Abstract
Diisocyanates are a group of chemicals that are widely used in occupational settings. They are known to induce various health effects, including skin- and respiratory tract sensitization resulting in allergic dermatitis and asthma. Exposure to diisocyanates has been studied in the past decades by using different types of biomonitoring markers and matrices. The aim of this review as part of the HBM4EU project was to assess: (i) which biomarkers and matrices have been used for biomonitoring diisocyanates and what are their strengths and limitations; (ii) what are (current) biomonitoring levels of the major diisocyanates (and metabolites) in workers; and (iii) to characterize potential research gaps. For this purpose we conducted a systematic literature search for the time period 2000–end 2018, thereby focussing on three types of diisocyanates which account for the vast majority of the total isocyanate market volume: hexamethylene diisocyanate (HDI), toluene diisocyanate (TDI), and 4,4′-methylenediphenyl diisocyanate (MDI). A total of 28 publications were identified which fulfilled the review inclusion criteria. The majority of these studies (93%) investigated the corresponding diamines in either urine or plasma, but adducts have also been investigated by several research groups. Studies on HDI were mostly in the motor vehicle repair industry [with urinary hexamethylene diamine result ranging from 0.03 to 146.5 µmol mol−1 creatinine]. For TDI, there is mostly data on foam production [results for urinary toluene diamine ranging from ~0.01 to 97 µmol mol−1 creatinine] whereas the available MDI data are mainly from the polyurethane industry (results for methylenediphenyl diamine range from 0.01 to 32.7 µmol mol−1 creatinine). About half of the studies published were prior to 2010 hence might not reflect current workplace exposure. There is large variability within and between studies and across sectors which could be potentially explained by several factors including worker or workplace variability, short half-lives of biomarkers, and differences in sampling strategies and analytical techniques. We identified several research gaps which could further be taken into account when studying diisocyanates biomonitoring levels: (i) the development of specific biomarkers is promising (e.g. to study oligomers of HDI which have been largely neglected to date) but needs more research before they can be widely applied, (ii) since analytical methods differ between studies a more uniform approach would make comparisons between studies easier, and (iii) dermal absorption seems a possible exposure route and needs to be further investigated. The use of MDI, TDI, and HDI has been recently proposed to be restricted in the European Union unless specific conditions for workers’ training and risk management measures apply. This review has highlighted the need for a harmonized approach to establishing a baseline against which the success of the restriction can be evaluated.
Keywords: biomarker, biomonitoring, diisocyanates, review, worker
Introduction
Diisocyanates are a group of chemicals containing two isocyanate functional groups (R–N=C=O). These low molecular weight compounds first alter a human protein before becoming allergenic. As such they are further known to induce various health effects, including skin and respiratory tract sensitization resulting in allergic dermatitis and asthma (DECOS, 2018). There is also concern of potential genotoxicity and carcinogenicity of diisocyanates, with the degradation products and metabolites of 4,4′-methylenediphenyl diisocyanate (MDI) and toluene diisocyanate (TDI) both being classified as mutagenic and carcinogenic (IARC, 1999; ECHA, 2005; DECOS, 2018).
The two major diisocyanates in the European market are MDI (CAS 101-68-8; 100 000–1 000 000 tonnes per annum; ECHA, 2019a) and TDI (CAS 584-84-9 for 2,4-TDI and CAS 26471-62-5 for the mixture of 2,4-TDI/2,6-TDI; 100 000–1 000 000 tonnes per annum; ECHA, 2019b,c). A third diisocyanate with widespread use, especially in vehicle paints, is hexamethylene diisocyanate (HDI; CAS 822-06-0; 10 000–100 000 tonnes per annum; ECHA, 2019d). In Europe, MDI, TDI, and HDI account for more than 95% of the volume of diisocyanate production (ECHA, 2017). Since there are no suitable alternatives for the majority of applications, the usage is not expected to decline in near future (ECHA, 2017). In addition to these three compounds, several oligomeric products (e.g. for HDI) and various other diisocyanates are registered in the European market [e.g. 1,5-naphthalene diisocyanate (NDI), CAS 3173-72-6; isophorone diisocyanate (IPDI), CAS 4098-71-9] and are manufactured and/or imported in smaller yet notable amounts.
Since diisocyanates are widely used in different applications in industry (including in the manufacturing of polyurethanes (PURs) and as hardeners in industrial paints, glues, varnishes, and resins), occupational exposure during production and handling of these materials is a concern (McDonald et al., 2005). Workplace exposure to diisocyanates has been studied historically by using different types of biomonitoring markers and matrices. Because of the diversity of industrial usages of diisocyanates, and the variety of biomarkers, we aimed to systematically identify and report relevant occupational biomonitoring studies reporting use of diisocyanates published between 2000 and 2018, focussed on addressing the following questions:
(1) Which biomarkers and matrices have been used for biomonitoring diisocyanates; what are their strengths and limitations?
(2) What are (current) biomonitoring levels of the major diisocyanates (and metabolites) in workers?
(3) What are potential research gaps with regard to studying diisocyanate biomonitoring levels?
Methods
A literature search was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology (Moher et al., 2009) from year 2000 to end 2018 in PubMed and Web of Science, using the following search terms for MDI, HDI, and TDI: (‘Occupational’ OR ‘worker*’) AND (‘biomonitor*’ OR ‘biomarker*’ OR ‘urin*’). The full chemical names were also used.
A total of 161 publications were retrieved. Publications were subsequently evaluated based on the abstract (independently by two reviewers). Publications were excluded if published in a language other than English, they reported in vitro or animal studies, or were mechanistic papers. Review papers identified from the 161 papers were screened for potential additional studies that were not retrieved via the systematic review. This evaluation process resulted in 59 publications being identified for full review by two reviewers. Upon full review, 31 studies were additionally excluded due to various criteria such as results being reported in earlier studies, no report of any biomarker results, volunteer studies, or method development papers. One paper by De Palma et al. (2012) could not be retrieved. One relevant paper, by Jones et al. (2017), was missed by the predefined search criteria. Twenty-eight publications were then taken forward to the next stage of the review process.
Data from these publications were extracted by using a bespoke template that collected information on: study type, study participants, chemicals investigated, type of biomarker and matrix, measurement techniques, and quality assurance. In order to compare results across studies, we standardized results as far as possible as µmol mol−1 creatinine for urine [converting any uncorrected values using an approximate creatinine value of 12 mmol l−1 (Cocker et al., 2011) and marking any approximate corrections as ~], nmol l−1 for plasma, and pmol g−1 for haemoglobin (Hb) and albumin.
The publications were reviewed and ranked independently by one of two reviewers using a modified version of the LaKind scoring criteria (Table 1; LaKind et al., 2014). The LaKind criteria were developed to assess study quality for non-persistent biomarker studies and were used here to give an indication of the overall quality of the study. This considers the specificity of the biomarkers used and the analytical techniques, the quality of the study design, sample handling, and quality assurance. A sample of papers (15, ~20%) was independently scored by both reviewers, with the results being compared for quality assurance purposes. Very few instances were identified where scoring was diametrically opposed (one reviewer scored Tier 1 (highest quality), the other Tier 3 (lowest quality). Both sets of scoring for these sample papers were reviewed by a third researcher with discussion on harmonizing scoring approaches for the remaining papers. Papers were scored against eight categories with the total score potentially ranging from 8 (highest quality) to 24 (lowest quality), see Table 1.
