Abstract
The amniote embryonic heart starts as a crescent of mesoderm that transitions through a midline linear heart tube in the course of developing into the four chambered heart. It is unusual in having to contract rhythmically while still undergoing extensive morphogenetic remodeling. Advances in imaging have allowed us to determine when during development this contractile activity starts. In the mouse, focal regions of contractions can be detected as early as the cardiac crescent stage. Calcium transients, required to trigger contraction, can be detected even earlier, prior to contraction. In this review, we outline what is currently known about how this early contractile function is initiated and the impact early contractile function has on cardiac development.
The role of the beating heart in sustaining life is so central that no other organ appears as commonly in prose and poetry, associated with not just vitality but also any number of other positive characteristics such as courage, honesty, perseverance, loyalty, and, of course, love. The rhythmic beating of the heart is so constant and all-pervasive during our life that we often take it for granted. In this review, we discuss what we currently know about when this rhythmic activity first starts.
The heart starts to develop in the mouse at approximately embryonic day 8.0 and in the human at around 16 days after fertilization. It is arguably the first organ to form and function during embryogenesis as it shows contractile activity already at these earliest stages (Fig. 1). In this review, we use “cardiac function” to mean the ability of cardiomyocytes to contract to produce force. This contractile function is critical in supplying the rapidly growing postimplantation embryo with sufficient oxygen and nutrients to develop properly. Because of the fundamental role of the heart, early perturbations in its development frequently result in embryonic lethality (Copp 1995). Indeed, congenital heart disease accounts for 10% of all spontaneous abortions, and heart defects are present in 1% of all live births (Triedman and Newburger 2016).
Figure 1.
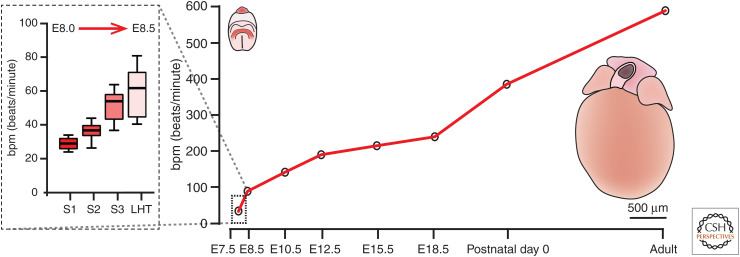
Increase in beat rate during the course of heart development. During mouse development, cardiac contractile activity starts at approximately embryonic day 8.0 at roughly 30 beats per minute (bpm) and increases in the adult mouse to 600 bpm. The dotted box shows the rapid increase in rate of contraction during the onset of cardiac function from cardiac crescent stages 1 through to the linear heart tube, roughly a 12-hour period from approximately E8.0 to E8.5. The images show, to scale, an embryo at E8.0 (at left, cardiac crescent in red) and an isolated adult mouse heart (at right). (LHT) Linear heart tube.
The embryonic heart is composed of maturing cardiomyocytes and cardiac progenitors that have yet to differentiate (Meilhac and Buckingham 2018). It also undergoes significant morphological changes, transitioning from a crescent into the linear heart tube (LHT). Recent live imaging experiments have reported two waves of development during this period, with an initial wave of differentiation followed by a second in which morphological changes occur (Ivanovitch et al. 2017). Among organs, the heart is unusual in having to contract rhythmically while still undergoing extensive remodeling. The coordinated changes in morphology, physiology, and cellular maturation that occur during the onset of cardiac function highlights how the development of form and function in the embryonic heart are inextricably linked. This raises important questions regarding when contractile activity is first initiated during development, the extent to which this influences the formation of cardiomyocytes, and its impact on subsequent cardiac morphogenesis. This is especially important as the forces exerted by cardiac contractions have been shown in several models to be required for proper heart development (Granados-Riveron and Brook 2012) and for modulating gene expression (Miyasaka et al. 2011) at later developmental stages.
Cardiac function is crucial not only for heart development but also for the development of the whole embryo, with knockout of key components of cardiac function leading to embryonic lethality around E9.5–E10.5 in the mouse (Table 1). Cardiac contraction is vital in providing nutrients to the developing embryo as well as influencing mechanosensory and calcium signaling. This leads to regulated gene expression and differentiation of cardiomyocytes, as well as other embryonic cell types such as endothelial and hematopoietic progenitors. In the LHT, contractile activity and circulation generate hemodynamic forces and wall sheer stress that guide the morphological changes that occur during cardiac looping (Hove et al. 2003). Hemodynamic forces generated by cardiac contraction are also necessary and sufficient to induce vessel remodeling in the mammalian yolk sac (Lucitti et al. 2007). It is also required for the distribution of hematopoietic progenitors throughout the fetus (Lux et al. 2007) and regulates their differentiation (North et al. 2009).
Table 1.
The role of ECC proteins during embryonic heart development
| Protein (gene) | Cardiac phenotype |
|---|---|
| NCX1 (Slc8a1) | Lethality E9 to E10; thin myocardium with reduced cardiomyocytes; no heartbeat observed (Wakimoto et al. 2000) |
| Lethality E9.0 to E9.5; underdeveloped heart with dilated pericardium; contractile although significantly slower than wild-type (WT) littermates; mutant and WT hearts indistinguishable at E8.5 (Cho et al. 2000) | |
| Lethality E11.5 to E13.5; lack of a spontaneous beating heart and organized myofibrils (Koushik et al. 2001) | |
| Cav1.2 (Cacna1c) | Lethality prior to E14.5; normal development until E12.5, contractile rate the same as WT littermates (Seisenberger et al. 2000) |
| Cav1.3 (Cacna1d) | Viable embryos; pronounced bradycardia and arrhythmias at rest in the adult; altered sinoatrial pacemaker activity (Platzer et al. 2000) |
| RyR2 (Ryr2) | Lethality E10.5 to E11.5; rhythmic contractions at E9.5, although irregularly arranged myocardium; no heartbeat at E10.5, anatomically similar to E9.5 (Takeshima et al. 1998) |
| SERCA2a (Atp2a2) | No mutant embryos detected with germline deletion (Periasamy et al. 1999); conditional myocardial deletion led to lethality at E11.5; circulation and heartbeat observed until E10.5 (Andersson et al. 2009) |
| Nav1.5 (Scn5a) | Lethality E10.5 to E11.5; some uncoordinated beating at E10.5, however, none observed at E11.5; reduced ventricular chamber size, trabeculation, and cardiomyocytes (Papadatos et al. 2002) |
| HCN4 (Hcn4) | Spontaneous beating E8.5–E10.5 although reduced rate of contraction; lethal between E9.5 and 11.5 (Stieber et al. 2003) |
| cTnT (Tnnt2) | Lack of heartbeat while Ca2+ transients still detected; no phenotypic differences until E8.75 leading to looping defects, sarcomere disassembly, and dilated hearts; lethality between E10.5 and 11.5 (Nishii et al. 2008) |
Summary of studies in which proteins required for excitation–contraction coupling (ECC) cardiac physiology and Ca2+ handling have been deleted in mice. All deletions are germline unless otherwise stated.
