lieberman J. 2014. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J Clin Invest 124: 5109–5128. 10.1172/JCI75695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Fraser K, Ghaddar B, Yang K, Kim E, Balaj L, Chiocca EA, Breakefield XO, Lee H, Weissleder R. 2018. Multiplexed profiling of single extracellular vesicles. ACS Nano 12: 494–503. 10.1021/acsnano.7b07060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, Kappes JC, Barnes S, Kimberly RP, Grizzle WE, et al. 2006. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol 176: 1375–1385. 10.4049/jimmunol.176.3.1375 [DOI] [PubMed] [Google Scholar]
- Liu X, Cao M, Palomares M, Wu X, Li A, Yan W, Fong MY, Chan WC, Wang SE. 2018. Metastatic breast cancer cells overexpress and secrete miR-218 to regulate type I collagen deposition by osteoblasts. Breast Cancer Res 20: 127 10.1186/s13058-018-1059-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Bowler N, Harshyne LA, Craig Hooper D, Krishn SR, Kurtoglu S, Fedele C, Liu Q, Tang H-Y, Kossenkov AV, et al. 2018. Exosomal αvβ6 integrin is required for monocyte M2 polarization in prostate cancer. Matrix Biol 70: 20–35. 10.1016/j.matbio.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL. 2012. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 151: 1542–1556. 10.1016/j.cell.2012.11.024 [DOI] [PubMed] [Google Scholar]
- Maji S, Chaudhary P, Akopova I, Nguyen PM, Hare RJ, Gryczynski I, Vishwanatha JK. 2017. Exosomal annexin II promotes angiogenesis and breast cancer metastasis. Mol Cancer Res 15: 93–105. 10.1158/1541-7786.MCR-16-0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCready J, Sims JD, Chan D, Jay DG. 2010. Secretion of extracellular hsp90α via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer 10: 294 10.1186/1471-2407-10-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, et al. 2014. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 26: 707–721. 10.1016/j.ccell.2014.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, et al. 2015. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523: 177–182. 10.1038/nature14581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minciacchi VR, Freeman MR, Di Vizio D. 2015. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol 40: 41–51. 10.1016/j.semcdb.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minciacchi VR, Spinelli C, Reis-Sobreiro M, Cavallini L, You S, Zandian M, Li X, Mishra R, Chiarugi P, Adam RM, et al. 2017. MYC mediates large oncosome-induced fibroblast reprogramming in prostate cancer. Cancer Res 77: 2306–2317. 10.1158/0008-5472.CAN-16-2942 [DOI] [PubMed] [Google Scholar]
- Morello M, Minciacchi VR, de Candia P, Yang J, Posadas E, Kim H, Griffiths D, Bhowmick N, Chung LW, Gandellini P, et al. 2013. Large oncosomes mediate intercellular transfer of functional microRNA. Cell Cycle 12: 3526–3536. 10.4161/cc.26539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroishi T, Hayashi T, Pan WW, Fujita Y, Holt MV, Qin J, Carson DA, Guan KL. 2016. The hippo pathway kinases LATS1/2 suppress cancer immunity. Cell 167: 1525–1539.e17. 10.1016/j.cell.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabet BY, Qiu Y, Shabason JE, Wu TJ, Yoon T, Kim BC, Benci JL, DeMichele AM, Tchou J, Marcotrigiano J, et al. 2017. Exosome RNA unshielding couples stromal activation to pattern recognition receptor signaling in cancer. Cell 170: 352–366.e13. 10.1016/j.cell.2017.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata R, Shimada H, Fernandez GE, Fanter R, Fabbri M, Malvar J, Zimmermann P, DeClerck YA. 2017. Contribution of neuroblastoma-derived exosomes to the production of pro-tumorigenic signals by bone marrow mesenchymal stromal cells. J Extracell Vesicles 6: 1332941 10.1080/20013078.2017.1332941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, Lochnit G, Preissner KT, Zoller M. 2010. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res 70: 1668–1678. 10.1158/0008-5472.CAN-09-2470 [DOI] [PubMed] [Google Scholar]
- O'Brien K, Rani S, Corcoran C, Wallace R, Hughes L, Friel AM, McDonnell S, Crown J, Radomski MW, O'Driscoll L. 2013. Exosomes from triple-negative breast cancer cells can transfer phenotypic traits representing their cells of origin to secondary cells. Eur J Cancer 49: 1845–1859. 10.1016/j.ejca.2013.01.017 [DOI] [PubMed] [Google Scholar]
- Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, Yoshida M, Tsuda H, Tamura K, Ochiya T. 2014. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal 7: ra63 10.1126/scisignal.2005231 [DOI] [PubMed] [Google Scholar]