Table 1.
Adaptation of LaKind scoring criteria for isocyanates mini-review. Each paper was scored from 1 (Tier 1) to 3 (Tier 3) for each of the eight components, giving total possible scores from 8 (highest quality) to 24 (lowest quality).
| Assessment component | Tier 1 | Tier 2 | Tier 3 |
|---|---|---|---|
| Study participants | >20 occupationally exposed individuals | 5–20 occupationally exposed individuals | Any other study (<5 occupationally exposed individuals, volunteers, general population) |
| Chemicals under investigation | HDI, TDI, and/or MDI | IPDI and NDI | Any other isocyanates |
| Exposure biomarker and matrix | Biomarker in a specified matrix has accurate and precise quantitative relationship with external exposure, internal dose, or target dose e.g. diamines and Hb adducts. | Evidence exists for a relationship between biomarker in a specified matrix and external exposure, internal dose, or target dose but limited application e.g. other protein adducts or conjugates. | Biomarker in a specified matrix is a poor surrogate (low accuracy and precision) for exposure/dose e.g. experimental biomarkers, non-specific markers such as general effect markers. |
| Biomarker specificity | Biomarker is derived from exposure to one parent chemical. | Biomarker is derived from a limited number of parent chemicals, such as diamines. | Biomarker is derived from multiple parent chemicals with varying types of adverse endpoints. |
| Technique | Instrumentation that provides unambiguous identification and quantitation of the biomarker at the required sensitivity [e.g. gas chromatography–mass spectrometry [GC–MS), GC–MS/MS, and liquid chromatography (LC)–MS/MS]. | Instrumentation that allows for identification of the biomarker with a high degree of confidence and the required sensitivity [e.g. GC–MS and GC–electron capture detector (GC–ECD)]. | Instrumentation that only allows for possible quantification of the biomarker but the method has known interferants (e.g. GC–FID, spectroscopy). |
| Method characteristics—Any specific weaknesses in study design leading to a Tier 3 score to be noted | Acceptable level of detection (LoD) | LoD above current state-of-the-art. | |
| Samples with a known history and documented stability data or those using real-time measurements. | Stability not specifically assessed, but samples were stored appropriately and analysed promptly. | Specific reason to query stability. E.g. samples with unknown history or known issues. | |
| Samples are contamination-free from time of collection to time of measurement (e.g. by use of certified analyte-free collection supplies and reference materials, and appropriate use of blanks both in the field and lab). Research includes documentation of the steps taken to provide the necessary assurance that the study data are reliable. | Study not using/documenting these procedures. | There are known contamination issues and no documentation that the issues were addressed. | |
| Quality assurance | Study has used external QA where appropriate | Some QA used (note details) | No QA |
| Matrix adjustment | Study includes results for adjusted and non-adjusted concentrations if adjustment is needed. 24 h total urine collection is considered Tier 1. | Study only provides results using one method (matrix-adjusted or not). | No established method for adjustment (e.g. adjustment for hair, saliva). |
Results
Overview of various biomarkers available
Several biomarkers and matrices have been used to study diisocyanate exposure. Considering the very high chemical reactivity of diisocyanates molecules due to the two NCO chemical groups, the direct analysis of the parent compounds in human matrices, like urine or blood, is not possible. For this reason, the biomonitoring techniques used to monitor diisocyanates exposure investigate the presence of products of chemical degradation, such as diamines in urine, or products of metabolism, such as acetylated amines or protein adducts in urine or blood samples. Here, we will provide an overview of the available biomarkers, including reported suitability and half-life.
Amines
The majority of the papers accessed for this review (>90%) studied the corresponding diamines in either urine or plasma. This is based on analysing isocyanate derived diamines released by hydrolysis of protein adducts in plasma or urine (Cocker, 2007). The corresponding diamines for the three diisocyanates in this review are hexamethylene diamine (HDA) for HDI, toluene diamine (TDA) for TDI (both isomers), and methylene dianiline (MDA) for MDI. Since the elimination half-lives of these derived diamines in urine are relatively short (2–5 h), urine samples should be collected at the end of exposure (i.e. end of workshift for occupational exposure). Indeed, most of the papers here report post-shift samples (‘spot’ samples in the case of urine) although some also report pre-shift and others after a weekend. Diamines are not specific, i.e. diamines themselves can also be common industrial chemicals. In addition, where the primary exposure is not to the diisocyanate monomer (particularly HDI exposures), it is unclear whether the diamine method is measuring only monomer exposure; data suggest that it may be more than just monomer but unlikely to include all oligomers/pre-polymers (Cocker, 2011).
Protein adducts
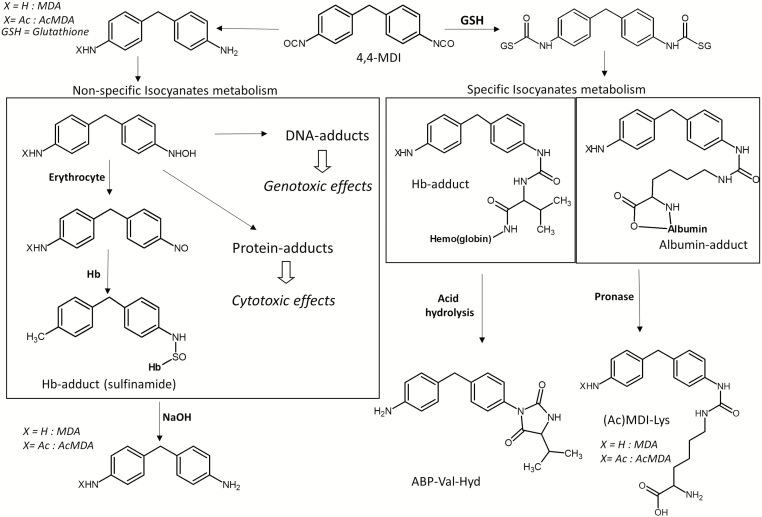
Protein adducts, isolated from blood samples, can be divided in two groups: albumin adducts and Hb adducts (Sabbioni et al., 2007). The first group can be produced by direct reaction of diisocyanates with the protein, or through an intermediate step where diisocyanates react with glutathione, in both cases adducts of albumin are formed (Fig. 1). These adducts are considered specific biomarkers of diisocyanates exposure; only molecules that contain one or more isocyanate (N=C=O) group can react directly with albumin or through an intermediate step with glutathione (Sabbioni et al., 2010). For albumin adducts, the most studied molecules are represented by MDI or acetyl-MDI reacting with lysine residues (MDI-Lys and AcMDI-Lys). It has been shown that albumin adducts, having a half-life of 20–25 days, could be used for the determination of short- to medium-term exposure to diisocyanates (Sabbioni et al., 2010).
Figure 1.
