Abstract
Calcium (Ca2+) ion microdomains are subcellular regions of high Ca2+ concentration that develop rapidly near open Ca2+ channels in the plasma membrane or internal stores and generate local regions of high Ca2+ concentration. These microdomains are remarkably versatile in that they activate a range of responses that differ enormously in both their temporal and spatial profile. In this review, we describe how Ca2+ microdomains generated by store-operated calcium channels, a widespread and conserved Ca2+ entry pathway, stimulate different signaling pathways, and how the spatial extent of a Ca2+ microdomain can be influenced by Ca2+ ATPase pumps.
Cytosolic Ca2+ controls a plethora of important physiological processes within cells, including secretion, energy production, motility, growth, and differentiation (Clapham 2007). The versatility of Ca2+ as an intracellular messenger nevertheless belies its complexity. A fundamental challenge inherent to the use of the pleiotropic Ca2+ message is to maintain specificity and thus ensure that only the intended Ca2+-dependent processes are activated, and not others, in response to a specific stimulus, but also to allow different stimuli that use the Ca2+ signal to evoke distinct responses.
DECODING THE Ca2+ SIGNAL: AMPLITUDE AND FREQUENCY MODULATION
Studies on many different cell types have established that both the amplitude and the temporal dynamics of the Ca2+ signal contribute to specificity (Berridge et al. 2003). Ca2+ sensors have different affinities for Ca2+ and, therefore, in a homogenous system, will be recruited in a sequential manner dictated by the magnitude or kinetics of the Ca2+ signal. Antigen-driven secretion in the RBL-2H3 mucosal mast cell line correlated well with the amplitude of the Ca2+ signal, suggesting an amplitude-driven response (Kim et al. 1997). Similarly, in B cells, the transcription factor nuclear factor (NF)-κB was stimulated by a high-amplitude Ca2+ spike, whereas another transcriptional regulator, nuclear factor of activated T cells (NFAT), was activated instead by a low-amplitude, but sustained, cytosolic Ca2+ signal (Dolmetsch et al. 1997). For a given Ca2+ increase, different Ca2+ sensors can be recruited in a manner dependent on the number of Ca2+-binding sites present. Many Ca2+ sensors have multiple Ca2+-binding sites. Perhaps the best-known example is calmodulin, which has two classical EF-hands on both the N- and C-lobes of the protein (Hoeflich and Ikura 2002). Increasing the number of identical Ca2+-binding sites on a sensor shifts the Ca2+ dependence by altering the relationship between free Ca2+ concentration and fractional occupancy of the sensor (Parekh 2011). For a sensor bearing one Ca2+-binding site with a KD of 1 µm, 50% saturation will occur in the presence of 1 µm Ca2+. In contrast, a sensor harboring four identical binding sites, each with a KD of 1 µm, will show only 10% occupancy at 1 µm. Therefore, simply increasing the number of Ca2+-binding sites on a sensor provides an effective mechanism through which different amplitudes of Ca2+ signal activate distinct Ca2+ sensors (Parekh 2011).
Specificity can also be imparted through the ability of different sensors to respond to different temporal patterns of Ca2+ signal. It has been shown that the duration of the Ca2+ signal is an important factor in contributing to specificity. In dog pancreatic duct epithelial cells, Ca2+ oscillations evoked by P2Y2 receptor activation were considerably less effective in driving exocytosis than a nonoscillatory, but longer duration Ca2+ signal, but which had the same amplitude as that of the oscillatory response (Jung et al. 2006). However, most studies that have addressed the role of the temporal profile of the Ca2+ signal in driving specificity have focused on cytosolic Ca2+ oscillations.
Cytosolic Ca2+ signals in nonexcitable cells often oscillate over time, both spontaneously and following stimulation of Gq-coupled receptors (Thomas et al. 1996; Parekh 2011). Receptor-evoked cytosolic Ca2+ oscillations, which can have periodicities from <10 sec to >400 sec, have been implicated in a wide range of cellular responses, including stimulation of exocytosis (Tse et al. 1993), mitochondrial metabolism (Hajnóczky et al. 1995), activation of different Ca2+-dependent transcription factors in a manner dependent on Ca2+ oscillation frequency (Dolmetsch et al. 1998), and neurite extension (Gu and Spitzer 1995). The frequency of the Ca2+ oscillations are decoded by sensors that change activity in response to the number of Ca2+ oscillations. Such sensors include conventional (Ca2+-dependent) protein kinase Cα, βI, βII, γ, and Ca2+/calmodulin-dependent protein kinase II (De Koninck and Schulman 1998; Oancea and Meyer 1998).
SPATIAL PROFILE: THE THIRD SOURCE OF INFORMATION IN A Ca2+ OSCILLATION
Cytosolic Ca2+ oscillations in response to receptor stimulation require activation of phospholipase Cβ by Gq-coupled receptors, or phospholipase Cγ by growth factor receptors (Berridge et al. 2003). Increased phospholipase C activity results in the hydrolysis of the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) in the inner leaflet of the plasma membrane (PM). PIP2 hydrolysis generates the second messenger inositol 1,4,5-trisphosphate (InsP3), which releases Ca2+ from the endoplasmic reticulum (ER) through opening of InsP3-gated Ca2+ channels, and diacylglycerol, which activates protein kinase C isoforms (Berridge 1987). The mechanisms that generate Ca2+ oscillations might be both cell-type- and agonist-dependent (Parekh 2011). In some cell types, InsP3 levels oscillate because of either feedback pathways that uncouple the receptor from phospholipase C (Woods et al. 1987; Kawabata et al. 1996) or because of the Ca2+ dependency of phospholipase C (Meyer and Stryer 1988). In other cell types, Ca2+ oscillations are thought to arise from coregulation of InsP3 receptors by both InsP3 and cytosolic Ca2+ (Bezprozvanny et al. 1991; Finch et al. 1991; Ivorra and Parker 1992). Low cytosolic Ca2+ concentrations (∼200 nm) facilitate InsP3-dependent Ca2+ release, whereas high concentrations (∼1 µm) inhibit it (Bezprozvanny et al. 1991). Irrespective of the mode of generation, in most cell types, cytosolic Ca2+ oscillations can be maintained, if only for a few minutes, in the absence of external Ca2+, showing that the primary mechanism for generating a Ca2+ oscillation is intracellular in origin (Thomas et al. 1996). However, Ca2+ oscillations do run down over time in Ca2+-free solution. This is because a fraction of Ca2+ released from the ER is extruded from the cell by the PM Ca2+ ATPase (PMCA) pump. This was nicely shown in a study by Tepikin and colleagues (1992), who measured simultaneously cytosolic and extracellular Ca2+ after placing pancreatic acinar cells in a small microdroplet. Stimulation with the physiological agonist cholecystokinin elicited oscillations in cytosolic Ca2+ and this was followed by pulsatile Ca2+ extrusion. The amount of Ca2+ extruded during the first Ca2+ oscillation was, on average, 39% of the total intracellular pool of mobilizable Ca2+ (Tepikin et al. 1992). Therefore, a sizeable fraction of Ca2+ released from the InsP3-sensitive store is exported by the PMCA pumps. The cytosolic Ca2+ oscillations in Ca2+-free solution run down over time because, after each spike, some Ca2+ is extruded from the cell and less Ca2+ is therefore available to refill the stores. To sustain oscillations requires Ca2+ influx and this is typically achieved through store-operated Ca2+ channels (Bird and Putney 2005).
Store-operated Ca2+ channels open following the loss of Ca2+ from within the ER, and the subsequent Ca2+ influx ensures the store is refilled with Ca2+ to support the next Ca2+ oscillation. The best understood store-operated channel is the Ca2+ release-activated Ca2+ (CRAC) channel (Hoth and Penner 1992; Parekh and Putney 2005). The molecular basis of the CRAC channel has been identified with the discoveries of STIM (Liou et al. 2005; Roos et al. 2005) and Orai proteins (Feske et al. 2006; Vig et al. 2006a,b; Zhang et al. 2006). STIM proteins are Ca2+ sensors that span the ER membrane (Lewis and Prakriya 2015). On store depletion, STIM proteins form multimers, which then migrate within the ER membrane to reach specialized ER–PM junctions located <20 nm below the PM (Wu et al. 2006). At these sites, STIM proteins capture and then gate open Orai proteins, which are the pore-forming subunit of the CRAC channel (Prakriya et al. 2006; Yeromin et al. 2006; Vig et al. 2006a).