In this review, we describe what is known about when and how the heart first establishes coordinated contractile activity. We start with a brief historic overview of pioneering studies in this area. This is followed by a description of the cellular machinery that generates the force required for contraction to occur and the mechanisms that ensure these forces are coordinated both within the cell and across the field of contractile cells. We conclude with a discussion of emerging approaches for disentangling the many questions that remain regarding how the heart first starts to beat.
ONSET OF CARDIAC FUNCTION
Fascination with when the heart starts to beat has existed for centuries, with the recurring theme that as technology has advanced, the precise stage at which it has been reported to start beating has become earlier and earlier. Contractile activity has been observed directly through the microscope (Sabin 1920; Nishii et al. 2006; Chen et al. 2015) or, more recently, inferred by using organic dyes to visualize the Ca2+ oscillations and voltage action potentials that control contraction (Kamino 1991). We have even been able to use atomic force microscopy to detect contractile activity at a stage when it is not visually detectible with an optical microscope (Chen et al. 2015).
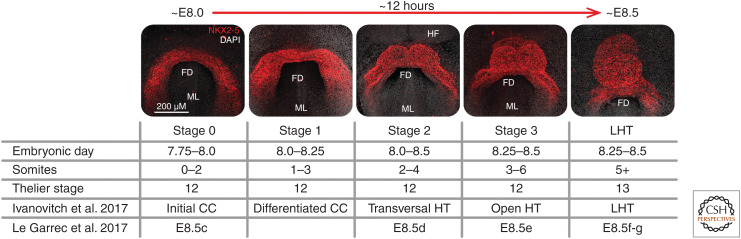
In addition to detection technology, refinements in staging the developing heart have allowed the onset of cardiac function to be more precisely defined (see below and Fig. 2). Older studies have used “embryonic day” or the number of somite pairs to stage heart development. Although these are useful for more general staging of the embryo, they do not offer the granularity required to accurately stage the embryonic heart during a time of rapid morphological change from the early cardiac crescent to LHT. Furthermore, somite number does not consistently correlate to the overall embryonic stage (Kaufman and Navaratnam 1981) or development of the heart because of normal variation in the rate of development of different structures in the embryo and differences in genetic background. This can lead to ambiguities because, for example, the three-somite stage may include embryos that range from the cardiac crescent to early LHT stages. To more accurately assess and describe changes in the forming heart, we and others have developed staging systems specific to the early heart (Tyser et al. 2016; Ivanovitch et al. 2017; Le Garrec et al. 2017). Based on morphological criteria, we divide the cardiac crescent into four distinct stages (stages 0 through 3) leading to the early LHT (Fig. 2).
Figure 2.
Staging system for cardiac development. We developed a numerical staging system to describe different phases of early cardiac development in a fine-grained manner. Stages 0 to 2 correspond to progressively more developed cardiac crescents (CC), whereas stage 3 represents a transition toward the linear heart tube (LHT) stage. Images are maximum-intensity projections of whole mount immunofluorescence showing the expression of NKX2-5, a marker of cardiac progenitors, highlighting changes in cardiac morphology during these stages. The table shows other staging systems for comparison, including two other recent systems specific to the heart. (FD) Foregut diverticulum, (ML) midline, (HF) headfold.
Modern studies of the embryonic onset of cardiac function were first undertaken in 1920 by Florence Sabin, a pioneer of women in science, being the first woman to hold a full professorship at Johns Hopkins University and the first woman elected to the American National Academy of Science in 1925. In her study published in 1920, Sabin used the chick embryo to investigate the origin of blood vessels and blood corpuscles (Sabin 1920). She cultured embryos hanging from a glass coverslip for up to 5 hours and observed them using a brightfield microscope. Although her primary focus was not on the heart, in these studies she first reported the onset of beating in the chick embryo at the 10-somite stage (∼HH10) at a single location along the right-hand margin of the medial primitive ventricle of an LHT that had already started the process of looping. She described how this initial slow rhythmic beating would spread from the caudal to rostral end of the LHT.
The first dedicated study into the initiation of cardiac contraction was conducted by Pattern and Kramer in 1933, again in the chick embryo. They reported that contractions started in the primitive ventricle of the LHT around the nine- or 10-somite stage (Patten and Kramer 1933). They differed from Sabin in that they observed several locations with “local twitching” but always within the right wall of the primitive ventricle as Sabin had found. When first detected, these twitches were not rhythmic, but soon became coordinated. Pattern and Kramer concluded that their findings differed from that of Sabin because they were able to detect contractions earlier, because of methodological advances and their ability to use enlarged film tracings to pick up less evident changes. They went on to describe how the contractile regions on the right side of the primitive ventricle become coordinated before the “restless” left side begins to contract synchronously with the right. At this point, the entire primitive ventricle contracts as one, but subsequently over the course of 1–2 hours the contractile activity changes to sweep through the ventricle in a caudal to rostral direction.