General overview of the metabolic pathway of 4,4-MDI as proposed by Gries and Leng (2013) and Sabbioni et al. (2010, 2017).
The second group of protein adducts, the Hb adducts, are formed through the reaction between diisocyanates and globin proteins. Hb adducts are also assumed to form through an intermediate step where diisocyanates react with glutathione (Gries and Leng, 2013). These Hb adducts can be measured directly (Gries and Leng, 2013) and are therefore specific, or they can be released by hydrolysis, resulting in diamines and are therefore non-specific. Hb has a lifetime of 120 days and its adducts therefore reflect longer-term exposure to diisocyanates (Flack et al., 2011).
Whilst the mechansims of sensitization are still unclear, it seems that glutathione conjugates may promote immune responses (Wisnewski et al., 2013) and therefore albumin adducts could potentially be considered biomarkers of effect as well as exposure.
Biomarker data
For amines data, the presented results focus on post-shift samples as these are most commonly reported and allow comparison between studies.
HDI
Reported biomonitoring levels
HDI is predominately used in spray paints within the motor vehicle repair (MVR) industry, see Table 2 for a summary of the studies identified. Seven studies were identified as being from the MVR sector, two of these are from European countries (UK and Netherlands) (Pronk et al., 2006; Jones et al., 2013). The number of workers in all these studies varies substantially from 45 (Netherlands) to 995 (UK). The Netherlands study (Pronk et al., 2006) observed urinary HDA levels up to 150.2 µg g−1 creatinine (146.5 µmol mol−1 creatinine). Sampling was carried out at multiple time points throughout the day with the highest mean exposures occurring between the early afternoon and evening. Mean exposures for these time points were all ~20 µmol mol−1 creatinine. This is high compared with the UK study results (Jones et al., 2013) where the maximum result was ~20 µmol mol−1 creatinine and to an Australian study of 196 MVR workers which had almost 100% ‘none detects’ with only three results above the limit of quantification (LoQ) of 0.5 µmol mol−1 creatinine (Hu et al., 2017). Also the Netherlands may be considered high in comparison to results from the USA (Gaines et al., 2010) that reported post-shift urine samples with HDA levels between <0.04 and 65.9 µg l−1 (~<0.03–47.3 µmol mol−1 creatinine). Other industry sectors have not been so well studied, with only two UK studies adding to the list. These were studies at small- and medium-sized enterprises (SMEs) producing PUR elastomer (Cocker et al., 2009) and a survey of SMEs with a variety of uses (Creely et al., 2006). Results were reported as total isocyanates (HDA + TDA + MDA + IPDA urine levels) and so it was not possible to compare HDI exposure with other studies. There were a low number of detects in the PUR study (Cocker et al., 2009), with 9 of the 13 positive sample results being above the UK biological monitoring guidance value of 1 µmol mol−1 creatinine showing potential for individual exposure in this industry (HSL, 2005).
Table 2.
Summary of HDI exposure studies for the main processes.
| Sector | Study populations (country, no. workers) | Biomonitoring data [expressed as range (median)] | Notable correlations/comments | LaKind scoringa | References |
|---|---|---|---|---|---|
| MVR | USA, 15 | Plasma HDA: 0.012–0.71 (0.061b) µg l−1 HDA-Hb: 1.3–37 (3.0b) ng g−1 Hb | Hb and plasma weakly associated. Air: correlated with cumulative exposure (Hb: r2 = 0.34, P < 0.05; P: r2 = 0.37, P < 0.05) 10× higher Hb adducts than plasma due to cumulative exposure and turnover times. Positive association between HDA-Hb adduct concentration and HDI exposure was strongest with cumulative dermal (N = 12, r2 = 0.32, P = 0.058), cumulative inhalation (N = 12, r2 = 0.35, P = 0.042), or cumulative air exposure (N = 12, r2 = 0.34, P = 0.048). | 15 | Flack et al. (2011) |
| USA, 46 | Plasma HDA: 0.02–0.92 µg l−1 | Inhalation correlation, r = 0.22, P = 0.026. | 14 | Flack et al. (2010) | |
| USA, 48 | Urine HDA: <0.04–65.9 µg l−1 (0.10b) [~0.03–47.2 µmol mol−1 cr.] | Dermal and inhalation exposure found to be significant predictors of urinary biomarker levels. | 12 | Gaines et al. (2010) | |
| USA, 15 | Urine TAHI: <LoD–1.99 µg l−1 (means) [<LoD–0.39 µmol mol−1 cr.] | TAHI reported for first time. Positive correlation between HDI isocyanurate exposure and total urine TAHI concentration (r = 0.14 with creatinine adjustment). | 13 | Robbins et al. (2018) | |
| Australia, 196 | Only 3 above LoQ (0.5 µmol mol−1 cr.) | Positive spray booths thought to be just as effective as negative because of high level of non-detects. | 11 External QA | Hu et al. (2017) | |
| UK, 995 | Pre-intervention: 1.34 (90%) µmol mol−1 cr. Intervention: 0.60 (90%) µmol mol−1 cr. Post-intervention: 0.68 (90%) µmol mol−1 cr. | Participants invited to a Safety and Health Awareness Day (SHAD). Samples taken before and after show lower results after the intervention. | 11 | Jones et al. (2013) | |
| Netherlands, 55 (10 workers from industrial paint shop) | Urine HDA: <2.9–146.5 µmol mol−1 cr. (means) | Higher levels of oligomers than monomers detected in air samples. Highest concentrations of HDA in urine seen in the afternoons and early evening. Dermal exposure was a predictor of the presence of HDA. | 11 24-h samples | Pronk et al. (2006) | |
| Other | UK, 71 | Urine HDA: 56 < LoD, 13 > LoD. 9 > Biological Monitoring Guidance Value<0.5–10.1 (1.8) µmol mol−1 cr. | About 25 companies were visited that were involved in the manufacture of PUR products. | 11 | Cocker et al. (2009) |
| UK, 67 | Urine HDA: results reported as total isocyanates, HDA most common detected n = 21 | Low airborne concentrations, only 20% above LoQ. Mixing and pouring tasks seen as a major potential source of exposure. Biased towards good practice. | 12 | Creely et al. (2006) |
aThe lower the LaKind score the better the overall quality (possible range 8–24).
bGeometric mean (rather than median).
One of the biomarkers reported for the first time was TAHI (trisaminohexyl isocyanurate), a specific metabolite to the oligomer HDI isocyanurate (Robbins et al., 2018). TAHI was detected in a third of the 111 exposed workers. Most of the data in the retrieved studies from the MVR described exposure to the HDI monomer (due to methods and standards not being available for oligomer exposure detection in urine prior to 2018) even though oligomers make up the bulk of 2-pack spray paints (Rosenberg and Savolainen, 1986; Fent et al., 2008). Pronk et al. (2006) also demonstrated significantly higher concentrations of HDI oligomers in personal inhalation samples when compared with HDI monomer. Mean values of NCO (isocyanate content) exposure for sprayers was 2.1 µg m−3 of NCO from monomer exposure and 116.3 µg m−3 of NCO from oligomer exposure.