Under physiological conditions of external Ca2+, Ca2+ entry through CRAC channels is required to counter PMCA pump-driven extrusion of cytosolic Ca2+ that has been released from the InsP3-sensitive Ca2+ store. Store-operated Ca2+ channels therefore sustain cytosolic Ca2+ oscillations in response to continual agonist stimulation. Consistent with this, inhibition of the channels or knockdown of Orai1 accelerates the rundown of Ca2+ oscillations in the presence of external Ca2+ (Wedel et al. 2007; Kar et al. 2012). If PMCA pumps export Ca2+ released during Ca2+ oscillations, then inhibition of Ca2+ extrusion even in the absence of Ca2+ entry, should support sustained oscillatory Ca2+ signals because any released Ca2+ would no longer be extruded from the cell and therefore would now be taken back into the stores by the Ca2+ ATPase pumps in the ER membrane. Bird and Putney (2005) developed a neat approach to show this. Rare-earth metal ions, such as Gd3+ and La3+, inhibit CRAC channels at low micromolar concentrations and block the PMCA ATPase pump at millimolar levels. In the presence of a low dose of Gd3+, cytosolic Ca2+ oscillations following muscarinic receptor activation in HEK293 cells ran down quickly as Ca2+ entry was suppressed. However, when a high dose of Gd3+ was used to block additionally the PMCA pumps, Ca2+ oscillations were maintained despite the full inhibition of CRAC channels (Bird and Putney 2005). Under these conditions, the PM is “tight” to Ca2+ flux; Ca2+ cannot enter through CRAC channels nor be extruded by the PMCA pump. Therefore, Ca2+ released from the ER can no longer be exported from the cell and is therefore returned to the organelle, in readiness for the next oscillatory cycle.
Di Capite et al. (2009) exploited PMCA pump inhibition to address whether Ca2+ oscillations of the same amplitude and frequency, regardless of how they were generated, were equally effective in activating downstream gene expression. Stimulation of cysteinyl leukotriene type I receptors in RBL-1 mast cells with the agonist leukotriene C4 (LTC4) evoked cytosolic Ca2+ oscillations that ran down either in the absence of external Ca2+ or after block of CRAC channels. However, the Ca2+ oscillations were sustained in the absence of external Ca2+, when the PMCA pump was inhibited by a high dose of La3+. These latter Ca2+ oscillations involve Ca2+ release from the ER but without any accompanying Ca2+ influx, whereas the oscillations in the presence of functional CRAC channels are comprised of Ca2+ release followed by Ca2+ influx. Only the latter situation results in elevation of the subplasmalemmal Ca2+ concentration, spatially restricted to the vicinity of the CRAC channels. A range of agonist concentrations was tested and it was found that, for any given concentration, the amplitude and frequency of the oscillations were indistinguishable between cells stimulated in the presence of external Ca2+ or in its absence (with PMCA pump inhibited to prevent loss of Ca2+ from the cell). However, Ca2+-dependent expression of the immediate early gene c-fos occurred only in response to those Ca2+ oscillations in which Ca2+ entry through CRAC channels was active (Di Capite et al. 2009). Activation of the Ca2+-dependent transcription factor NFAT also showed a similar dependency on CRAC channel activation during oscillatory Ca2+ signals evoked by LTC4 (Kar et al. 2011). These results identified a major role for the spatial profile of the Ca2+ oscillation in excitation–transcription coupling driven by Ca2+ microdomains near open CRAC channels.
Very close to the mouth of an open Ca2+ channel, intracellular Ca2+ chelators are not fast enough to prevent the increase in the local Ca2+ concentration but reduce the lateral spread of the microdomain to an extent determined by the on-rate of the chelator for Ca2+ (Neher 1998) The chelator EGTA binds Ca2+ considerably more slowly than BAPTA. Although both reduce the bulk increase in Ca2+ similarly (as they have comparable equilibrium affinities for Ca2+), only BAPTA is fast enough to reduce the extent of the Ca2+ microdomain (Neher 1998; Parekh 2008). Loading the cytosol of RBL cells with EGTA had no inhibitory effect on LTC4-driven c-fos expression, whereas BAPTA suppressed c-fos induction (Di Capite et al. 2009). Because BAPTA is fast enough to constrain Ca2+ entry to within a few nanometers of the pore, these results support a central role for local Ca2+ entry, and not global Ca2+ oscillations, in activation of gene expression. An important factor in determining the size of a Ca2+ microdomain is the unitary current through the Ca2+ channel. The single channel flux depends on the electrochemical gradient for Ca2+. Manipulation of either the electrical or chemical driving forces for Ca2+ entry through CRAC channels impaired c-fos expression, despite such maneuvers having little effect on the bulk cytosolic Ca2+ increase (Ng et al. 2009). In addition to c-fos and NFAT, several other pathways are activated by local Ca2+ entry through CRAC channels. These are described below.
In Madin–Darby canine kidney cells, bradykinin-induced oscillations in cytosolic Ca2+ concentration were dependent on Ca2+-permeation through connexin 43 hemichannels expressed in the PM (De Bock et al. 2012). In contrast, ATP-evoked oscillations in cytosolic Ca2+ concentration were unaffected by manipulations that inhibited connexin function. Therefore, in some cell types, connexin hemichannels might contribute to Ca2+ oscillations in an agonist-specific context.
TARGETS FOR LOCAL Ca2+ ENTRY THROUGH CRAC CHANNELS
Adenylyl Cyclase
The first signaling pathway that was shown to be activated by local Ca2+ microdomains arising through store-operated Ca2+ channels was the adenylyl cyclase/cAMP (Fig. 1). Four isoforms of membrane-bound adenylyl cyclase are regulated by cytosolic Ca2+; adenylyl cyclases 1 and 8 are activated by Ca2+-calmodulin, whereas isoforms 5 and 6 are directly inhibited. In C2-2B glioma cells, Ca2+ entry was evoked following store-depletion with thapsigargin, a sesquiterpene lactone extracted from the plant Thapsia garganica, which potently inhibits the Ca2+ ATPase pump of the ER Ca2+ store. When the pump is inhibited, Ca2+ can no longer be taken back into the store and, in the presence of continuous flux of Ca2+ out of the ER through poorly characterized leak channels, the store gradually depletes of Ca2+ and this leads to the opening of CRAC channels. Application of thapsigargin in the glioma cells led to stimulation of type I adenylyl cyclase and inhibition of the type 6 isoform (Chiono et al. 1995; Fagan et al. 1996). In contrast, Ca2+ release evoked by thapsigargin, Gq-coupled receptor agonist or ionomycin all failed to alter adenylyl cyclase activity despite raising bulk Ca2+ to a higher level. Regulation of adenylyl cyclase activity by store-operated Ca2+ entry was suppressed by cytosolic BAPTA but not EGTA (Fagan et al. 1998), consistent with local regulation by Ca2+. Opening of a different type of Ca2+-permeable ion channel, a transient receptor potential canonical (TRPC) channel, increased subplasmalemmal Ca2+ and thereby bulk Ca2+ but failed to alter enzyme activity (Shuttleworth and Thompson 1999). Therefore, local Ca2+ entry through CRAC channels regulates adenylyl cyclase, suggesting the channel and enzyme colocalize. Strong evidence in support of this has been provided by a combination of pull-down and Förster resonance energy transfer (FRET) studies, which show close association between adenylyl cyclase 8 and Orai1 (Willoughby et al. 2012). Mutagenesis studies identified an arginine-rich sequence in the amino terminus of Orai1 (R28-R29, R31-R32-R33) that was required for binding to adenylyl cyclase 8 (Willoughby et al. 2012). The enzyme is therefore located close to the mouth of the channel, enabling it to respond rapidly and with high fidelity to the local Ca2+ increase.
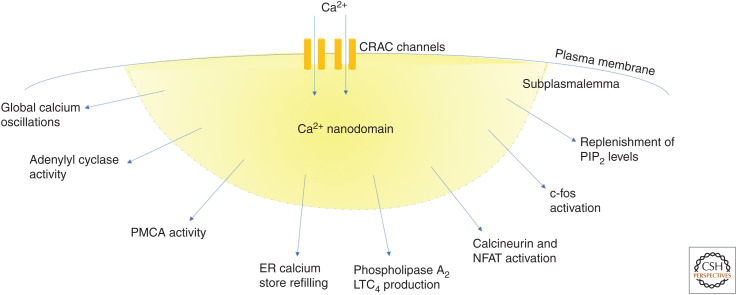
Figure 1.
Cartoon summarizes various responses activated by local calcium (Ca2+) entry through Ca2+ release-activated Ca2+ (CRAC) channels. PMCA, Plasma membrane Ca2+ ATPase; ER, endoplasmic reticulum; LTC4, leukotriene C4; NFAT, nuclear factor of activated T cells.