The first studies in mammals were conducted by Goss in 1938 using rat embryos. He reported that contractile activity began at E9.5 (equivalent approximately to E8.5 in mice) in the lateral ventricular myocardium of the left LHT. These first contractions had a regular rhythm and a rate of 37 to 42 beats per minute (bpm) (Goss 1938). He further described pacemaker-like activity in regions on either side of the embryonic midline, suggesting that in contrast to the chick, contractile function in rats commenced prior to the formation of the LHT at around the two- to three-somite stage (Goss 1952).
These earlier studies relied on the use of brightfield microscopy to determine the onset of contraction. However, as new fluorescence imaging technologies developed, voltage and Ca2+-sensitive dyes were used to measure action potentials and Ca2+ transients in early embryonic hearts. Kamino and colleagues showed, using voltage-sensitive dyes, that spontaneous electrical activity could be observed in the chick cardiac primordium at the six- and early seven-somite stage and in rat at the three-somite stage (Kamino 1991). More recently, Ca2+ transients were reported in the cardiac crescent of rat embryos (Kobayashi et al. 2011).
As the mouse became the established model for studying mammalian development, staging systems based on the number of somite pairs were created to compare development of different species (Kaufman and Navaratnam 1981). Using this staging approach, the onset of cardiac contraction in the mouse was placed at the three- to four-somite stage, corresponding roughly to the LHT at E8.5 (Navaratnam et al. 1986). Later studies in mouse embryos reported that contractile activity could be first detected in the cardiac crescent at the three-somite stage initiating at ∼30 bpm (Nishii et al. 2006). This study showed that contractions initiated on either the left or right side and that independent regions had different beating rates.
Most recently, we have shown that contraction initiates earlier, in the lateral regions of the early cardiac crescent that we term “stage 1” corresponding with the one- to three-somite stage embryo (Fig. 2). We do not observe contractions or sarcomeric banding at earlier stages (stage 0 and earlier), consistent with the notion that assembly of the contractile machinery into sarcomeres is required to generate sufficient spatial displacement to be detected optically. When first detected, these contractions are ∼30 bpm but increase to >60 bpm with formation of the LHT (Tyser et al. 2016). This indicates that cardiomyocytes start contracting at the very earliest stages of heart development, essentially as soon as they are formed and assemble mature sarcomeres (Fig. 3).
Figure 3.
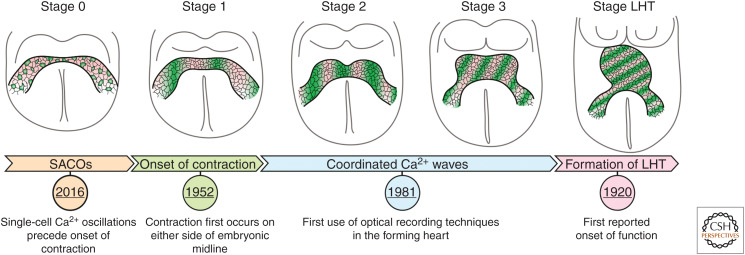
Changes in Ca2+ dynamics during the onset of cardiac function. Schematic of the changes that occur during development of the cardiac crescent. Green shading represents changes in the cytoplasmic level of Ca2+. At stage 0, prior to the onset of function, spontaneous asynchronous calcium oscillations (SACOs) are observed in single cells. At stage 1, contractions can first be observed with Ca2+ transients propagating through the cardiac crescent in a lateral orientation. At later stages, the rate of Ca2+ transient propagation continues to increase in line with the rate of contraction. At the linear heart tube (LHT) stage, Ca2+ transients begin to propagate in a more caudal to rostral direction.
Coordinated Ca2+ transients are also required for rhythmic contraction. At stage 0, prior to the onset of contraction, we detected spontaneous asynchronous calcium oscillations (SACOs) in immature cardiomyocytes (discussed later in this review), suggesting that as cells mature, the molecular machinery for Ca2+ handling is put in place slightly before the contractile machinery is assembled into sarcomeres. Although we cannot exclude the possibility that contractile activity exists earlier than stage 1, it is unlikely, given that cardiac progenitors lack the sarcomeric assemblies required for contraction and do not have coordinated Ca2+ activity.
MOLECULAR UNDERPINNING OF CARDIAC FUNCTION
Cardiomyocytes have a highly defined structure that allows them to respond to rhythmic electrical excitation to generate the force required to circulate blood around the body. Immature cardiomyocytes/progenitors must develop both the apparatus needed to generate physical contraction and the ability to regulate contraction in a synchronized manner. These two requirements are met, respectively, by the formation of sarcomeres and the coordinated activity of channels, pumps, and exchangers to modulate cytosolic Ca2+ levels.
The contractile apparatus of cardiomyocytes, the myofibrils, are long structures composed of bundles of myosin, actin, and titin, making up the thick, thin, and elastic filaments, respectively (Clark et al. 2002; Ehler 2016). These filaments are arranged in a repeating manner along the length of the myofibril in units called sarcomeres. The alignment of sarcomeres across myofibrils is what gives cardiomyocytes their characteristic striated appearance. Contraction of myofibrils relies on the binding of Ca2+ to the troponin complex, leading to a conformational change that causes myosin heads to flex and slide along the actin filaments, shortening sarcomere length and resulting in the generation of contractile force (Metzger and Westfall 2004). For contraction to occur in nascent cardiomyocytes, this highly organized contractile machinery needs to first form. Analyses over the course of cardiac crescent to LHT formation have shown that contractile proteins required for function are expressed in a region- and time-dependent manner (Tyser et al. 2016). One of the earliest contractile proteins to be expressed is cardiac troponin T, which is expressed throughout the cardiac crescent prior to the onset of function (Ivanovitch et al. 2017). In contrast, other sarcomeric proteins such as sarcomeric-α actinin and myomesin are initially only detected in the more lateral regions of the cardiac crescent prior to the onset of contraction, in the regions in which contractile function is first initiated (Tyser et al. 2016). These regional differences in the expression of contractile proteins could be the result of a mediolateral gradient of maturation within the cardiac crescent, with more lateral cells being the first to mature sufficiently to assemble functional sarcomeres and therefore being the first to display contractile function. More detailed fate mapping of the cardiac mesoderm, which gives rise to the crescent, will need to be conducted to test this possibility.