Correlations
Some studies show a relationship between plasma levels of HDA and air levels of HDI, such as a US MVR study (Flack et al., 2010) here plasma HDA levels were weakly correlated (r = 0.22) with personal inhalation exposure samples. A study in the following year (Flack et al., 2011) from the same researchers showed that plasma HDA correlated best with inhalation exposure when taking cumulative exposure into account, r = 0.61. This was also observed for Hb adducts, however Hb and plasma levels were not correlated, most likely due to the different turnover times of these proteins in the body. Gaines et al. (2010) and Pronk et al. (2006) reported that dermal, as well as inhalation exposure, was a significant predictor of urinary biomarker levels.
TDI
Reported biomonitoring levels
TDI is a volatile diisocyanate used in foam blowing, glues/adhesives, and lacquers, see Table 3 for a summary of the studies identified.
Table 3.
Summary of TDI exposure studies for the main processes.
| Sector | Study populations (country, no. workers) | Biomonitoring data [expressed as range (median)] | Notable correlations/comments | LaKind scoringa | References |
|---|---|---|---|---|---|
| Continuous foam production | Poland (n = 20) | Sum-TDA (U) = <0.01–3.9 µmol mol−1 cr. | Positive for geometric mean (GM) in each group (r 0.9) U_totTDA (µmol mol−1) = 0.10777_TDI (µg m−3) + 0.2178 [5 ppb TDI = 4.1 µmol mol−1 cr.] RPE used and observed to impact TDA(U) results (no correlation between individual urinary TDA concentrations and TDI air concentrations). | 12 | Swierczynska-Machura et al. (2015) |
| UK (n = 26, 13 handlers, 13 non) | Sum-TDA (U) = <~0.4 to 7 (2.21) µmol mol−1 cr. (handlers) | No correlation between post-shift urinary TDA concentration and airborne TDI concentrations (r = 0.027). Dermal considered a significant factor—urine 20× higher for same airborne exposure. | 16 Methodology not well described | Austin (2007) | |
| Belgiumb (n = 9) | Sum-TDA (U) = 4.4–142.6 (18.01b) µg l−1 [21 samples] [~3 to ~97 (~12.3) µmol mol−1 cr.] | TDA (µg g−1) = 0.547_TDI (µg m−3)–1.636, r = 0.917 [5 ppb TDI =19.2 µg g−1, 17.8 µmol mol−1 cr.] Proposed measuring ‘increase over shift’ to exclude accumulation. | 12 External QA | Geens et al. (2012) | |
| Finland (n = 17) | Sum-TDA (U) = <0.05 to 39 µmol mol−1 cr. | Good correlation between airborne TDI and urinary TDA in post-shift samples (r = 0.91 and 0.86 for the two different factories studied). | 13 | Kääriä et al. (2001) | |
| Sweden (n = 6) | 2,4-TDA(U)/2,6-TDA(U) 0.5–5.4/0.2–4.7 µg l−1 [~0.3–3.7/0.14–3.2 µmol mol−1 cr.] 2,4-TDA(P)/2,6-TDA(P) 0.1–14/0.7–12 µg l−1 | Samples taken Monday morning so results not comparable to other studies. Only reported levels ‘above reference value’. | 12 Creatinine and specific gravity | Tinnerberg et al. (2014) | |
| Swedenc (n = 4 in 2000, n = 6 in 2005) | 2,4-TDA (U) ~ 0–10 µmol mol−1 cr. 2,6-TDA (U) ~ 0–35 µmol mol−1 cr. 2,4-TDA (P)/2,6_pTDA (µg l−1) In 2000: 2.9–27.2 (7.0)/8.2–62.1 (30.8) In 2005: 0.5–1.3 (1.0)/2–11.8 (4.0) | Urine results only presented graphically. | 14 Small study | Tinnerberg and Mattsson (2008) | |
| Finland (n = 17) | Sum-TDA(U) 0.2–39(4.9) µmol mol−1 cr. Sum-TDA(P) 0.4–70.8 (5.6) µg l−1 Sum_TDA(Hb) 0.012–0.33 (0.047) (nmol g−1) | Air TDI and plasma TDA correlated (r = 0.91). Plasma and urine TDA well correlated (r = 0.97). | 13 | Säkkinen et al. (2011) | |
| UK (n = 71) | Sum-TDA(U) <0.5–15.5 (1.3) µmol mol−1 cr. 2,6-TDA(U) <0.5–13.2 (0.8) µmol mol−1 cr. 2,4-TDA (U) <0.5–5.6 (0.7) µmol mol−1 cr. | The companies visited were involved in the manufacture of PUR products. | 11 | Cocker et al. (2009) | |
| UK (n = 90) | Sum-TDA(U) (µmol mol−1 cr.) <0.4–6.5 (90%, median <LOD) | Positive association observed in 4 pairs of samples (air and urine). Air levels <LoD at 2/5 sites and only 1/11 samples >WEL (20 µg m−3 NCO)— no further data. 446 samples analysed of which 280 were below the detection limit. Follow-up to Cocker et al. (2009). | 12 | Keen et al. (2012) | |
| Mixed | Sweden (n = 136) (including: moulding-, continuous foam-, and flame-lamination plants) | 2,6-TDA(U) <0.05–43.1 µg l−1 [~<0.03–29.3 µmol mol−1 cr.] 2,6-TDA(P) <0.05–62.1 µg l−1 | Correlation (r > 0.86) with air levels for both urine and plasma for same-day samples. Same sites as Sennbro et al. (2004). | 12 | Littorin et al. (2007) |
| Sweden (n = 81) | 2,4-TDA(U)/2,6-TDA(U)/Sum-TDA(U) <0.1–47 (4.5)/<0.1–115 (3.7)/<0.1–162 (9.7) µg l−1 [~<0.07–32 (3.1)/ −78 (2.5)/−110 (6.6) µmol mol−1 cr.] 2,4-TDA(P)/2,6-TDA(P)/Sum-TDA(P) <0.1–31 (7.4)/<0.1–42 (6.1)/<0.1–70 (14) µg l−1 | High correlations between air exposure and urinary biomarker levels (ranging from 0.75 to 0.88) or plasma biomarker levels (ranging from 0.50 to 0.78). 2,6-TDA(U) (µg l−1) = 2.7_TDI(ppb) + 0.02 (r = 0.88) [5 ppb = 97 µg l−1, ~66 µmol mol−1 cr.] | 13 | Sennbro et al. (2004) | |
| Japanb (n = 18) (spraying urethane paints) | Individual results not reported except graphically, 2,6-TDA(U) <19 µmol mol−1 cr. | 2,6-TDA(U) (µg g−1) = 6.6_TDI (ppb) –1.43 (r = 0.91) [5 ppb = 29 µmol mol−1 cr.] | 13 Both raw and corrected data | Sakai et al. (2005) | |
| Finlandb (n = 6, car repair; n = 15 other PUR processes) | Car repair Sum-TDA(U) <0.02–0.76 (0.23) µmol mol−1 cr. Other processes <0.02–0.17 (0.07) µmol mol−1 cr. | Other processes included milling and turning of PUR-coated metal cylinders, injection moulding of thermoplastic PUR, welding of district heating pipes and joint welding of PUR floor covering. | 11 | Rosenberg et al. (2002) |
aThe lower the LaKind score the better the overall quality (possible range 8–24).
bGeometric mean rather than median.
cCountry of origin assumed from authors’ affiliation, not specifically stated in paper.