Adenylyl cyclases can also be activated following store depletion in a STIM1-dependent manner independent of Ca2+ influx (Lefkimmiatis et al. 2009). Store depletion with ionomycin or chelation of free Ca2+ within the store by TPEN both raised cAMP levels in the absence of external Ca2+ or after strongly buffering cytosolic Ca2+ with BAPTA. STIM1 has also been found to activate adenylyl cyclase in an Orai1-independent manner during melanogenesis (Motiani et al. 2018). In this study, STIM1 was found to interact with adenylyl cyclase 6 at ER–PM junctions after store depletion.
Ca2+-Dependent Phospholipase A2 and Metabolic Coupling
In several types of immune cell, Ca2+ influx stimulates de novo synthesis of the proinflammatory LTC4, LTD4, and LTE4. Leukotrienes are produced from metabolism of arachidonic acid by 5-lipoxygenase enzyme. Ca2+ entry through CRAC channels increased arachidonic acid levels through stimulation of Ca2+-dependent cytosolic phospholipase A2 (cPLA2) and this led to an increase in leukotriene production (Chang and Parekh 2004). Ca2+ release from the stores was unable to activate this pathway. Stimulating cells with thapsigargin in the absence of external Ca2+ and when PMCA pump activity was suppressed raised bulk cytosolic Ca2+ to levels higher than that achieved following store-operated Ca2+ entry, but the former was still unable to stimulate arachidonic acid production (Chang et al. 2008). Therefore, cPLA2 is activated by local Ca2+ entry. Manipulations that significantly reduced the unitary flux through CRAC channels, but which had little effect on bulk cytosolic Ca2+, impaired arachidonic acid production, as predicted for a pathway stimulated by Ca2+ microdomains. Consistent with this, loading the cytosol with the slow Ca2+ chelator EGTA had little effect on cPLA2 activated by CRAC channels (Chang et al. 2008). The underlying signal transduction pathway linking local Ca2+ entry to cPLA2 and 5-lipoxygenase involved recruitment of Ca2+-dependent protein kinases α and β1, which in turn activated the MAP kinase ERK (Chang et al. 2006). Activated ERK metabolically coupled the cPLA2 and 5-lipoxygenase pathways. ERK phosphorylated serine 505 of cPLA2, increasing its activity and thus generating more arachidonic acid. At the same time, ERK increased translocation of 5-lipoxygenase to the nuclear membrane, where it bound to, and was activated by, the nuclear membrane protein 5-lipoxygenase-activating protein. Through ERK activity, local Ca2+ entry through CRAC channels is therefore able to coordinate production of arachidonic acid with its subsequent metabolism to cysteinyl leukotrienes (Chang et al. 2006).
Regulation of PIP5 Kinase
InsP3 has a relatively short lifetime in the cytosol, as it is sequentially dephosphorylated within a few seconds via a series of inositol phosphatases to myoinositol monophosphate (Allbritton et al. 1992). The latter is metabolized to inositol by inositol monophosphatases and this step is inhibited by Li+ (Berridge et al. 1982). Inositol then combines with cytidine diphosphate diacylglycerol to form phosphatidylinositol (PI), which is phosphorylated by PIP4 and PIP5 kinases to make PIP2. Blocking inositol monophosphatases by elevating intracellular Li+ depletes PIP2 levels and therefore impairs the production of InsP3, and this has been proposed as a possible mechanism to explain the mood-stabilizing effect of Li+ treatment on bipolar disorder (Berridge et al. 1982). Ca2+ oscillations evoked by LTC4 in RBL cells ran down in the presence of Li+, indicating depletion of PIP2 during continuous exposure to agonist (Alswied and Parekh 2015). In the blowfly salivary gland, a seminal study by Berridge and Fain (1979) showed that the decline in Ca2+ flux to continuous exposure to serotonin could be overcome by addition of exogenous inositol. In Li+-treated RBL cells, exposure to inositol prevented rundown of Ca2+ oscillations to LTC4 (Alswied and Parekh 2015). Application of exogenous phosphatidylinositol 4-phosphate (PI4P) also sustained the cytosolic Ca2+ oscillations in Li+-treated cells, consistent with the need to replenish PIP2 under these conditions. Sustained Ca2+ oscillations were evoked by LTC4 in the absence of external Ca2+ provided PMCA pumps were blocked. These oscillations also ran down in the presence of Li+ but could no longer be rescued by inositol or PI4P (Alswied and Parekh 2015). Therefore Ca2+ influx is required to replenish the PIP2 pool used by LTC4. In support of this, block of CRAC channels prevented exogenous inositol and PI4P from sustaining Ca2+ oscillations in Li+-treated cells. PI4P is converted to PIP2 by PIP5 kinases. In cells in which PI5P kinase 1α or 1γ had been knocked down, Ca2+ oscillations ran down quickly and could not be rescued by PI4P (Alswied and Parekh 2015). Therefore, Ca2+ entry through CRAC channels replenishes PIP2 levels by enhancing PIP5 kinase activity. This was a local effect because oscillatory Ca2+ release in the absence of external Ca2+ was unable to replenish PIP2 levels despite raising cytosolic Ca2+ to levels that matched those seen in the presence of external Ca2+.
Ca2+-Dependent Transcription Factors c-fos and NFAT
As described in an earlier section, local Ca2+ entry through CRAC channels during cytosolic Ca2+ oscillations leads to expression of c-fos and activates NFAT. The molecular mechanisms driving these responses have been teased apart in mast cells. For induction of c-fos expression, Ca2+ microdomains near open CRAC channels activate the nonreceptor tyrosine kinase Syk (Ng et al. 2008, 2009). Immunocytochemical and pull-down experiments have shown that Syk associates with Orai1 and remains so after activation of Ca2+ entry (Samanta et al. 2015). Syk phosphorylates, and thereby activates, the transcription factor Stat5 (Ng et al. 2009), mainly Stat5a (Yeh and Parekh 2015). Phosphorylated Stat5 dimerizes and then migrates into the nucleus to increase c-fos transcription.
A slightly different mechanism couples CRAC channel Ca2+ microdomains to NFAT1-4 activation. In resting cells, these NFAT proteins are extensively phosphorylated and trapped within the cytosol (Hogan et al. 2003). Dephosphorylation is mediated by the Ca2+-dependent protein phosphatase 2B, calcineurin, the target for immunosuppressants cyclosporine and tacrolimus. Following a local increase in cytosolic Ca2+ near CRAC channels, Ca2+-calmodulin binds to calcineurin to increase enzyme activity. Dephosphorylation of NFAT by active calcineurin exposes a nuclear localization sequence, which enables NFAT to migrate into the nucleus. As in some other cell types (Li et al. 2012), a fraction of the cellular pools of calcineurin and NFAT are associated with the PM through binding to the anchoring protein AKAP79 in RBL cells (Kar et al. 2014). Under nonstimulated conditions, Orai1 and AKAP79 do not coimmunoprecipitate but do so after store depletion (Kar et al. 2014). In this way, calcineurin is brought close to the Ca2+ microdomain and, once activated, has immediate access to its target NFAT.
CLUSTERING OF CRAC CHANNELS ENHANCES SIGNALING STRENGTH OF LOCAL Ca2+
As the preceding examples show, Ca2+ microdomains near open CRAC channels activate a diverse range of downstream signaling pathways. In resting cells, the channel pore-forming subunit Orai1 is distributed throughout the PM. However, after store depletion, STIM1 proteins oligomerize and migrate to peripheral ER located just below the PM. At these specialized ER–PM junctions, STIM1 proteins bind to and gate Orai1, resulting not only in channel activation but also a clustered distribution of the channels (Lewis and Prakriya 2015). This spatial rearrangement of the CRAC channels raises further questions; first, what is the signaling advantage conferred by such clustering of CRAC channels? Second, how high can local Ca2+ reach at these regions? Finally, what is the role of Ca2+ extrusion in controlling the size of the local Ca2+ signal?
Samanta et al. (2015) compared the ability of mutant CRAC channels that did not redistribute to ER–PM junctions, with a similar number of wild-type channels that did relocate, to activate c-fos and NFAT. Both mutant and wild-type channels evoked similar increases in bulk cytosolic Ca2+ (Samanta et al. 2015). Channel relocalization to ER–PM junctions led to more robust transcription factor activation and subsequent gene expression, identifying a significant benefit to CRAC channel clustering.