Expression of contractile proteins is not in itself sufficient for contractile activity—they need to be assembled into sarcomeres. Prior to contraction, a number of sarcomeric proteins can be observed in the cardiac crescent, but not in the banded pattern characteristic of sarcomeres. As the crescent develops rapidly over the time course of 1–2 hours, one can see the emergence of sarcomere banding in a small number of cells in the lateral regions of the stage 1 crescent, highlighting the formation of functional sarcomeres and corresponding with the onset of function (Tyser et al. 2016). Subsequently, as cardiac development progresses through stages 2 and 3, the expression of contractile proteins continues to increase and sarcomeric banding rapidly spreads across the crescent, resulting in increased contraction.
In addition to sarcomeric organization, the other aspect crucial to cardiac function is the ability to modulate the cytoplasmic level of Ca2+, which is what actually triggers contraction. When contractile components such as cardiac troponin T (encoded by Tnnt2) are knocked out, the onset of contraction is prevented but Ca2+ transients still persist, the heart develops normally until E8.75, and the embryo dies between E10.5 and E11.5 (Nishii et al. 2008). In contrast, when the Na+/Ca2+ exchanger, a key complex required for Ca2+ homeostasis, is knocked out, both Ca2+ transients and contraction fail, resulting in embryonic lethality as early as E9.5 (Wakimoto et al. 2000). This highlights that, for the onset of cardiac function, it is crucial that both the contractile apparatus and the ability to dynamically regulate cytosolic Ca2+ are present.
CHANGING CARDIOMYOCYTE PHYSIOLOGY IN THE DEVELOPING HEART
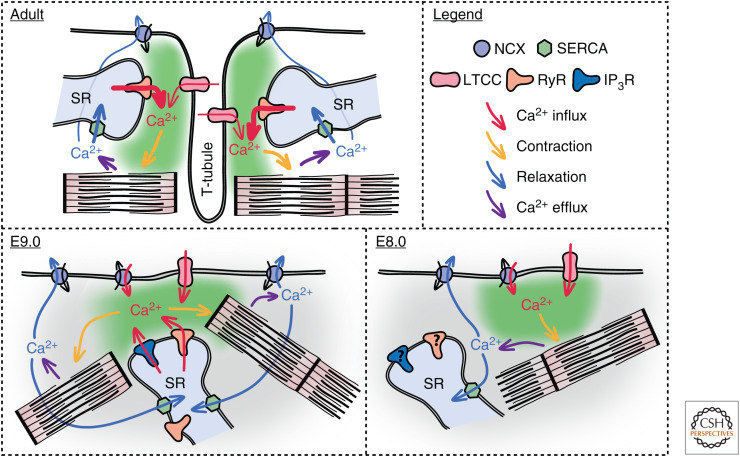
For rhythmic contractions to occur, the intracellular concentration of Ca2+ must increase and decrease in a periodic manner, to regulate myosin–actin interaction. For the onset of cardiac function, cardiac progenitors, therefore, must have the ability to dynamically regulate the concentration of cytosolic Ca2+. In adult cardiomyocytes, rapid changes in concentration of intracellular Ca2+ are coupled to coordinated electrical excitation in a process termed excitation contraction coupling (ECC), predominantly relying on Ca2+ release from the sarcoplasmic reticulum (SR) (Fig. 4; Bers 2002).
Figure 4.
The mechanism of Ca2+ regulation is significantly different in adult compared to embryonic cardiomyocytes. In the adult cardiomyocyte, contraction occurs via excitation–contraction coupling (ECC) in which an action potential triggers the opening of L-type Ca2+ channels (LTCCs) causing Ca2+ influx, which leads to the release of Ca2+ from the sarcoplasmic reticulum (SR) via ryanodine receptors (RyRs). The increase in cytoplasmic Ca2+ leads to contraction of myofibrils. For relaxation to occur, the Ca2+ is removed from the cytoplasm; the majority of Ca2+ is pumped back into the SR via SERCA while some is removed by the Na+/Ca2+ exchanger (NCX). In E9.0 cardiomyocytes, Ca2+ transients are driven by the spontaneous release of Ca2+ from the SR via RyRs and inositol 3-phosphate receptors (IP3Rs). This leads to Ca2+ efflux via NCX, which, because of its electrogenic properties, triggers membrane depolarization leading to the opening of LTCCs and further Ca2+ influx. It is also reported that NCX has a role in Ca2+ influx because of its ability to work in both forward (Ca2+ efflux) and reverse (Ca2+ influx) modes. During the onset of function (E8.0), both NCX and LTCCs are required for the generation of Ca2+ transients. However, current evidence suggests that both RyRs and IP3Rs are not involved at this stage, with plasmalemmal Ca2+ flux being the main mechanism for changing cytoplasmic Ca2+ levels.
Although ECC has been well characterized in mature cardiomyocytes, it is less well defined in embryonic cardiomyocytes during the onset of function. Targeted disruption of genes encoding ECC proteins in mice has shown that the early contractile activity of immature cardiomyocytes evidently does not require ECC (Table 1). In the majority of these transgenic models, the immature heart remained contractile until ∼E11.5, highlighting physiological differences in the mechanism by which cytosolic Ca2+ levels are regulated in the adult versus embryonic cardiomyocyte. These differences are also manifested in the resting membrane potential of adult cardiomyocytes (−75 mV) compared to E8.5 immature embryonic cardiomyocytes (−33.2 mV) (Sasse et al. 2007).
Historically, two opposing mechanisms have been proposed for Ca2+ handling in immature cardiomyocytes. Initially, it was suggested that increases in the concentration of cytosolic Ca2+ were solely the result of plasmalemmal Ca2+ entry with little or no contribution from SR stores (Nakanishi et al. 1988; Takeshima et al. 1998). However, later studies in embryonic cardiomyocytes showed that Ca2+ transients can also be driven by SR Ca2+ release (Viatchenko-Karpinski et al. 2002; Méry et al. 2005). Most recently, studies have shown that a combination of both plasmalemmal Ca2+ influx and spontaneous SR Ca2+ release are required for function in early embryonic cardiomyocytes (Sasse et al. 2007; Rapila et al. 2008; Karppinen et al. 2014).