- Ortiz A, Gui J, Zahedi F, Yu P, Cho C, Bhattacharya S, Carbone CJ, Yu Q, Katlinski KV, Katlinskaya YV, et al. 2019. An interferon-driven oxysterol-based defense against tumor-derived extracellular vesicles. Cancer Cell 35: 33–45.e6. 10.1016/j.ccell.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, et al. 2010. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 12: 19–30; sup pp. 11–13 10.1038/ncb2000 [DOI] [PubMed] [Google Scholar]
- Palma J, Yaddanapudi SC, Pigati L, Havens MA, Jeong S, Weiner GA, Weimer KM, Stern B, Hastings ML, Duelli DM. 2012. microRNAs are exported from malignant cells in customized particles. Nucleic Acids Res 40: 9125–9138. 10.1093/nar/gks656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BT, Johnstone RM. 1983. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33: 967–978. 10.1016/0092-8674(83)90040-5 [DOI] [PubMed] [Google Scholar]
- Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, et al. 2009. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem 284: 34211–34222. 10.1074/jbc.M109.041152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, et al. 2012. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18: 883–891. 10.1038/nm.2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigati L, Yaddanapudi SC, Iyengar R, Kim DJ, Hearn SA, Danforth D, Hastings ML, Duelli DM. 2010. Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS ONE 5: e13515 10.1371/journal.pone.0013515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaila B, Lyden D. 2009. The metastatic niche: adapting the foreign soil. Nat Rev Cancer 9: 285–293. 10.1038/nrc2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci F, Garris C, Lai CP, Newton A, Pfirschke C, Engblom C, Alvarez D, Sprachman M, Evavold C, Magnuson A, et al. 2016. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science 352: 242–246. 10.1126/science.aaf1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman A, Bandari SK, Liu J, Mobley JA, Brown EE, Sanderson RD. 2016. Fibronectin on the surface of myeloma cell-derived exosomes mediates exosome-cell interactions. J Biol Chem 291: 1652–1663. 10.1074/jbc.M115.686295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowits G, Gerçel-Taylor C, Day JM, Taylor DD, Kloecker GH. 2009. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer 10: 42–46. 10.3816/CLC.2009.n.006 [DOI] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. 1996. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183: 1161–1172. 10.1084/jem.183.3.1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappa G, Santos MF, Green TM, Karbanová J, Hassler J, Bai Y, Barsky SH, Corbeil D, Lorico A. 2017. Nuclear transport of cancer extracellular vesicle-derived biomaterials through nuclear envelope invagination-associated late endosomes. Oncotarget 8: 14443–14461. 10.18632/oncotarget.14804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riches A, Campbell E, Borger E, Powis S. 2014. Regulation of exosome release from mammary epithelial and breast cancer cells—a new regulatory pathway. Eur J Cancer 50: 1025–1034. 10.1016/j.ejca.2013.12.019 [DOI] [PubMed] [Google Scholar]
- Santos MF, Rappa G, Karbanová J, Kurth T, Corbeil D, Lorico A. 2018. VAMP-associated protein-A and oxysterol-binding protein-related protein 3 promote the entry of late endosomes into the nucleoplasmic reticulum. J Biol Chem 293: 13834–13848. 10.1074/jbc.RA118.003725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Pochampally R, Watabe K, Lu Z, Mo Y-Y. 2014. Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol Cancer 13: 256 10.1186/1476-4598-13-256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr., Carter BS, Krichevsky AM, Breakefield XO. 2008. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10: 1470–1476. 10.1038/ncb1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ZJ, Lee C, Rojalin T, Carney RP, Hazari S, Knudson A, Lam K, Saari H, Ibañez EL, Viitala T, et al. 2015. Single exosome study reveals subpopulations distributed among cell lines with variability related to membrane content. J Extracell Vesicles 4: 28533 10.3402/jev.v4.28533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM. 2015. Directional cell movement through tissues is controlled by exosome secretion. Nat Commun 6: 7164 10.1038/ncomms8164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauro BJ, Mathias RA, Greening DW, Gopal SK, Ji H, Kapp EA, Coleman BM, Hill AF, Kusebauch U, Hallows JL, et al. 2013. Oncogenic H-ras reprograms Madin-Darby canine kidney (MDCK) cell-derived exosomal proteins following epithelial-mesenchymal transition. Mol Cell Proteomics 12: 2148–2159. 10.1074/mcp.M112.027086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DD, Gercel-Taylor C. 2008. microRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 110: 13–21. 10.1016/j.ygyno.2008.04.033 [DOI] [PubMed] [Google Scholar]
- Théry C, Zitvogel L, Amigorena S. 2002. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2: 569–579. 10.1038/nri855 [DOI] [PubMed] [Google Scholar]