Continuous foam production has been the most studied individual process [six papers from five different European Union (EU) countries], although the number of workers per study was generally small (N < 30) (Table 3). Maximum observed results ranged from 3.9 µmol mol−1 creatinine (Swierczynska-Machura et al., 2015) to 97 µmol mol−1 creatinine (Geens et al., 2012).
Moulding processes have been studied in larger (n = 18–90) worker populations than for continuous foam production (n = 4–26) but were only reported in two countries: the UK (Cocker et al., 2009; Keen et al., 2012) and Sweden (Sennbro et al., 2004; Tinnerberg et al., 2014). If mixed processes (which include some moulding companies but results are not reported separately) are included (Littorin et al., 2007; Säkkinen et al., 2011) then three countries are covered (UK, Sweden, and Finland) and maximum results for urinary TDA range from ~3.2 (Tinnerberg et al., 2014) to ~110 µmol mol−1 creatinine (Sennbro et al., 2004). If the Sennbro study (2004) is excluded, the results are more comparable across studies, with maximum urine TDA levels of ~3.2 (Tinnerberg et al., 2014), >6.5 (Keen et al., 2012), only 90th percentile reported, 15.5 (Cocker et al., 2009), ~29.3 (Littorin et al., 2007), and 39 (Säkkinen et al., 2011) being reported; all results in µmol mol−1 creatinine.
There were very few studies looking at other uses of TDI, such as glues, spray adhesives, or heat guns; these were sometimes included in ‘mixed’ studies involving multiple sites but the results were not reported separately. Sakai et al. (2005) reported on urethane spray painting for lacquering musical instruments. This study showed a good correlation between urine TDA and airborne TDI (r > 0.9 for post-shift creatinine-corrected urinary 2,6-TDA and 2,6-TDI). Results up to 19 µmol mol−1 creatinine were reported. One study (Rosenberg et al., 2002) examined thermal degradation processes such as cutting, welding, and grinding. Airborne levels were generally less than 5% of the occupational exposure limit (OEL) and urine TDA levels were very low (all less than 1 µmol mol−1 creatinine).
Correlations
Except where respiratory protective equipment (RPE) use or skin contact was significant (and noted), there was generally a strong correlation (r > 0.8) between urine TDA and airborne TDI (Sennbro et al., 2004; Sakai et al., 2005; Littorin et al., 2007; Säkkinen et al., 2011; Geens et al., 2012; Swierczynska-Machura et al., 2015). However, the resulting estimates of a urine TDA level from a 5 ppb TDI exposure varied significantly from 4.1 µmol mol−1 creatinine (Swierczynska-Machura et al., 2015); (only based on GM correlations) to ~66 µmol mol−1 creatinine (Sennbro et al., 2004).
Fewer data were presented on correlation with air monitoring in other studies compared with continuous foam production (Table 3) although Sennbro et al. (2004), Littorin et al. (2007), and Säkkinen et al. (2011) all report positive correlations (r > 0.75).
No specific albumin adducts of TDI have been measured in plasma samples, only plasma TDA was assessed after alkaline or acid hydrolysis. Säkkinen et al. (2011) analysed 2,4-TDA (6–270 nmol l−1) and 2,6-TDA (3–310 nmol l−1) in plasma after acid hydrolysis with sulphuric acid 3 M (100°C for 16 h) and found a very good correlation (r = 0.91) between the plasma total TDA and the airborne TDI. Sennbro et al. (2004), Littorin et al. (2007), and Tinnerberg et al. (2014) used basic hydrolysis with 0.3 M NaOH (24 h) in order to measure plasma concentration of TDA. Similar to Säkkinen et al. (2011), both Sennbro et al. (2004) and Littorin et al. (2007) found same range levels of plasma TDA as well as good correlations with the air levels of TDI (r = 0.50 for 2,4-TDA and 0.78 for 2,6-TDA; and r = 0.72 for 2,4-TDA and 0.86 for 2,6-TDA, respectively).
As for albumin adducts, no direct measure of TDI Hb adducts has been performed. Säkkinen et al. (2011), after isolation of globin and acid hydrolysis with sulphuric acid 3 M (100°C for 16 h), were able to quantify 2,4-TDA (13–120 pmol g−1) and 2,6-TDA (12–210 pmol g−1) in globin, as representative for Hb adducts, but no correlation was observed between the Hb total TDA and airborne TDI. This might be, amongst others, due to the relatively low number of detects amongst the occupationally exposed workers (n = 4 for 2,4-TDA and n = 10 for 2,6-TDA), or Hb total TDA might be a reflection of long-term exposure unlike airborne TDI measurements.
MDI
Reported biomonitoring levels
Available studies on MDI (Table 4) are mostly from the PUR sector with five papers classified under PUR production/use (Rosenberg et al., 2002; Sennbro et al., 2006; Robert et al., 2007; Cocker et al., 2009; Keen et al., 2012). Exposures reported in studies from France (Robert et al., 2007) and Sweden (Sennbro et al., 2006) are in reasonable agreement; urine levels of MDA up to 33.7 µg g−1 creatinine (19.2 µmol mol−1 creatinine) in the Robert et al. (2007) study and 78 µg l−1 (~32.6 µmol mol−1 creatinine) in the Sennbro et al. (2006) study. Low levels were reported in the UK PUR elastomer industry with only 6 of the 71 workers sampled having detectable exposures (<0.5–0.7 µmol mol−1 creatinine) in one study (Cocker et al., 2009) and a reported 90th percentile of 0.5 µmol mol−1 creatinine in a second study (Keen et al., 2012). A study conducted in Finland (Rosenberg et al., 2002) examined exposure to thermal degradation products of PURs in a number of processes including grinding and welding in MVR, milling and turning of PUR-coated metal cylinders, injection moulding, welding, and cutting heating pipes, joint welding, and heat-flexing of PUR floor covering. Exposures were low overall (0.01–3.1 µmol mol−1 creatinine) with pipe layers receiving the highest exposures.
Table 4.