The earliest detailed electrophysiological analysis of immature cardiomyocytes used cardiomyocytes cultured for between 12 to 70 hours after being isolated from embryonic hearts between E8.5 and E9.5, roughly 12 hours after the in vivo onset of function (Reppel et al. 2007; Rapila et al. 2008; Liang et al. 2010). Using such a system, the mechanism by which contraction is regulated was shown to originate in the spontaneous release of Ca2+ from SR stores via ryanodine (RyRs) and inositol 3-phosphate receptors (IP3Rs) (Fig. 4; Sasse et al. 2007; Rapila et al. 2008). This spontaneous release of Ca2+ elevates the concentration of cytosolic Ca2+ leading to the removal of Ca2+ via the Na+/Ca2+ exchanger. The Na+/Ca2+ exchanger is electrogenic and its removal of Ca2+ creates a small inward current, which leads to membrane potential depolarization and the activation of voltage-gated Na+ and Ca2+ channels, thereby generating an action potential. Whereas early embryonic cardiomyocytes are thought to be voltage-independent Ca2+ oscillators, the generation of such action potentials is thought to provide a mechanism for the synchronization of electrical and mechanical signals (Sasse et al. 2007; Rapila et al. 2008). Studies on early immature cardiomyocytes at the same stage have also demonstrated that not only is the Na+/Ca2+ exchanger important for the efflux of Ca2+ following cytosolic increases in concentration but it can also play a role in Ca2+ influx at more positive membrane potentials, thereby having a fundamental role in Ca2+ homeostasis and the onset of cardiac function, especially in light of the more depolarized resting membrane potential at these stages (Linask et al. 2001; Reppel et al. 2007).
More recently, we have examined the molecular basis for Ca2+ regulation in the intact cardiac crescent during the onset of function and subsequent maturation of early cardiac progenitors, using ex vivo whole embryo Ca2+ imaging. We find a shift between mechanisms through which Ca2+ transients are generated, with limited contribution from the SR (Fig. 4). Early function in the stages 1 to 2 cardiac crescent can be inhibited by blocking both the Na+/Ca2+ exchanger and L-type channels. However, as the crescent matures toward the LHT, blocking the Na+/Ca2+ exchanger no longer has an inhibitory effect on the generation of Ca2+ transients while blocking the L-type channel continues to do so. Blocking Ca2+ release from SR stores by inhibiting RyRs and IP3Rs did not have an effect on Ca2+ transient generation at early crescent stages, indicative of a limited role for the SR at this stage. Inhibition of these receptors, however, prevents contraction shortly thereafter (at the looping LHT stage at ∼E8.5), consistent with previous reports that Ca2+ transients in the E8.5 heart originate from SR through RyR- and IP3R-mediated Ca2+ release (Sasse et al. 2007; Rapila et al. 2008). The absence of a role for the SR in generating Ca2+ transients in the cardiac crescent may be because the SR is functionally immature in these early cardiomyocytes, potentially because of an absence of functional RyR and IP3Rs. Alternatively, this early stage might represent a period of SR Ca2+ filling, during which the concentration of SR Ca2+ is increasing but is not yet at a level where spontaneous Ca2+ release occurs. Detailed analyses of SR Ca2+ concentration in isolated nascent cardiomyocytes cells is needed to clarify between these possibilities. Together, these data suggest that during the onset of cardiac function, the initial Ca2+ transients are derived from sarcolemmal Ca2+ flux and that the SR is still maturing until LHT stages whereupon SR-derived Ca2+ oscillations predominate. Further electrophysiological characterization, ideally on cardiomyocytes within the context of the intact crescent, is required to verify this shift in cellular Ca2+-handling mechanisms.
SPONTANEOUS ASYNCHRONOUS Ca2+ OSCILLATIONS PRECEDE OVERT CONTRACTILE ACTIVITY
Synchronized Ca2+ transients can be detected in the early cardiac crescent at stage 1, often before the onset of contractile activity. However, even before the appearance of these synchronized Ca2+ transients, nascent cardiomyocytes in the early stage 0 cardiac crescent display SACOs in the absence of contractile activity (Tyser et al. 2016). SACOs show a range of Ca2+ dynamics with variable frequencies and durations. The rate of these oscillations range from two to less than one oscillation per minute, significantly slower than the rate at which contractile activity initiates (30 bpm). SACOs could be inhibited by blocking the Na+/Ca2+ exchanger, which in adult cardiomyocytes is primarily responsible for Ca2+ efflux, suggesting as previously reported that the Na+/Ca2+ exchanger could be responsible for oscillations in immature cardiomyocytes, having a role in both Ca2+ influx and efflux (Reppel et al. 2007). Interestingly inhibition of the voltage-gated L-type calcium channel did not inhibit SACOs, suggesting these oscillations may be generated in a voltage-independent manner. Whether the SR has a role in generating these oscillations is unclear, although it has been shown that inhibition of RyRs and IP3Rs did not prevent SACOs in the forming crescent, suggesting that SACOs are generated by sarcolemmal rather than SR-generated Ca2+ flux.
The observation that SACOs at stage 0 preceded the onset of coordinated Ca2+ transients in the stage 1 cardiac crescent, just 1–2 hours later, raises several fundamental questions regarding how synchronization occurs, when SACOs first originate in cardiac progenitors and whether these early SACOS play any direct role in cardiomyocyte development. In this context, it is interesting to note that Ca2+ signaling within early cardiac progenitors appears to be important in promoting maturation into cardiomyocytes. In addition to its role in contraction, Ca2+ is a crucial second messenger involved in a number of different signaling pathways (Pucéat and Jaconi 2005). Transgenic mice in which SR Ca2+ homeostasis was dysregulated show embryonic malformations, impaired Ca2+ signaling, and compromised Ca2+-dependent transcription (Mesaeli et al. 1999). The release of Ca2+ from SR stores in E9.0 cardiomyocytes leads to changes in Ca2+-dependent signaling pathways, alters localization of histone deacetylases, and regulates expression of cardiac developmental genes (Karppinen et al. 2018). In immature cardiomyocytes at E8.0, Na+/Ca2+ exchanger function is essential not only for generating early Ca2+ transients but also for cardiomyocyte differentiation and correct phosphorylation of CamKinase (Tyser et al. 2016), which is known to regulate gene expression by phosphorylating various transcription factors, thereby linking cardiac cellular physiology and gene transcription (Molkentin 2006). Misregulation of this process can have profound consequences. Abnormal Ca2+ signaling in adult cardiomyocytes has been implicated in some types of pathological hypertrophy associated with heart failure. This is understood to happen as a result of the abnormal reactivation of genes normally only expressed during development, such as Mef2c, Nkx2-5, and Myh7, that are required for cardiomyocyte differentiation.