- Thompson CA, Purushothaman A, Ramani VC, Vlodavsky I, Sanderson RD. 2013. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J Biol Chem 288: 10093–10099. 10.1074/jbc.C112.444562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkach M, Théry C. 2016. Communication by extracellular vesicles: where we are and where we need to go. Cell 164: 1226–1232. 10.1016/j.cell.2016.01.043 [DOI] [PubMed] [Google Scholar]
- Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, Lötvall J, Nakagama H, Ochiya T. 2015. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun 6: 6716 10.1038/ncomms7716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trams EG, Lauter CJ, Salem N Jr, Heine U. 1981. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta 645: 63–70. 10.1016/0005-2736(81)90512-5 [DOI] [PubMed] [Google Scholar]
- Vader P, Mol EA, Pasterkamp G, Schiffelers RM. 2016. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev 106: 148–156. 10.1016/j.addr.2016.02.006 [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659. 10.1038/ncb1596 [DOI] [PubMed] [Google Scholar]
- van Niel G, D'Angelo G, Raposo G. 2018. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19: 213–228. 10.1038/nrm.2017.125 [DOI] [PubMed] [Google Scholar]
- Villarroya-Beltri C, Baixauli F, Gutiérrez-Vázquez C, Sánchez-Madrid F, Mittelbrunn M. 2014. Sorting it out: regulation of exosome loading. Semin Cancer Biol 28: 3–13. 10.1016/j.semcancer.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Gilkes DM, Takano N, Xiang L, Luo W, Bishop CJ, Chaturvedi P, Green JJ, Semenza GL. 2014. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci 111: E3234–E3242. 10.1073/pnas.1410041111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. 2010. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res 70: 9621–9630. 10.1158/0008-5472.CAN-10-1722 [DOI] [PubMed] [Google Scholar]
- Weis SM, Cheresh DA. 2011. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med 17: 1359–1370. 10.1038/nm.2537 [DOI] [PubMed] [Google Scholar]
- Welsh JA, Kepley J, Rosner A, Horak P, Berzofsky JA, Jones JC. 2018. Prospective use of high-refractive index materials for single molecule detection in flow cytometry. Sensors (Basel) 18: E2461 10.3390/s18082461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Somlo G, Yu Y, Palomares MR, Li AX, Zhou W, Chow A, Yen Y, Rossi JJ, Gao H, et al. 2012. De novo sequencing of circulating miRNAs identifies novel markers predicting clinical outcome of locally advanced breast cancer. J Transl Med 10: 42 10.1186/1479-5876-10-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, Cheng Z, Shah SV, Wang GJ, Zhang L, et al. 2009. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer 124: 2621–2633. 10.1002/ijc.24249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Wu X, Zhou W, Fong MY, Cao M, Liu J, Liu X, Chen CH, Fadare O, Pizzo DP, et al. 2018. Cancer-cell–secreted exosomal miR-105 promotes tumour growth through the MYC-dependent metabolic reprogramming of stromal cells. Nat Cell Biol 20: 597–609. 10.1038/s41556-018-0083-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Chen J, Su F, Yu B, Su F, Lin L, Liu Y, Huang J-D, Song E. 2011. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer 10: 117 10.1186/1476-4598-10-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang XF. 2015. A niche role for cancer exosomes in metastasis. Nat Cell Biol 17: 709–711. 10.1038/ncb3181 [DOI] [PubMed] [Google Scholar]
- Zhang JX, Huang XX, Cai MB, Tong ZT, Chen JW, Qian D, Liao YJ, Deng HX, Liao DZ, Huang MY, et al. 2012. Overexpression of the secretory small GTPase Rab27B in human breast cancer correlates closely with lymph node metastasis and predicts poor prognosis. J Transl Med 10: 242 10.1186/1479-5876-10-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, Li P, Li M, Wang X, Zhang C, et al. 2015. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 527: 100–104. 10.1038/nature15376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, Mark MT, Molina H, Martin AB, Bojmar L, et al. 2018. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol 20: 332–343. 10.1038/s41556-018-0040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, Marini JC, Tudawe T, Seviour EG, San Lucas FA, et al. 2016. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. eLife 5: e10250 10.7554/eLife.10250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O'Connor ST, Chin AR, et al. 2014. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 25: 501–515. 10.1016/j.ccr.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, Oeh J, Modrusan Z, Bais C, Sampath D, et al. 2012. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J 31: 3513–3523. 10.1038/emboj.2012.183 [DOI] [PMC free article] [PubMed] [Google Scholar]