Summary of MDI exposure studies for the main processes.
| Sector | Study populations (country, no. workers) | Biomonitoring data [expressed as range (median)] | Notable correlations/comments | LaKind scoringa | References |
|---|---|---|---|---|---|
| (Rigid) foam production | Finland, n = 57 | Urine: 0.015–1.4 (0.13) µmol mol−1 cr. Plasma: 1.8–2.6 µg l−1 18–37 pmol g−1 (Hb) | Airborne levels (very low or not detected) and task time not associated with urinary biomarker levels. | 13 | Säkkinen et al. (2011) |
| Sweden, n = 18 | Urine: 0.5–8.4 µg l−1 [~0.2–3.5 µmol mol−1 cr.] Plasma: 0.4–19.4 µg l−1 | Plasma and urinary MDA correlated after 2 days of no exposure (P > 0.986). | 12 Creatinine and specfic gravity | Tinnerberg et al. (2014) | |
| PUR industry (generic) | France, n = 169 (19 factories) | Urine: <0.1–23.6 µg l−1 [<0.5–19.25 µmol mol−1 cr.] | Association with skin exposure. Elevated pre-shift levels but not cumulative. Higher MDI % in formulations not associated with higher results. | 13 Both raw and corrected data | Robert et al. (2007) |
| Sweden, n = 18 | Urine: 0.3–78 (2) µg l−1 [~0.13–32.7 (0.8) µmol mol−1 cr.] Plasma: 0.2–74 (0.7) µg l−1 | Weak but significant correlations with air. P < 0.01 (U), P < 0.05 (P) | 12 Both raw and corrected data | Sennbro et al. (2006) | |
| UK, n = 71 | Urine: <0.5–0.7 µmol mol−1 cr. (only 6+ve/71 results above LoD) | Low levels of isocyanate exposure in the PUR elastomer industry. | 11 | Cocker et al. (2009) | |
| UK, n = 90 | Urine: 56/326 > LoD, 90% 0.5 µmol mol−1 cr. (median < LOD) | 12 | Keen et al. (2012) | ||
| Finland, n = 21 | Urine: <0.01–3.1 µmol mol−1 cr. | Low exposures but highest levels seen in pipe layers. | 11 | Rosenberg et al. (2002) | |
| PUR industry (glue) | Sweden, n = 150 | Urine: <LoD–1.8 µg l−1 [~<LoD–0.8 µmol mol−1 cr.] <LoD-9.4 (heat) µg l−1 | Higher exposure levels when using heated glue. | 11 External QA | Littorin et al. (2000) |
| Construction | Switzerlandb, n = 65 | Urine: MDA 0.003–3.2 µg l−1 [~0.001–1.3 µmol mol−1 cr.] Median (P90) Hb-MDA: 0 (0.177) pmol g−1 Hb Hb-AcMDA: 2 positive, 2.3 and 3.7 pmol g−1 Hb | U-MDA-tot correlates with U-AcMDA and Hb-MDA with r = 0.86 and r = 0.39, respectively (P < 0.01). U-AcMDA correlates with Hb-MDA with r = 0.47, (P < 0.01). U-MDA correlates with Hb-MDA (r = 0.38, P = 0.05). | 14 Methodology not well described | Sabbioni et al. (2007) |
| Switzerlandb, n = 65 | Albumin: MDI-Lys 0–899.4 fmol mg−1 AcMDI-Lys: 0–51.2 fmol mg−1 | Correlation MDI-Lys with MDA-Hb, r = 0.295 (P < 0.05) Same workers as Sabbioni et al. (2007) MDI-Lys levels were compared in a subgroup of construction workers (n = 19) which were analysed prior to isocyanate exposure and after 4–7 months of isocyanate exposure; the MDI-Lys levels increased significantly (Wilcoxon sign test, P < 0.01). | 12 | Sabbioni et al. (2010) | |
| Finland, n = 21 | Urine: <0.1–0.2 µmol mol−1 cr. Dermal: 88% <2 µg MDI 10 cm−2 on hand | Effect of RPE lowering exposure seen in post-shift samples but not evening and following morning samples indicating 2 routes of exposure, dermal and inhalation. | 12 Both raw and corrected data | Henriks-Eckerman et al. (2015) | |
| Other | Switzerlandbn = 27 (chemical industry) | Urine: MDA 0–10.2 (1.7) nmol l−1 [~0–0.9 (0.142) µmol mol−1 cr.] Albumin: MDI-Lys 0–138 pmol g−1 AcMDI-Lys: 25.6 pmol g−1 (1 +ve) | Correlation MDI-Lys with MDA-Hb, r = 0.382 (P < 0.05). | 12 | Sabbioni et al. (2010) |
| Switzerland, n = 73 (urethane mould production) | Albumin: MDI-Lys = 191 pmol g−1 (mean) (based on 4 workers with asthma who reported that their last activity with MDI was >3 months ago) MDI-Lys = 750 pmol g−1 (mean) (n = 5) | Workers with confirmed asthma had significantly higher adduct levels than healthy worker. | 14 Methodology not well described | Sabbioni et al. (2017) | |
| Germanyb, n = 25 | Urine: <500–124 490 pmol g−1 creatinine [0.004–1.1 µmol mol−1 cr.] Hb: MDA <0.35–1.12 pmol g−1 ABP-Val-Hyd 0.15–16.2 pmol g−1 | No exposure assessment; measurement of Hb adducts and ABP-Val-Hyd reflect long term exposure (up to 120 days) | 12 | Gries and Leng (2013) |
aThe lower the LaKind score the better the overall quality (possible range 8–24).
bCountry of origin assumed from authors’ affiliation, not specifically stated in paper.
One paper was identified from Sweden (Littorin et al., 2000) in which 150 workers were using glue containing MDI, with some of these workers using the glue heated. Maximum MDA urine levels from workers using heated glue were over five times higher than those using non-heated glue, 9.4 µg l−1 (~3.9 µmol mol−1 creatinine) and 1.8 µg l−1 (~0.8 µmol mol−1 creatinine), respectively.
Three papers were identified from the construction industry (Sabbioni et al., 2007, 2010; Henriks-Eckerman et al., 2015). Urine MDA levels were low for both study groups: 0.017–16.4 nmol l−1 (~0.001–1.4 µmol mol−1 creatinine) in a Switzerland study (Sabbioni et al., 2007) and 0.1–0.2 µmol mol−1 creatinine in a Finnish study (Henriks-Eckerman et al., 2015). Workers were reported to be involved in a range of activities, including spray foaming which could generate higher exposures, but RPE was used in the majority of cases.
In the 2007 study from Sabbioni, MDA in urine from acid hydrolysis correlated well with acetyl-MDA (acMDA), released by alkaline hydrolysis, and Hb-MDA with r = 0.86 and r = 0.39, respectively (P < 0.01). Inhalation and dermal were both identified as routes of exposure in the study of Finnish workers (Henriks-Eckerman et al., 2015); this was also the only study to report dermal concentrations (88% workers <2 µg MDI 10 cm−2 on hand). A French PUR study of 169 workers also noted evidence of dermal exposure (Robert et al., 2007).
Specific albumin adducts (MDI-Lys and AcMDI-Lys) have been measured by Sabbioni et al. (2010) in two groups of workers: construction workers (n = 65) and workers of a non-specified MDI plant (n = 27). The specific biomarker MDI-Lys was found in 63% of the construction workers, in 64% of the plant workers, and in only 15% for the control group. In Sabbioni et al. (2017) a higher concentration (750 pmol g−1) was found in workers with asthma who had recently worked with MDI compared with asthmatic workers who had not used MDI for more than 3 months (MDI-Lys 191 pmol g−1). These MDI-Lys biomarkers are more specific than hydrolysed MDA levels (as they are not confounded with MDA itself) and can be used as exposure biomarkers. Sabbioni et al. (2007) found that these biomarkers accumulate in the body over time. Elevated levels were observed in workers samples after working with MDI over a period of 4–7 months.