Calculations suggest that about five endogenous CRAC channels occupy each ER–PM junction in a T lymphocyte or RBL cell after store depletion, assuming all the junctions are occupied and contain a similar number of channels (Hogan 2015; Samanta et al. 2015). Assuming the channels are noncoupled, the mean distance between any one CRAC channel picked at random and its nearest neighbor in a junction is ∼47 nm. Simulations suggest, for channels spaced 47 nm apart, that the spatial profile of local Ca2+ is elevated in the mid-range of the junction and shows two peaks; a large one of ∼6 µm corresponding to a single channel and a second, smaller peak that represents spillover from a couple of proximal channels (Samanta et al. 2015). If CRAC channels colocalize at a junction then the minimum distance between two adjacent pores would be ∼6.3 nm, based on a lineal measure from juxtaposition of the crystal structures of the hexameric channel. The spatial profile for five such colocalized channels was considerably altered; now, local Ca2+ concentration increased to ∼13 µm, almost 30-fold higher than the corresponding bulk cytosolic Ca2+ concentration (Samanta et al. 2015).
The narrow gap between peripheral ER and the PM excludes mitochondria from being localized adjacent to CRAC channels at the junctions. Therefore, other Ca2+ clearance pathways have the potential to regulate directly the size and expanse of the local Ca2+ signal. In many nonexcitable cells that rely heavily on store-operated Ca2+ entry such as lymphocytes and mast cells, PM Na+-Ca2+ exchangers are either absent or weak. Therefore, the PMCA pump would be a potentially important candidate for shaping local Ca2+ signals that arise from open CRAC channels.
CROSS TALK BETWEEN CRAC CHANNELS AND PMCA PUMPS
Studies on Ca2+ clearance pathways in Jurkat T lymphocytes identified important functional interaction between Ca2+ entry through CRAC channels and PMCA pump activity (Bautista et al. 2002). In cells kept in Ca2+-free solution in the presence of thapsigargin, readmission of external Ca2+ led to a cytosolic Ca2+ increase caused by Ca2+ entry through CRAC channels. Subsequent removal of external Ca2+ resulted in a bi-exponential decay of the Ca2+ signal. Information on Ca2+ clearance rates could be extracted from the decay phase of the Ca2+ by applying linear fits to the initial phases or exponential fits to the whole process. Following exposure to the combination of antimycin A and oligomycin (to depolarize mitochondria and thus suppress mitochondrial Ca2+ buffering), brief readmission of external Ca2+ to thapsigargin-treated cells led to an increase in cytosolic Ca2+, which then fell mono-exponentially caused by PMCA pump activity with a time constant of ∼40 sec for small Ca2+ increases less than ∼0.5 µm and this decreased to ∼25 sec when cytosolic Ca2+ rose to >1.5 µm. The rate of Ca2+ clearance rose as the duration of the Ca2+ pulse was prolonged, increasing approximately fivefold when Ca2+ pulses lasted >60 sec, a process that was termed modulation (Bautista et al. 2002). Recovery from modulation was considerably slower than its onset, with a time constant of ∼240 sec, thereby imparting a form of memory to the PMCA pump of the previous Ca2+ elevation. By applying a Michaelis–Menten type model, it was found that modulation increased both the maximal rate of the PMCA pump and reduced the KM. Subsequent experiments established a major role for local Ca2+ influx through CRAC channels and not an increase in bulk Ca2+ in driving development of the modulation process (Bautista and Lewis 2004). Modulation therefore increases Ca2+ efflux rate to counter prolonged Ca2+ influx through CRAC channels and thus plays an important role in shaping the spatiotemporal profile of the Ca2+ signal.
The mechanism underlying modulation of the PMCA pump in T cells was not identified but two possibilities were considered (Bautista and Lewis 2004). One involved physical coupling between PMCA pumps and CRAC channels perhaps involving the PDZ domain of the PMCA4b (the major isoform expressed in T cells) and Dlg proteins. Physical tethering of the pump to the CRAC channel would expose the pump directly to the Ca2+ microdomains that enhance modulation. The second possibility was that coupling between the channels and the pumps was indirect, mediated by a diffusible molecule. In this scenario, a Ca2+ sensor would detect the local Ca2+ near CRAC channels and then diffuse to more remote PMCA pumps to induce modulation.
Subsequent studies in T cells do not support physical coupling between the PMCA pump and CRAC channels, at least under physiological conditions (Quintana et al. 2011). Stimulation of T cells by engaging the T-cell receptor induces the formation of the immunological synapse, which reflects tight apposition of a T cell with an antigen-presenting cell. The synapse is the site where the T-cell receptor is triggered by its antigen ligand and where Orai1 channels relocalize and cluster. Analysis of the distribution of Orai1 and PMCA4b in T cells using total internal reflection microscopy revealed that the two proteins did not colocalize (Quintana et al. 2011). Orai1 was found within the immunological synapse, whereas PMCA4b was confined to the periphery of the synapse. Despite this spatial separation, Ca2+ entry though CRAC channels was still able to induce pump modulation (Quintana et al. 2011).
A plausible diffusible signal linking CRAC channels to PMCA pumps is calmodulin. Several PMCA pump isoforms including PMCA4b bind calmodulin, and Ca2+-calmodulin reduces KM and increases VMax, mirroring the changes observed in modulation. Studies with a fluorescent calmodulin protein showed that calmodulin binding to PMCA pump was slow, developing over several tens of seconds (Penheiter et al. 2003). This is consistent with the observed temporal induction of modulation in T cells, where modulation developed over tens of seconds (Bautista et al. 2002).
Can calmodulin regulation account for modulation of PMCA pumps by CRAC channels? Interestingly, an earlier study by Caride et al. (2001) reported Ca2+-dependent memory to PMCA4b and that this modulation involved calmodulin. In their study, they expressed PMCA pump isoforms in Sf9 insect cells and measured ATPase activity in microsomal membranes. These investigators used a two-pulse protocol to show induction of Ca2+-dependent memory. Microsomes were first exposed to 500 nm Ca2+ for 300 sec and then rapidly perfused with 50 nm Ca2+. PMCA2b showed prolonged memory of the high Ca2+ pulse in that pump activity declined in low Ca2+ with a time constant of ∼80 sec. In contrast, PMCA4b activity declined considerably more quickly, with a time constant of ∼30 sec (Caride et al. 2001). Studies with a calmodulin-binding peptide suggested that the memory process was a consequence of calmodulin remaining bound to the PMCA pump for several seconds after switching from high (500 nm) to low (50 nm) Ca2+. However, the kinetics of calmodulin regulation of PMCA4b in microsomes do not match the time course of modulation in T lymphocytes. Although both have a broadly similar activation time course, reversal of modulation because of calmodulin dissociation is almost an order of magnitude faster in microsomes (Caride et al. 2001) than in T cells (Bautista et al. 2002). One possible explanation for this mismatch is that interaction between PMCA pumps and the cytoskeleton or other regulatory proteins is lost in the microsomal system and such interactions might affect calmodulin dissociation from PMCA pumps. Another possibility is that in intact cells calmodulin binding might render the pump susceptible to some form of posttranslational modification, trapping it in place. Finally, modulation might not involve calmodulin but another protein that regulates PMCA pump activity. One possible candidate is neuroplastin, an immunoglobulin superfamily protein. In T cells, neuroplastin was found to associate with both PMCA1 and PMCA4 isoforms as well as regulate the levels of pump expression (Korthals et al. 2017). Using cryo-electron microscopy, the structure of PMCA1 in complex with neuroplastin was reported to 4.1 Å resolution (Gong et al. 2018). The transmembrane domain of neuroplastin interacted with transmembrane domain 10 of PMCA1 as well as the linker region between transmembrane domains 8 and 9. Neuroplastin binding induces a marked structural rearrangement of the pump, exposing the cytosolic Ca2+-binding site. An interesting hypothesis has been put forward that suggests binding of PMCA interacting proteins such as STIM1 and POST might alter the association between neuroplastin and the pump (Go and Soboloff 2018). Such a rearrangement could mask or impair access to the cytosolic Ca2+-binding site, reducing pump activity. Whether the PMCA modulation seen in T cells involves neuroplastin is at present unclear.