FUTURE PROSPECTS
Investigating the onset of cardiac function in vivo is technically challenging because of the size and accessibility of mammalian embryos. Embryonic stem cells (ESCs) have emerged as a useful in vitro model for studying differentiation and cellular function. Since the development of induced pluripotent stem cells (iPSCs), they, along with ESC-derived cardiomyocytes, have become widely used as models to investigate cardiomyocyte development. Early studies using mouse (mESCs) have shown that they can differentiate into functional cardiomyocytes in vitro and recapitulate the pre- and postgastrulation cardiogenic events that occur in vivo (Mercola et al. 2011; Mummery et al. 2012; Burridge et al. 2012). Although these models lack the morphological cues of in vivo developing embryos at the transcriptional and phenotypic level, they are similar, expressing physiologically relevant proteins in a stage-specific manner. They also provide a practical means of studying initiation of cardiac function during human development, which would otherwise be intractable given the various challenges associated with human embryo research. In terms of cardiac function, mESC-derived cardiomyocytes start contracting at ∼30 bpm, a rate similar to the first contractions detected in intact embryos (Tyser et al. 2016). This suggests that there is an intrinsic rate of Ca2+ handling during the transition of cardiac progenitors into functional immature cardiomyocytes and that ESC-based models faithfully capture events occurring in the embryo.
One of the most striking biological phenomena during the initiation of cardiac function is the observation of SACOs prior to coordinated Ca2+ transients and contractile activity, raising questions regarding their onset, synchronization, and biological context. To understand these questions, it is important to use live imaging of embryo development using novel techniques such as light sheet microscopy (McDole et al. 2018) in combination with live reporters. Genetically encoded Ca2+ reporters have been widely used in the field of neuroscience to investigate neural activity within distinct cell populations (Chen et al. 2013). Applying these tools to heart development will allow Ca2+ dynamics to be visualized and monitored in intact embryos during formation of the cardiac crescent, something not possible with traditional dyes, and allow us to address how SACOs become coordinated to produce synchronized Ca2+ transients.
Over the last 5 years, there has been an explosion in the use of single-cell sequencing to explore cellular heterogeneity within tissues and gene expression of individual cells in different tissue types. Recent papers have applied this technique to the developing embryo, covering stages in which the onset of function occurs (de Soysa et al. 2019; Pijuan-Sala et al. 2019). Using these data sets along with bioinformatic techniques, it should be possible to determine the order in which genes required for sarcomere formation or SACO synchronization are expressed during early cardiac development. Any in silico analyses will need to be validated using fundamental physiological approaches to piece together how cardiac function is initiated. Such insights will contribute to a deeper understanding of how cardiac contractile function is triggered and facilitates efforts in regenerative medicine focused on promoting functional integration of de novo–generated cardiomyocytes into a damaged heart.
ACKNOWLEDGMENTS
R.C.V.T. is funded through a British Heart Foundation Immediate Fellowship FS/18/24/33424. The work in the S.S. group is funded through a Wellcome Senior Investigator Award 103788/Z/14/Z and Wellcome Strategic Awards 105031/C/14/Z and 108438/Z/15/Z.
Footnotes
Editors: Benoit G. Bruneau and Paul R. Riley
Additional Perspectives on Heart Development and Disease available at www.cshperspectives.org
REFERENCES
- Andersson KB, Finsen AV, Sjåland C, Winer LH, Sjaastad I, Ødegaard A, Louch WE, Wang Y, Chen J, Chien KR, et al. 2009. Mice carrying a conditional Serca2flox allele for the generation of Ca2+ handling-deficient mouse models. Cell Calcium 46: 219–225. 10.1016/j.ceca.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. 2002. Cardiac excitation–contraction coupling. Nature 415: 198–205. 10.1038/415198a [DOI] [PubMed] [Google Scholar]
- Burridge PW, Keller G, Gold JD, Wu JC. 2012. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell 10: 16–28. 10.1016/j.stem.2011.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. 2013. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499: 295–300. 10.1038/nature12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Miranda AMA, Bub G, Srinivas S. 2015. Detecting cardiac contractile activity in the early mouse embryo using multiple modalities. Front Physiol 5: 508 10.3389/fphys.2014.00508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho CH, Kim SS, Jeong MJ, Lee CO, Shin HS. 2000. The Na+–Ca2+ exchanger is essential for embryonic heart development in mice. Mol Cells 10: 712–722. [DOI] [PubMed] [Google Scholar]
- Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. 2002. Striated muscle cytoarchitecture: an intricate web of form and function. Annu Rev Cell Dev Biol 18: 637–706. 10.1146/annurev.cellbio.18.012502.105840 [DOI] [PubMed] [Google Scholar]
- Copp AJ. 1995. Death before birth: clues from gene knockouts and mutations. Trends Genet 11: 87–93. 10.1016/S0168-9525(00)89008-3 [DOI] [PubMed] [Google Scholar]