Gries and Leng (2013) have found a higher concentration of the specific MDI biomarker ABP-Val-Hyd in exposed workers (0.15–16.2 pmol g−1) compared with non-exposed workers (below LoQ). Säkkinen et al. (2011) measuring MDA after acid hydrolysis of globin, found the same order of magnitude between exposed and non-exposed workers; this is most likely due to the hydrolysis releasing non-specific adducts and converting them to MDA.
Correlations
Although MDI is less volatile than HDI and TDI, correlations with air levels were still observed. Sennbro et al. (2006) reported weak but significant correlations (r > 0.5) with air levels for both urine (P < 0.01) and plasma (P < 0.05) biomarkers. The Finnish study from the construction industry (Henriks-Eckerman et al., 2015) observed that the use of RPE led to lower post-shift urine levels of MDA.
Säkkinen et al. (2011) reported urine MDA levels of workers (n = 65) at 0.015–1.4 µmol mol−1 creatinine in post-shift samples and plasma concentrations of MDA at 9–13 nmol l−1. There were insufficient positive plasma results to investigate correlations between urine and plasma levels of MDA. Tinnerberg et al. (2014) reported MDA urine levels of workers (n = 21) at 0.5–8.4 µg l−1 (~0.2–3.5 µmol mol−1 creatinine) and plasma concentrations of MDA at 0.4–19.4 µg l−1 (2.0–98.0 nmol l−1). Significant correlations (P < 0.01) were seen between plasma MDA and urinary MDA levels (r = 1.000, 0.988, and 0.986 for unadjusted, specific gravity adjusted, and creatinine adjusted urine, respectively) (Tinnerberg et al., 2014).These results cannot be directly compared with the Säkkinen study as the samples were taken on a Monday morning after a weekend of no exposure.
Discussion
Biomarkers for biomonitoring of diisocyanates
The majority of the papers (>90%) assessed for this review studied the corresponding diamines in either urine or plasma. Where both have been studied, a good correlation (r > 0.85) is generally seen between diamines in urine and plasma (Sennbro et al., 2003; Tinnerberg and Mattsson, 2008; Tinnerberg et al., 2014) although significant individual variation was observed and correlations could not be compared between studies due to sample collection differences or the way the data were reported. Aside from the diamine biomarkers, the quantity of identified literature published is limited. The use of adducts has been investigated by several research groups although different adducts have been used such as diisocyanate-specific Hb adducts, diamine-specific Hb adducts, and diisocyanate-specific albumin adducts. One major benefit of the adduct biomarkers is the ability to study exposures over the longer term; amines are really only representing same day or previous day exposures whereas adducts can represent exposures over weeks (albumin) and months (Hb). It is probably necessary to consider both peak and chronic exposures in the assessment of occupational asthma risk as the exact mechanisms of sensitization and exacerbation are still unclear. Development of more specific biomarkers (U-TAHI for HDI-isocyanurate, ABP-Val-Hyd for MDI, and MDI-lysine for MDI) is reported by individual papers. These are yet to be used widely and there are potential issues with standards (they are not commercially available yet) so demonstrating comparability of results will be difficult initially. To date there are few data correlating adducts to diamines or airborne levels so these cannot be interpreted with respect to risk at this time. Although the LaKind scoring criteria provide useful clarity on the strengths and weaknesses of papers when considering papers as part of a review, the ‘total score’ is less useful as papers that have different strengths may have the same ‘total score’. The ‘total score’ should therefore not be the sole consideration when differentiating papers. Despite this, the best quality papers can be identified by a low score. From this review, it is clear that many papers still do not adequately present methodological details (often citing another paper with no relevant details in the present paper). Other factors, such as quality assurance and sample handling, are also often not reported although they may have in fact been done. There is therefore a need for journal reviewers to request these details more often. Given the widespread facility to submit supplementary data for papers, more reporting of both corrected and uncorrected urine data should be encouraged because there is still not always a consensus on whether particular biomarkers are best reported in one or other format. Some criteria (such as study size) do not reflect quality per se but rather the generalizability of the study results to other scenarios.
Reported biomonitoring levels
With regard to our second aim: we have provided an overview of available studies on reported exposure in available diisocyanate biomonitoring studies. About half of the studies were prior to 2010 hence perhaps do not reflect current workplace exposures, especially given subsequent EU restrictions and more recent product developments (ECHA, 2010). Also we noticed that a substantial number of studies measured airborne levels below the OEL. This indicates that secondary exposures such as cutting, welding, and grinding (after curing) are likely to be minimal. Exposure levels can only be considered for the urinary and plasma diamines where there is a sizeable body of data. There is large variability within and between studies and across sectors making it difficult to identify high-risk sectors. The observed large variability indicates that elevated exposures are most likely due to individual (whether worker or workplace) factors rather than reflecting an industry or a process as a whole.
Austin (2007) observed that ‘handlers’ of foam blocks had much greater urinary TDA levels (by up to 20 times) for the same airborne TDI exposure as ‘non-handlers’. This finding points to the potential for dermal TDI uptake although co-exposure to TDA (known to be well absorbed through the skin and shown to be generated in foam blowing processes (Jones et al., 2017) possibly contributed to the body burden.
The short half-lives of amines and the timing of sampling in relation to the activities are probably also influential and another factor that could contribute to the observed large variability is the use of RPE; when comparing the studies on HDI in the MVR we noticed that virtually all sprayers in the UK study wore air-fed visors whereas these were not used in the Netherlands. Swierczynska-Machura et al. (2015) also stated that the lack of observed correlation with air levels in their study could be due to the use of RPE.
Another possibility for the observed variability is the potential issue with comparing TDI results across studies with regard to the different sample hydrolysis procedures. Generally, one of three different conditions is applied (acid hydrolysis for 1.5 h, acid hydrolysis for 16 h, or alkaline hydrolysis for up to 24 h). Alkaline hydrolysis has been reported to give higher results as more adducts are released. Note, e.g., that Geens et al. (2012) and Kääriä et al. (2001) used the higher release alkaline method in comparison to the other TDI biomonitoring studies (Table 3).
A couple of papers (Sennbro et al., 2003; Sakai et al., 2005) examined different hydrolysis conditions and concluded that the results from the different techniques are well correlated but that alkaline hydrolysis can result in ~50% higher results in urine and ~10% higher in plasma. Such differences are not so extreme as to negate a comparison of the different studies, processes and exposure risks so we have not ‘corrected’ reported results for hydrolysis method. Furthermore, although methods are well described and published by respected institutes, the lack of reported external quality assurance means that direct equivalence of the studies cannot be assumed. Although this issue is not specific to diisocyanates, it is an area where journals could seek to improve reporting. It also highlights the value of studies such as HBM4EU (www.hbm4eu.eu) and DEMOCOPHES (Casteleyn et al., 2015) where significant effort has gone into ensuring that results from different studies/countries within the project are comparable.