Can PMCA pump modulation itself be regulated? An elegant study in T cells showed that PMCA pump modulation was suppressed by mitochondrial Ca2+ buffering under physiological conditions (Quintana et al. 2011). Following induction of the immunological synapse, Orai1 accumulated at the synapse, as observed by others (Barr et al. 2008; Lioudyno et al. 2008), whereas mitochondria and PMCA pumps were confined to the fringes. There was little colocalization between Orai1 channels and either mitochondria or PMCA pumps, but considerably more overlap between mitochondria and PMCA protein (Quintana et al. 2011). In contrast, following stimulation with thapsigargin, which does not induce synapse formation, PMCA pumps showed a homogeneous distribution through the PM and areas of colocalization between the pumps and Orai1 clusters were observed (Quintana et al. 2011). This might reflect the fact that both proteins are abundant in T cells and so some colocalization might occur by chance owing to random positioning of the proteins in the membrane. Collectively, these data show that PMCA pumps redistribute away from Orai1 after synapse formation and instead are positioned close to mitochondria. Mitochondria are rapid and high-capacity Ca2+ buffers and their juxtaposition with PMCA proteins raises the possibility that mitochondrial Ca2+ uptake might siphon Ca2+ away from the PMCA pump, suppressing the development of modulation. This turned out to be the case; no modulation occurred following Ca2+ entry through Orai1 channels, provided mitochondria were able to take up local Ca2+(Quintana et al. 2011). Mitochondrial depolarization, which inhibits Ca2+ uptake into the organelle, enabled PMCA pump modulation to occur. In contrast, in thapsigargin-treated cells, modulation developed normally. Therefore, the rearrangement of Orai1 channels, PMCA pumps, and mitochondria at the immunological synapse enables mitochondria to remove Ca2+ from the vicinity of the pumps and thereby suppress the development of modulation.
A further twist to how CRAC channels and PMCA pumps interact has been provided by the report that STIM1 suppresses pump activity in T cells (Ritchie et al. 2012). Activation of Jurkat T cells with the agonist PHA led to strong up-regulation of STIM1 and PMCA4 proteins. These investigators adopted the protocol for measuring PMCA pump activity previously described (Bautista et al. 2002), wherein CRAC channels were activated by exposure to thapsigargin and pump activity was measured following removal of external Ca2+, and found that Ca2+ decay was a double exponential process, as also shown in previous reports from T cells (Ritchie et al. 2012). Ca2+ clearance was initially fast, with a half-time of ∼12 sec and this was followed by a much slower clearance phase, with a half-time of ∼80 sec. Increased levels of STIM1, either by PHA activation or simply by transfection of STIM1 plasmid, slowed Ca2+ clearance with a pronounced effect on the second phase. Whereas the half-time of the first phase increased slightly from 12 sec to 18 sec after PHA treatment, the second phase increased from 80 sec to 142 sec. Studies with truncated constructs identified the carboxy-terminal domain of STIM1 close to the poly lysine tail as being responsible for this inhibitory action (Ritchie et al. 2012). It is unclear whether STIM1 directly binds to the PMCA pump but immunoprecipitation experiments in the absence of PHA revealed significant interaction of STIM1 and PMCA under resting conditions, and this was not altered much by PHA stimulation (Ritchie et al. 2012). The amount of colocalization between STIM1 and PMCA pump was measured using fluorescence microscopy and only minimal overlap was observed in cells under resting conditions, but strong colocalization was seen after PHA-induced activation. Thapsigargin had no effect on STIM1/PMCA colocalization, suggesting STIM1 regulation of PMCA pump was independent of store depletion. Collectively, these results suggest that STIM1 impairs PMCA pump activity and therefore serves to sustain Ca2+ entry through CRAC channels independent of store depletion.
Interestingly, in the study by Ritchie et al. (2012), Ca2+ clearance kinetics was biphasic even after inhibition of mitochondrial Ca2+ uptake. This is different from previous reports in T cells that showed inhibition of mitochondrial Ca2+ uptake by depolarization with antimycin A and oligomycin to impair flux through the uniporter channel switched the biphasic decay to a monophasic event (Bautista et al. 2002; Bautista and Lewis 2004; Quintana et al. 2011). The slow second-phase reflected slow release of Ca2+ from mitochondria after the organelle had been loaded with Ca2+ during store-operated Ca2+ influx (Bautista et al. 2002). In contrast, RU360, considered a membrane permeable inhibitor of the uniporter channel, had no effect on Ca2+ clearance kinetics compared with control cells (Ritchie et al. 2012).
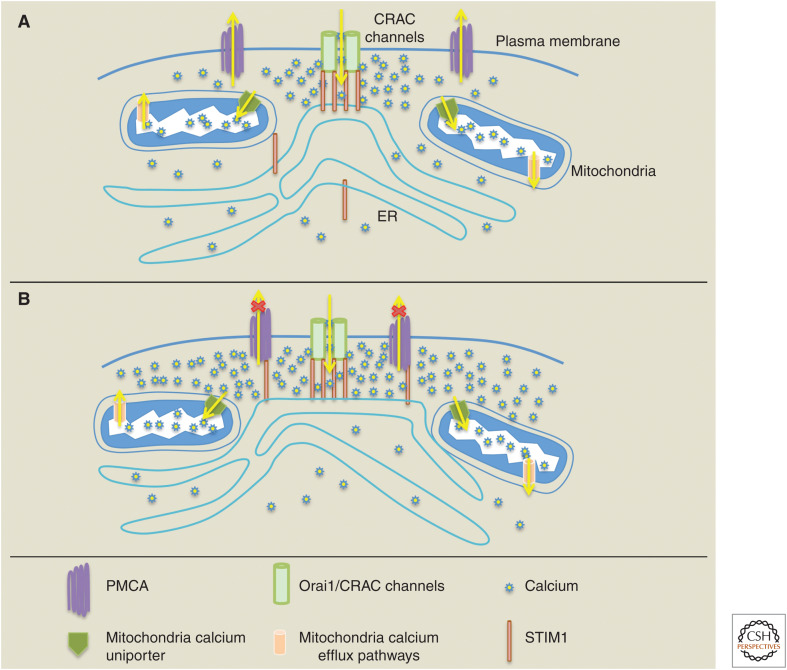
In T cells, there are therefore two models for how PMCA pumps impact on local Ca2+ concentration at the immunological synapse (Fig. 2). The models yield the same output, namely, PMCA activity is reduced after CRAC channel activation, but differ mechanistically in how this is accomplished. The Quintana et al. model (Fig. 2A) proposes that PMCA pump activity is low because mitochondria buffer Ca2+ at the edges of the synapse, in which the pumps are located, and the reduction in local Ca2+ both directly reduces PMCA pump activity and suppresses modulation from developing (Quintana et al. 2011). The Ritchie et al. model (Fig. 2B) posits that PMCA pump activity is low because of STIM1 inhibition of the transporter (Ritchie et al. 2012). The STIM1 block is presumably constitutive because the coimmunoprecipitation data suggested PMCA pump and STIM1 already interacted at rest and no further association was seen following stimulation with thapsigargin (Ritchie et al. 2012). If PMCA pumps are blocked by STIM1 at the synapse, then they should not be able to develop modulation. However, the data from Quintana et al. (2011) show robust modulation at the immunological synapse after mitochondrial depolarization. Now, local Ca2+ can increase sufficiently to stimulate the modulation process, an observation that is not predicted by model 2.
Figure 2.
The cartoon summarizes two models (A,B) for how the plasma membrane Ca2+ ATPase (PMCA) pump activity is regulated at the immunological synapse in T cells (see text for further details). CRAC, Ca2+ release-activated Ca2+; ER, endoplasmic reticulum.
The models could be reconciled by postulating a Ca2+-dependence to STIM1 block of the PMCA pump. During the fast phase of cytosolic Ca2+ clearance, cytosolic Ca2+ levels are high and this might prevent STIM1 from inhibiting PMCA pump activity. However, during the slow phase of Ca2+ clearance cytosolic Ca2+ is considerably reduced, and this could lead to STIM1-mediated PMCA inhibition. Such a mechanism could also explain why modulation occurs following mitochondrial depolarization (Figs. 1 and 2A); the elevated local Ca2+ concentration will no longer enable STIM1 to block PMCA pumps. It will be interesting to see in the future whether there is a Ca2+ dependence to STIM1 block of the PMCA pump or whether other factors account for the differences between the two models. Regardless, the PMCA pump has emerged as an important factor that can help shape local Ca2+ signals near CRAC channels and thus impact on their signaling strength. How CRAC channel-dependent Ca2+ microdomains communicate with PMCA pumps and whether such functional coupling can be regulated by stimulation of cell-surface receptors are interesting questions for the future.