- de Soysa TY, Ranade S, Okawa S, Ravichandran S, Huang Y, Salunga H, Schricker A, del Sol A, Gifford C, Srivastava D. 2019. Single-cell analysis of cardiogenesis reveals basis for organ-level developmental anomalies. Nature 572: 120–124. 10.1038/s41586-019-1414-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehler E. 2016. Cardiac cytoarchitecture—why the “hardware” is important for heart function! Biochim Biophys Acta 1863: 1857–1863. 10.1016/j.bbamcr.2015.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss CM. 1938. The first contractions of the heart in rat embryos. Anat Rec 70: 505–524. 10.1002/ar.1090700502 [DOI] [Google Scholar]
- Goss C. 1952. Development of the median coordinated ventricle from the lateral hearts in rat embryos with three to six somites. Anat Rec 112: 761–796. 10.1002/ar.1091120405 [DOI] [PubMed] [Google Scholar]
- Granados-Riveron JT, Brook JD. 2012. The impact of mechanical forces in heart morphogenesis. Circ Cardiovasc Genet 5: 132–142. 10.1161/CIRCGENETICS.111.961086 [DOI] [PubMed] [Google Scholar]
- Hove JR, Köster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. 2003. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421: 172–177. 10.1038/nature01282 [DOI] [PubMed] [Google Scholar]
- Ivanovitch K, Temiño S, Torres M. 2017. Live imaging of heart tube development in mouse reveals alternating phases of cardiac differentiation and morphogenesis. eLife 6: e30668 10.7554/eLife.30668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamino K. 1991. Optical approaches to ontogeny of electrical activity and related functional organization during early heart development. Physiol Rev 71: 53–91. 10.1152/physrev.1991.71.1.53 [DOI] [PubMed] [Google Scholar]
- Karppinen S, Rapila R, Mäkikallio K, Hänninen SL, Rysä J, Vuolteenaho O, Tavi P. 2014. Endothelin-1 signalling controls early embryonic heart rate in vitro and in vivo. Acta Physiol 210: 369–380. 10.1111/apha.12194 [DOI] [PubMed] [Google Scholar]
- Karppinen S, Hänninen SL, Rapila R, Tavi P. 2018. Sarcoplasmic reticulum Ca2+-induced Ca2+ release regulates class IIa HDAC localization in mouse embryonic cardiomyocytes. Physiol Rep 6: e13522 10.14814/phy2.13522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MH, Navaratnam V. 1981. Early differentiation of the heart in mouse embryos. J Anat 133: 235–246. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Maeda S, Ichise N, Sato T, Iwase T, Seki S, Yamada Y, Tohse N. 2011. The beginning of the calcium transient in rat embryonic heart. J Physiol Sci 61: 141–149. 10.1007/s12576-010-0131-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koushik SV, Wang J, Rogers R, Moskophidis D, Lambert NA, Creazzo TL, Conway SJ. 2001. Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. FASEB J 15: 1209–1211. 10.1096/fj.00-0696fje [DOI] [PubMed] [Google Scholar]
- Le Garrec JF, Domínguez JN, Desgrange A, Ivanovitch KD, Raphaël E, Bangham JA, Torres M, Coen E, Mohun TJ, Meilhac SM. 2017. A predictive model of asymmetric morphogenesis from 3D reconstructions of mouse heart looping dynamics. eLife 6: e28951 10.7554/eLife.28951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Halbach M, Hannes T, Fleischmann BK, Tang M, Schunkert H, Hescheler J, Reppel M. 2010. Electrophysiological basis of the first heart beats. Cell Physiol Biochem 25: 561–570. 10.1159/000315075 [DOI] [PubMed] [Google Scholar]
- Linask KK, Han MD, Artman M, Ludwig CA. 2001. Sodium-calcium exchanger (NCX-1) and calcium modulation: NCX protein expression patterns and regulation of early heart development. Dev Dyn 221: 249–264. 10.1002/dvdy.1131 [DOI] [PubMed] [Google Scholar]
- Lucitti JL, Jones EAV, Huang C, Chen J, Fraser SE, Dickinson ME. 2007. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 134: 3317–3326. 10.1242/dev.02883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux C, Yoshimoto M, McGrath K, Conway S, Palis J, Yoder M. 2007. All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood 111: 3435–3438. 10.1182/blood-2007-08-107086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDole K, Guignard L, Amat F, Berger A, Malandain G, Royer LA, Turaga SC, Branson K, Keller PJ. 2018. In toto imaging and reconstruction of post-implantation mouse development at the single-cell level. Cell 175: 859–876.e33. 10.1016/j.cell.2018.09.031 [DOI] [PubMed] [Google Scholar]
- Meilhac SM, Buckingham ME. 2018. The deployment of cell lineages that form the mammalian heart. Nat Rev Cardiol 15: 705–724. 10.1038/s41569-018-0086-9 [DOI] [PubMed] [Google Scholar]
- Mercola M, Ruiz-lozano P, Schneider MD. 2011. Cardiac muscle regeneration: lessons from development. Genes Dev 25: 299–309. 10.1101/gad.2018411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méry A, Aimond F, Ménard C, Mikoshiba K, Michalak M, Pucéat M. 2005. Initiation of embryonic cardiac pacemaker activity by inositol 1,4,5-trisphosphate-dependent calcium signaling. Mol Biol Cell 16: 2414–2423. 10.1091/mbc.e04-10-0883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesaeli N, Nakamura K, Zvaritch E, Dickie P, Dziak E, Krause KH, Opas M, MacLennan DH, Michalak M. 1999. Calreticulin is essential for cardiac development. J Cell Biol 144: 857–868. 10.1083/jcb.144.5.857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger JM, Westfall MV. 2004. Covalent and noncovalent modification of thin filament action: the essential role of troponin in cardiac muscle regulation. Circ Res 94: 146–158. 10.1161/01.RES.0000110083.17024.60 [DOI] [PubMed] [Google Scholar]
- Miyasaka KY, Kida YS, Banjo T, Ueki Y, Nagayama K, Matsumoto T, Sato M, Ogura T. 2011. Heartbeat regulates cardiogenesis by suppressing retinoic acid signaling via expression of miR-143. Mech Dev 128: 18–28. 10.1016/j.mod.2010.09.002 [DOI] [PubMed] [Google Scholar]