INTRACELLULAR Orai1 CHANNELS
Although the majority of Orai channels are in the PM, a fraction is found on intracellular organelles. That these Orai proteins function as intracellular Ca2+ release channels was elegantly shown by Hille and colleagues (Dickson et al. 2012). Secretory granules bud from the trans-Golgi network and after maturation carry cargo to the PM, which is released by exocytosis. Free Ca2+ concentration within a secretory granule is ∼70 µm (Dickson et al. 2012), around three orders of magnitude larger than resting cytosolic free Ca2+. Using a FRET cameleon probe targeted to the secretory granule lumen, Dickson and colleagues found that stimulation of cell-surface Gq-coupled P2Y receptors led to slow release of Ca2+ from secretory granules into the cytosol. Three pieces of evidence suggested this form of Ca2+ release was mediated by Orai1 channels (Dickson et al. 2012). First, Ca2+ mobilization from the granules was prevented by stimulating P2Y receptors in the absence of external Ca2+. Second, the CRAC channel blocker BTP2 prevented Ca2+ release from the granules and third, expression of a nonconducting dominant-negative Orai1 channel (Orai1-E106A) prevented Ca2+ release from the granules during Gq-coupled P2Y receptor stimulation (using ATP). Confocal colocalization analysis using Orai1–GFP and tPA-mcherry (a marker for secretory granules) revealed a fraction of Orai1 channels resided on secretory granules. In contrast, there was little colocalization between STIM1 and secretory granules at rest. However, P2Y receptor stimulation increased colocalization between STIM1 and secretory granules and this was prevented by removal of external Ca2+. To explain these findings, these investigators proposed a very interesting model. Following stimulation, STIM1–Orai1 clusters formed below the PM. Subsequent store-operated Ca2+ entry raised local Ca2+ and this led to disaggregation of some STIM1 molecules from Orai1. The released STIM1 proteins were able, after a delay, to bind to and activate low-density Orai1 channels on secretory granules. Because the Ca2+ concentration within a granule is considerably lower than extracellular Ca2+ concentration (∼70 µm compared with 1–2 mm), the local Ca2+ near open Orai channels in the granule membrane will be much lower than the corresponding level at the PM and so STIM1 will presumably remain associated with granule Orai1 for longer.
What could be the physiological role of Orai1-dependent Ca2+ release from secretory granules? Because these Orai1 channels are activated by STIM1 resident in the ER membrane, Ca2+ release from the granules could contribute to effective refilling of the ER with Ca2+.
Another nice example of local Ca2+ signals generated by intracellular Orai1 stems from studies in neutrophils (Nunes et al. 2012). These phagocytes ingest foreign particles and degrade them within phagocytic vesicles. STIM1 was found to sustain phagocytosis by bringing thin ER cisternae to phagosomes, which then formed tight ER–phagosomal junctions. Local periphagosomal Ca2+ signals were seen in intact cells following particle ingestion and the frequency of these signals was significantly reduced by STIM1 ablation (Nunes et al. 2012). The local Ca2+ signals arose from phagosomal store-operated Orai1 channels that were activated by STIM1. These findings provide new potential targets for the treatment of infections caused by intracellular pathogens.
TUNNELING OF LOCAL Ca2+ ENTRY NEAR Orai1 CHANNELS THROUGH THE ER
Local Ca2+ entry through CRAC channels can be delivered to distant targets through a mechanism that bypasses diffusion through the cytosol. This pathway involves Ca2+ tunneling through the ER and was first described in pancreatic acinar cells (Mogami et al. 1997). In these polarized epithelial cells, store-operated Ca2+ entry occurs at the basolateral membrane. Local Ca2+ influx is then rapidly pumped into basolateral ER by SERCA pumps. The ER in acinar cells is a contiguous organelle, protruding into the apical pole where trypsin-containing secretory granules are confined (Park et al. 2000). InsP3 receptors are found at high density in the apical pole. The ER has a low Ca2+-buffering capacity compared with the cytosol (Mogami et al. 1999), and therefore Ca2+ that is taken up into the ER at the basolateral pole diffuses rapidly to the apical portion where it can be released into the cytosol through InsP3 receptors. In this way, local Ca2+ influx at the basolateral region can drive Ca2+-dependent exocytosis at the apical end. Ca2+ tunneling also couples store-operated Ca2+ entry to Ca2+-activated Cl− channels in Xenopus oocytes (Courjaret and Machaca 2014). In this system, the Ca2+ channels and Ca2+-activated Cl− channels are located apart but local Ca2+ influx is taken up into the ER by SERCA pumps and then released by InsP3 receptors positioned close to the Cl− channels, reminiscent of tunneling in acinar cells (Petersen et al. 2017). In mast cells, stimulation of Gq-coupled cysteinyl leukotriene type 1 receptors with the agonist LTC4 generates oscillations in nuclear Ca2+ that arise from opening of InsP3 receptors in the inner nuclear membrane and maintains NFAT4 transcription factor activity and subsequent cytokine gene expression (Kar et al. 2016). The oscillations run down rapidly in the presence of the CRAC channel blocker BTP2 or when external Ca2+ is removed. Ca2+ influx contributes little to the amplitude or decay rate of each cytoplasmic Ca2+ oscillation evoked by LTC4 (Di Capite et al. 2009). That the nuclear store, which is contiguous with the ER, is replenished by Ca2+ flux through CRAC channels at the cell surface is therefore another example of long-range Ca2+ tunneling.
CONCLUSION
Ca2+ microdomains near open Ca2+-permeable ion channels that populate the PM or internal Ca2+ stores regulate a range of cellular responses over a broad temporal bandwidth. Recent work has led to a remarkable wave of progress in understanding how these local Ca2+ signals are decoded by cells and how different types of Ca2+ channel tap into different intracellular signaling pathways. Recent advances in superresolution microscopy combined with increasingly sophisticated structural and biochemical approaches should provide more detailed insight into the properties of local Ca2+ signals and how they might be manipulated in the treatment of human disease.
ACKNOWLEDGMENTS
This work was supported by a Medical Research Council (MRC) Programme Grant (Grant No. LO1047X) to A.B.P. P.B. was a recipient of a European Molecular Biology Organization (EMBO) Long Term Fellowship.
Footnotes
Editors: Geert Bultynck, Martin D. Bootman, Michael J. Berridge, and Grace E. Stutzmann
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
- Allbritton NL, Meyer T, Stryer L. 1992. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science 258: 1812–1815. 10.1126/science.1465619 [DOI] [PubMed] [Google Scholar]
- Alswied A, Parekh AB. 2015. Ca2+ influx through store-operated calcium channels replenishes the functional phosphatidylinositol 4,5-bisphosphate pool used by cysteinyl leukotriene type I receptors. J Biol Chem 290: 29555–29566. 10.1074/jbc.M115.678292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr VA, Bernot KM, Srikanth S, Gwack Y, Balagopalan L, Regan CK, Helman DJ CL, Oh-Hora M S, Rao A, et al. 2008. Dynamic movement of the calcium sensor STIM1 and the calcium channel Orai1 in activated T-cells: Puncta and distal caps. Mol Biol Cell 19: 2802–2817. 10.1091/mbc.e08-02-0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Lewis RS. 2004. Modulation of plasma membrane calcium-ATPase activity by local calcium microdomains near CRAC channels in human T cells. J Physiol 556: 805–817. 10.1113/jphysiol.2003.060004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Hoth M, Lewis RS. 2002. Enhancement of calcium signalling dynamics and stability by delayed modulation of the plasma-membrane calcium-ATPase in human T cells. J Physiol 541: 877–894. 10.1113/jphysiol.2001.016154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. 1987. Inositol trisphosphate and diacylglycerol: Two interacting second messengers. Annu Rev Biochem 56: 159–193. 10.1146/annurev.bi.56.070187.001111 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Fain JN. 1979. Inhibition of phosphatidylinositol synthesis and the inactivation of calcium entry after prolonged exposure of the blowfly salivary gland to 5-hydroxytryptamine. Biochem J 178: 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Downes CP, Hanley MR. 1982. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J 206: 587–595. 10.1042/bj2060587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. 2003. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517–529. 10.1038/nrm1155 [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE. 1991. Bell-shaped calcium-response curves of Ins(1,4,5)P3– and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351: 751–754. 10.1038/351751a0 [DOI] [PubMed] [Google Scholar]
- Bird GS, Putney JWJ. 2005. Capacitative calcium entry supports calcium oscillations in human embryonic kidney cells. J Physiol 562: 697–706. 10.1113/jphysiol.2004.077289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caride AJ, Penheiter AR, Filoteo AG, Bajzer Z, Enyedi A, Penniston JT. 2001. The plasma membrane calcium pump displays memory of past calcium spikes. J Biol Chem 276: 39797–39804. 10.1074/jbc.M104380200 [DOI] [PubMed] [Google Scholar]
- Chang WC, Parekh AB. 2004. Close functional coupling between Ca2+ release-activated Ca2+ channels, arachidonic acid release and leukotriene secretion. J Biol Chem 279: 29994–29999. 10.1074/jbc.M403969200 [DOI] [PubMed] [Google Scholar]
- Chang WC, Nelson C, Parekh AB. 2006. Ca2+ influx through CRAC channels activates cytosolic phospholipase A2, leukotriene C4 secretion and expression of c-fos through ERK-dependent and independent pathways in mast cells. FASEB J 20: 2381–2383. 10.1096/fj.06-6016fje [DOI] [PubMed] [Google Scholar]
- Chang WC, Di Capite J, Singaravelu K, Nelson C, Halse V, Parekh AB. 2008. Local Ca2+ influx through Ca2+ release-activated Ca2+ (CRAC) channels stimulates production of an intracellular messenger and an intercellular pro-inflammatory signal. J Biol Chem 283: 4622–4631. 10.1074/jbc.M705002200 [DOI] [PubMed] [Google Scholar]
- Chiono M, Mahey R, Tate G, Cooper DMF. 1995. Capacitative Ca2+ entry exclusively inhibits cAMP synthesis in C6-2B glioma cells. Evidence that physiologically evoked Ca2+ entry regulates Ca2+-inhibitable adenylyl cyclase in non-excitable cells. J Biol Chem 270: 1149–1155. 10.1074/jbc.270.3.1149 [DOI] [PubMed] [Google Scholar]
- Clapham DE. 2007. Calcium signaling. Cell 131: 1047–1058. 10.1016/j.cell.2007.11.028 [DOI] [PubMed] [Google Scholar]
- Courjaret R, Machaca K. 2014. Mid-range Ca2+ signalling mediated by functional coupling between store-operated Ca2+ entry and IP3-dependent Ca2+ release. Nat Commun 5: 3916 10.1038/ncomms4916 [DOI] [PubMed] [Google Scholar]
- De Bock M, Wang N, Bol M, Decrock E, Ponsaerts R, Bultynck G, Dupont G, Leybaert L. 2012. Connexin 43 hemichannels contribute to cytoplasmic Ca2+ oscillations by providing a bimodal Ca2+-dependent Ca2+ entry pathway. J Biol Chem 287: 12250–12266. 10.1074/jbc.M111.299610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck P, Schulman H. 1998. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science 279: 227–230. 10.1126/science.279.5348.227 [DOI] [PubMed] [Google Scholar]
- Di Capite J, Ng SW, Parekh AB. 2009. Decoding of cytoplasmic Ca2+ oscillations through the spatial signature drives gene expression. Curr Biol 19: 853–858. 10.1016/j.cub.2009.03.063 [DOI] [PubMed] [Google Scholar]
- Dickson EJ, Duman JG, Moody MW LC, Hille B. 2012. Orai-STIM–mediated Ca2+ release from secretory granules revealed by a targeted Ca2+ and pH probe. Proc Natl Acad Sci 109: E3539–E3548. 10.1073/pnas.1218247109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. 1997. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386: 855–858. 10.1038/386855a0 [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Xu K, Lewis RS. 1998. Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392: 933–936. 10.1038/31960 [DOI] [PubMed] [Google Scholar]
- Fagan KA, Mahey R, Cooper DMF. 1996. Functional co-localization of transfected Ca2+-stimulable adenylyl cyclases with capacitative Ca2+ entry sites. J Biol Chem 271: 12438–12444. 10.1074/jbc.271.21.12438 [DOI] [PubMed] [Google Scholar]
- Fagan KA, Mons N, Cooper DMF. 1998. Dependence of the Ca2+-inhibitable adenylyl cyclase of C6–2B glioma cells on capacitative Ca2+ entry. J Biol Chem 273: 9297–9305. 10.1074/jbc.273.15.9297 [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SV, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. 2006. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441: 179–185. 10.1038/nature04702 [DOI] [PubMed] [Google Scholar]
- Finch EA, Turner TJ, Goldin SM. 1991. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science 252: 443–446. 10.1126/science.2017683 [DOI] [PubMed] [Google Scholar]
- Go CK, Soboloff J. 2018. Hold the door: hPMCA1/neuroplastin interactions regulate Ca2+ binding site accessibility. Cell Calcium 76: 135–136. 10.1016/j.ceca.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, Chi X, Ren K, Huang G, Zhou G, Yan N, Lei J, Zhou Q. 2018. Structure of the human plasma membrane Ca2+ ATPase 1 in complex with its obligatory subunit neuroplastin. Nat Commun 9: 3623 10.1038/s41467-018-06075-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Spitzer NC. 1995. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature 375: 784–787. 10.1038/375784a0 [DOI] [PubMed] [Google Scholar]
- Hajnóczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. 1995. Decoding of cytosolic calcium oscillations in the mitochondria. Cell 82: 415–424. 10.1016/0092-8674(95)90430-1 [DOI] [PubMed] [Google Scholar]
- Hoeflich KP, Ikura M. 2002. Calmodulin in action: Diversity in target recognition and activation mechanisms. Cell 108: 739–742. 10.1016/S0092-8674(02)00682-7 [DOI] [PubMed] [Google Scholar]
- Hogan PG. 2015. The STIM1–ORAI1 microdomain. Cell Calcium 58: 357–367. 10.1016/j.ceca.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan PG, Chen L, Nardone J, Rao A. 2003. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev 17: 2205–2232. 10.1101/gad.1102703 [DOI] [PubMed] [Google Scholar]
- Hoth M, Penner R. 1992. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 355: 353–356. 10.1038/355353a0 [DOI] [PubMed] [Google Scholar]
- Ivorra I, Parker I. 1992. Inhibition by Ca2+ of inositol trisphosphate-mediated Ca2+ liberation: A possible mechanism for oscillatory release of Ca2+. Proc Natl Acad Sci 87: 260–264. 10.1073/pnas.87.1.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SR, Kim K, Hille B, Nguyen TD, Koh DS. 2006. Pattern of Ca2+ increase determines the type of secretory mechanism activated in dog pancreatic duct epithelial cells. J Physiol 163–178. 10.1113/jphysiol.2006.114876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar P, Nelson C, Parekh AB. 2011. Selective activation of the transcription factor NFAT1 by calcium microdomains near Ca2+ release-activated Ca2+ (CRAC) channels. J Biol Chem 286: 14795–14803. 10.1074/jbc.M111.220582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar P, Bakowski D, Di Capite J, Nelson C, Parekh AB. 2012. Different agonists recruit different stromal interaction molecule proteins to support cytoplasmic Ca2+ oscillations and gene expression. Proc Natl Acad Sci 109: 6969–6974. 10.1073/pnas.1201204109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar P, Samanta K, Kramer H, Morris O, Bakowski D, Parekh AB. 2014. Dynamic assembly of a membrane signaling complex enables selective activation of NFAT by Orai1. Curr Biol 24: 1361–1368. 10.1016/j.cub.2014.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar P, Mirams GR, Christian HC, Parekh AB. 2016. Control of NFAT isoform activation and NFAT-dependent gene expression through two coincident and spatially segregated intracellular Ca2+ signals. Mol Cell 64: 746–759. 10.1016/j.molcel.2016.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata S, Tsutsumi R, Kohara A, Yamaguchi T, Nakanishi S, Okada M. 1996. Control of calcium oscillations by phosphorylation of metabotropic glutamate receptors. Nature 383: 89–92. 10.1038/383089a0 [DOI] [PubMed] [Google Scholar]
- Kim TD, Eddlestone GT, Mahmoud SF, Kuchtey J, Fewtrell C. 1997. Correlating Ca2+ responses and secretion in individual RBL-2H3 mucosal mast cells. J Biol Chem 272: 31225–31229. 10.1074/jbc.272.50.31225 [DOI] [PubMed] [Google Scholar]
- Korthals M, Langnaese K, Smalla KH, Kaehne T, Herrera-Molina R, Handschuh J, Lehmann AC, Mamula D, Naumann M, Seidenbecher C, et al. 2017. A complex of neuroplastin and plasma membrane Ca2+ ATPase controls T cell activation. Sci Rep 7: 8358 10.1038/s41598-017-08519-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkimmiatis K, Srikanthan M, Maiellaro I, Moyer MP, Curci S, Hofer AM. 2009. Store-operated cyclic AMP signalling mediated by STIM1. Nat Cell Biol 11: 433–442. 10.1038/ncb1850 [DOI] [PubMed] [Google Scholar]