- Molkentin JD. 2006. Dichotomy of Ca2+ in the heart: contraction versus intracellular signaling. J Clin Invest 116: 623–626. 10.1172/JCI27824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. 2012. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res 111: 344–358. 10.1161/CIRCRESAHA.110.227512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi T, Seguchi M, Takao A. 1988. Development of the myocardial contractile system. Experientia 44: 936–944. 10.1007/BF01939887 [DOI] [PubMed] [Google Scholar]
- Navaratnam V, Kaufman MH, Skepper JN, Barton S, Guttridge KM. 1986. Differentiation of the myocardial rudiment of mouse embryos: an ultrastructural study including freeze-fracture replication. J Anat 146: 65–85. [PMC free article] [PubMed] [Google Scholar]
- Nishii K, Shibata Y. 2006. Mode and determination of the initial contraction stage in the mouse embryo heart. Anat Embryol (Berl) 211: 95–100. 10.1007/s00429-005-0065-x [DOI] [PubMed] [Google Scholar]
- Nishii K, Morimoto S, Minakami R, Miyano Y, Hashizume K, Ohta M, Zhan DY, Lu QW, Shibata Y. 2008. Targeted disruption of the cardiac troponin T gene causes sarcomere disassembly and defects in heartbeat within the early mouse embryo. Dev Biol 322: 65–73. 10.1016/j.ydbio.2008.07.007 [DOI] [PubMed] [Google Scholar]
- North TE, Goessling W, Peeters M, Li P, Ceol C, Lord AM, Weber GJ, Harris J, Cutting CC, Huang P, et al. 2009. Hematopoietic stem cell development is dependent on blood flow. Cell 137: 736–748. 10.1016/j.cell.2009.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadatos GA, Wallerstein PMR, Head CEG, Ratcliff R, Brady PA, Benndorf K, Saumarez RC, Trezise AEO, Huang CLH, Vandenberg JI, et al. 2002. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc Natl Acad Sci 99: 6210–6215. 10.1073/pnas.082121299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten BM, Kramer TC. 1933. The initiation of contraction in the embryonic chick heart. Am J Anat 53: 349–375. 10.1002/aja.1000530302 [DOI] [Google Scholar]
- Periasamy M, Reed TD, Liu LH, Ji Y, Loukianov E, Paul RJ, Nieman ML, Riddle T, Duffy JJ, Doetschman T, et al. 1999. Impaired cardiac performance in heterozygous mice with a null mutation in the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 (SERCA2) gene. J Biol Chem 274: 2556–2562. 10.1074/jbc.274.4.2556 [DOI] [PubMed] [Google Scholar]
- Pijuan-Sala B, Griffiths JA, Guibentif C, Hiscock TW, Jawaid W, Calero-Nieto FJ, Mulas C, Ibarra-Soria X, Tyser RCV, Ho DLL, et al. 2019. A single-cell molecular map of mouse gastrulation and early organogenesis. Nature 566: 490–495. 10.1038/s41586-019-0933-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. 2000. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 102: 89–97. 10.1016/S0092-8674(00)00013-1 [DOI] [PubMed] [Google Scholar]
- Pucéat M, Jaconi M. 2005. Ca2+ signalling in cardiogenesis. Cell Calcium 38: 383–389. 10.1016/j.ceca.2005.06.016 [DOI] [PubMed] [Google Scholar]
- Rapila R, Korhonen T, Tavi P. 2008. Excitation–contraction coupling of the mouse embryonic cardiomyocyte. J Gen Physiol 132: 397–405. 10.1085/jgp.200809960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppel M, Sasse P, Malan D, Nguemo F, Reuter H, Bloch W, Hescheler J, Fleischmann BK. 2007. Functional expression of the Na+/Ca2+ exchanger in the embryonic mouse heart. J Mol Cell Cardiol 42: 121–132. 10.1016/j.yjmcc.2006.10.008 [DOI] [PubMed] [Google Scholar]
- Sabin F. 1920. Studies on the origin of blood-vessels and of red blood-corpuscles as seen in the living blastoderm of chicks during the second day of incubation. Contrib Embryol 9: 213–262. [Google Scholar]
- Sasse P, Zhang J, Cleemann L, Morad M, Hescheler J, Fleischmann BK. 2007. Intracellular Ca2+ oscillations, a potential pacemaking mechanism in early embryonic heart cells. J Gen Physiol 130: 133–144. 10.1085/jgp.200609575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seisenberger C, Specht V, Welling A, Platzer J, Pfeifer A, Kühbandner S, Striessnig J, Klugbauer N, Feil R, Hofmann F. 2000. Functional embryonic cardiomyocytes after disruption of the L-type α1C (Cav1.2) calcium channel gene in the mouse. J Biol Chem 275: 39193–39199. 10.1074/jbc.M006467200 [DOI] [PubMed] [Google Scholar]
- Stieber J, Herrmann S, Feil S, Löster J, Feil R, Biel M, Hofmann F, Ludwig A. 2003. The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci 100: 15235–15240. 10.1073/pnas.2434235100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima H, Komazaki S, Hirose K, Nishi M, Noda T, Iino M. 1998. Embryonic lethality and abnormal cardiac myocytes in mice lacking ryanodine receptor type 2. EMBO J 17: 3309–3316. 10.1093/emboj/17.12.3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triedman JK, Newburger JW. 2016. Trends in congenital heart disease. Circulation 133: 2716–2733. 10.1161/CIRCULATIONAHA.116.023544 [DOI] [PubMed] [Google Scholar]
- Tyser RCV, Miranda AMA, Chen CM, Davidson SM, Srinivas S, Riley PR. 2016. Calcium handling precedes cardiac differentiation to initiate the first heartbeat. eLife 5: e17113 10.7554/eLife.17113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viatchenko-Karpinski S, Fleischmann BK, Liu Q, Sauer H, Gryshchenko O, Ji GJ, Hescheler J. 2002. Intracellular Ca2+ oscillations drive spontaneous contractions in cardiomyocytes during early development. Proc Natl Acad Sci 96: 8259–8264. 10.1073/pnas.96.14.8259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakimoto K, Kobayashi K, Kuro-o M, Yao A, Iwamoto T, Yanaka N, Kita S, Nishida A, Azuma S, Toyoda Y, et al. 2000. Targeted disruption of Na+/Ca2+ exchanger gene leads to cardiomyocyte apoptosis and defects in heartbeat. J Biol Chem 275: 36991–36998. 10.1074/jbc.M004035200 [DOI] [PubMed] [Google Scholar]