- Lewis RS, Prakriya M. 2015. Store-operated calcium channels. Physiol Rev 95: 1383–1436. 10.1152/physrev.00020.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Pink MD, Murphy JG, Stein A, Dell'Acqua ML, Hogan PG. 2012. Balanced interactions of calcineurin with AKAP79 regulate Ca2+-calcineurin-NFAT signaling. Nat Struct Mol Biol 19: 337–345. 10.1038/nsmb.2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr, Meyer T. 2005. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ infux. Curr Biol 15: 1235–1241. 10.1016/j.cub.2005.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioudyno MI, Kozak JA, Penna A, Safrina O, Zhang SL, Sen D, Roos J, Stauderman KA, Cahalan MD. 2008. Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc Natl Acad Sci 105: 2011–2016. 10.1073/pnas.0706122105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Stryer L. 1988. Molecular model for receptor-stimulated calcium spiking. Proc Natl Acad Sci 85: 5051–5055. 10.1073/pnas.85.14.5051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogami H, Nakano K, Tepikin AV, Petersen OH. 1997. Ca2+ flow via tunnels in polarized cells: Recharging of apical Ca2+ stores by focal Ca2+ entry through basal membrane patch. Cell 88: 49–55. 10.1016/S0092-8674(00)81857-7 [DOI] [PubMed] [Google Scholar]
- Mogami H, Gardner J, Gerasimenko OV, Camello P, Petersen OH, Tepikin AV. 1999. Calcium binding capacity of the cytosol and endoplasmic reticulum of mouse pancreatic acinar cells. J Physiol 518: 463–467. 10.1111/j.1469-7793.1999.0463p.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motiani RK, Tanwar J, Raja DA, Vashisht A, Khanna S, Sharma S, Srivastava S, Sivasubbu S, Natarajan VT, Gokhale RS. 2018. STIM1 activation of adenylyl cyclase 6 connects Ca2+ and cAMP signaling during melanogenesis. EMBO J 37: e97597 10.15252/embj.201797597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. 1998. Vesicle pools and Ca2+ microdomains: New tools for understanding their roles in neurotransmitter release. Neuron 20: 389–399. 10.1016/S0896-6273(00)80983-6 [DOI] [PubMed] [Google Scholar]
- Ng SW, DiCapite JL, Singaravelu K, Parekh AB. 2008. Sustained activation of the tyrosine kinase Syk by antigen in mast cells requires local Ca2+ influx through Ca2+ release-activated Ca2+ channels. J Biol Chem 283: 31348–31355. 10.1074/jbc.M804942200 [DOI] [PubMed] [Google Scholar]
- Ng SW, Nelson C, Parekh AB. 2009. Coupling of Ca2+ microdomains to spatially and temporally distinct cellular responses by the tyrosine kinase Syk. J Biol Chem 284: 24767–24772. 10.1074/jbc.M109.011692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes P, Cornut D, Bochet V, Hasler U, Oh-Hora M, Waldburger JM, Demaurex N. 2012. STIM1 juxtaposes ER to phagosomes, generating Ca2+ hotspots that boost phagocytosis. Curr Biol 22: 1990–1997. 10.1016/j.cub.2012.08.049 [DOI] [PubMed] [Google Scholar]
- Oancea E, Meyer T. 1998. Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell 95: 307–318. 10.1016/S0092-8674(00)81763-8 [DOI] [PubMed] [Google Scholar]
- Parekh AB. 2008. Ca2+ microdomains near plasma membrane Ca2+ channels: Impact on cell function. J Physiol 586: 3043–3054. 10.1113/jphysiol.2008.153460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB. 2011. Decoding cytosolic Ca2+ oscillations. Trends Biochem Sci 36: 78–87. 10.1016/j.tibs.2010.07.013 [DOI] [PubMed] [Google Scholar]
- Parekh AB, Putney JWJ. 2005. Store-operated calcium channels. Physiol Rev 85: 757–810. 10.1152/physrev.00057.2003 [DOI] [PubMed] [Google Scholar]
- Park MK, Petersen OH, Tepikin AV. 2000. The endoplasmic reticulum as one continuous Ca2+ pool: Visualization of rapid Ca2+ movements and equilibration. EMBO J 19: 5729–5739. 10.1093/emboj/19.21.5729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penheiter AR, Bajzer Z, Filoteo AG, Thorogate R, Torok K, Caride AJ. 2003. A model for the activation of plasma membrane calcium pump isoform 4b by calmodulin. Biochemistry 42: 12115–12124. 10.1021/bi027098+ [DOI] [PubMed] [Google Scholar]
- Petersen OH, Courjaret R, Machaca K. 2017. Ca2+ tunnelling through the ER lumen as a mechanism for delivering Ca2+ entering via store-operated Ca2+ channels to specific target sites. J Physiol 595: 2999–3014. 10.1113/JP272772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. 2006. Orai1 is an essential pore subunit of the CRAC channel. Nature 443: 230–233. 10.1038/nature05122 [DOI] [PubMed] [Google Scholar]
- Quintana A, Pasche M, Junker C, Al-Ansary D, Rieger H, Kummerow C, Nunez L, Villalobos C, Meraner P, Becherer U, et al. 2011. Calcium microdomains at the immunological synapse: How Orai channels, mitochondria and calcium pumps generate local calcium signals for efficient T-cell activation. EMBO J 30: 3895–3912. 10.1038/emboj.2011.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie MF, Samakai E, Soboloff J. 2012. STIM1 is required for attenuation of PMCA-mediated Ca2+ clearance during T-cell activation. EMBO J 31: 1123–1133. 10.1038/emboj.2011.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, et al. 2005. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169: 435–445. 10.1083/jcb.200502019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta K, Kar P, Mirams GR, Parekh AB. 2015. Ca2+ channel re-localization to plasma-membrane microdomains strengthens activation of Ca2+-dependent nuclear gene expression. Cell Rep 12: 203–216. 10.1016/j.celrep.2015.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth TJ, Thompson JL. 1999. Discriminating between capacitative and arachidonate-activated Ca2+ entry pathways in HEK293 cells. J Biol Chem 274: 31174–31178. 10.1074/jbc.274.44.31174 [DOI] [PubMed] [Google Scholar]
- Tepikin AV, Voronina SG, Gallacher DV, Petersen OH. 1992. Pulsatile Ca2+ extrusion from single pancreatic acinar cells during receptor-activated cytosolic Ca2+ spiking. J Biol Chem 267: 14073–14076. [PubMed] [Google Scholar]
- Thomas AP, Bird GS, Hajnóczky G, Robb-Gaspers LD, Putney JWJ. 1996. Spatial and temporal aspects of cellular calcium signaling. FASEB J 10: 1505–1517. 10.1096/fasebj.10.13.8940296 [DOI] [PubMed] [Google Scholar]
- Tse A, Tse FW, Almers W, Hille B. 1993. Rhythmic exocytosis stimulated by GnRH-induced Ca2+ oscillations in rat gonadotropes. Science 260: 82–84. 10.1126/science.8385366 [DOI] [PubMed] [Google Scholar]
- Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, et al. 2006a. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol 16: 2073–2079. 10.1016/j.cub.2006.08.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, et al. 2006b. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312: 1220–1223. 10.1126/science.1127883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedel B, Boyles RR, Putney JW, Bird GS. 2007. Role of the store-operated calcium entry proteins Stim1 and Orai1 in muscarinic cholinergic receptor-stimulated calcium oscillations in human embryonic kidney cells. J Physiol 579: 679–689. 10.1113/jphysiol.2006.125641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby D, Everett KL, Halls ML, Pacheco J, Skroblin P, Vaca L, Klussmann E, Cooper DM. 2012. Direct binding between Orai1 and AC8 mediates dynamic interplay between Ca2+ and cAMP signaling. Sci Signal 5: ra29 10.1126/scisignal.2002299 [DOI] [PubMed] [Google Scholar]
- Woods NM, Cuthbertson KS, Cobbold PH. 1987. Phorbol-ester-induced alterations of free calcium ion transients in single rat hepatocytes. Biochem J 246: 619–623. 10.1042/bj2460619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MM, Buchanan J, Luik RM, Lewis RS. 2006. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol 174: 803–813. 10.1083/jcb.200604014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh YC, Parekh AB. 2015. Distinct structural domains of caveolin-1 independently regulate Ca2+ release-activated Ca2+ channels and Ca2+ microdomain-dependent gene expression. Mol Cell Biol 35: 1341–1349. 10.1128/MCB.01068-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. 2006. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 443: 226–229. 10.1038/nature05108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yeromin AV, Zhang XHF, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. 2006. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci 103: 9357–9362. 10.1073/pnas.0603161103 [DOI] [PMC free article] [PubMed] [Google Scholar]