Research gaps
We have identified a number of research gaps based on an analysis of the available data. Firstly, much of the focus of diisocyanate exposure assessment has been, naturally, inhalation exposure (given it causes occupational asthma); however, several studies (Austin, 2007; Robert et al., 2007; Robbins et al., 2018) indicate the possibility of dermal absorption or the need to further investigate routes of exposure (Keen et al., 2012). As these studies have used the diamine biomarkers, it is not certain that any dermal uptake is from the intact diisocyanates. Obviously one of the primary advantages of using biomonitoring is that exposures when relying on RPE or where skin uptake is possible are difficult to assess by other means. Further, in general, we found that most studies showed a fair correlation between urinary amines levels and airborne measurements. However, in some cases where dermal exposure was likely or RPE was used, the correlation was weaker. This also illustrates the added value of taking biomonitoring samples.
Furthermore, the vast majority of papers discovered were studying HDI, TDI, and/or MDI. There were brief mentions of NDI (Tinnerberg and Mattsson, 2008; Tinnerberg et al., 2014) and IPDI (Creely et al., 2006); however, these were not a focus of the review and so not specific search terms in the initial literature search. One of the new ‘innovations’ in isocyanate use has been the introduction of so-called ‘blocked isocyanates’ into paints and coatings, where the –NCO group is chemically blocked until the reaction process (which usually takes place in an enclosed booth or oven). This is proposed to be a safer use although the diisocyanate is still released during the reaction process. No papers were found looking specifically at the exposures from blocked diisocyanates so the assumption of safer use has not been demonstrated and may warrant investigation.
Lastly, we looked into potential research gaps with regard to industrial sectors studied. For TDI, there is a reasonable amount of data on flexible foam production (Poland, Sweden, UK, Belgium, and Finland). Less data are available for the uses of TDI in rigid PUR manufacture and the use of heated glues. Littorin et al. (2000) suggest that heated glues may present a greater exposure risk than unheated glues.
The available MDI data are mainly from the PUR industry with just one study looking at exposure from glues and two concerned with the construction sector where there are potentially several different sources of exposure such as sprayed insulation. Paint spraying in MVR has long been considered potentially high risk due to the very high concentrations of HDI that have been measured in spray booths (hundreds to thousands of µg m−3, well in excess of current exposure limits). Whilst MVR has been well studied globally (including in the Netherlands and UK in Europe), exposures in other transport sectors such as aerospace, shipping, and large commercial vehicles have not been widely reported, with data only available from Netherlands (Pronk et al., 2006). These sectors may present different exposure issues as the use of enclosed spray booths is impractical. The most relevant industrial uses of diisocyanates have been reported to be in the manufacturing of: diisocyanate compounds themselves, PUR and PUR composite materials, foam (spray foam applications), coatings, and adhesives (RAC/SEAC, 2017). The direct manufacture of PUR plastic materials accounts for more than 90% of the use of diisocyanates. We did not find any European studies on manufacture of spray adhesives or coatings; hence these sectors might also be worthwhile investigating.
The use of MDI, TDI, and HDI has been recently proposed to be restricted in the EU unless specific conditions for workers’ training and risk management measures apply (RAC/SEAC, 2017/2018). The aim of the restriction is not, however, to ban the use of diisocyanates but rather to improve the control of diisocyanate use by obligatory training for good working practices and risk management. There is evidence that exposures can be well controlled within a study population e.g. 98% of 196 workers less than LoQ (Hu et al., 2017), >90% of 995 workers less than Great Britain guidance value of 1 µmol mol−1 creatinine (Jones et al., 2013), both for HDI in MVR. However, there are also examples of significantly elevated results of up to 100 µmol TDA mol−1 creatinine (Geens et al., 2012; Sennbro et al., 2004), which are well in excess of the American Conference of Governmental Industrial Hygienists guidance value (ACGIH, 2019) of 4.6 µmol mol−1 creatinine, meaning that health effects cannot be ruled out. This is the first time that this type of restriction has been proposed at the EU level and there might be an interest to follow-up on the effectiveness of the restriction if implemented. This review has highlighted the wide range of results that can be found within workers, workplaces, and sectors. If the restriction proposal on diisocyanates does come into force, it should have an impact on the exposure to diisocyanates, but small and medium enterprises (with the cost implications of the restriction and being traditionally hard to reach) may still pose a challenge. Therefore, a follow-up on the effectivity especially in SMEs is of high interest to evaluate whether exposures overall have been reduced through improved training (the restriction proposal), as has been seen to some extent in the UK (Piney, 2015).
Conclusion
Based on a systematic review we provide a comprehensive summary on available biomarkers and matrices for diisocyanate exposure. Further, we summarized available occupational diisocyanates biomonitoring studies published since 2000 including reported exposure levels, and discussed the studies in detail. Although biomonitoring studies have a number of advantages over external exposure measurements—for example when dermal exposure is likely or when respiratory protection is used—this review has highlighted the need for a harmonized approach to study and report biomonitoring levels; also to provide a baseline against which the success of the recently proposed restriction can be evaluated. We identified several knowledge gaps which could further aid studying diisocyanates biomonitoring levels: (i) the development of specific biomarkers is promising (e.g. to study oligomers of HDI which have been largely neglected to date) but needs more research before they can be widely applied, (ii) a more uniform approach of analytical methods would make comparisons between studies easier, and (iii) dermal absorption seems a possible exposure route and needs to be further investigated.
Funding
The HBM4EU project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement no. 733032 and received co-funding from the author’s organizations and/or ministries. All the experts that have provided their comments and were involved in the preparation and translation of the information material and different standard operating procedures for this study are acknowledged and greatly appreciated by the (co-)authors of this manuscript.
Disclaimer
HSE’s contribution to this publication was co-funded by the Health and Safety Executive (HSE) of Great Britain. Its contents, including any opinions and/or conclusions expressed, are those of the authors alone and do not necessarily reflect HSE policy.
Conflict of interest
None declared.
References
- ACGIH (2019) TLVs and BEIs. Available at https://www.acgih.org/forms/store/ProductFormPublic/2019-tlvs-and-beis. Accessed 2 February 2020.
- Austin S. (2007) Biological monitoring of TDI-derived amines in polyurethane foam production. Occup Med; 57: 444–8. [DOI] [PubMed] [Google Scholar]
- Casteleyn L, Dumez B, Becker K et al. (2015). A pilot study on the feasibility of European harmonized human biomonitoring: strategies towards a common approach, challenges and opportunities. Environ Res; 141: 3–14. [DOI] [PubMed] [Google Scholar]
- Cocker J. (2007) Biological monitoring for isocyanates. Ann Occup Hyg; 57: 391–3. [DOI] [PubMed] [Google Scholar]
- Cocker J. (2011) Biological monitoring for isocyanates. Ann Work Expos Health; 55: 127–31. [DOI] [PubMed] [Google Scholar]
- Cocker J, Cain J, Baldwin P et al. (2009) A survey of occupational exposure to 4,4′-methylene-bis (2-chloroaniline) (MbOCA) in the UK. Ann Occup Hyg; 53: 499–507. [DOI] [PubMed] [Google Scholar]
- Cocker J, Mason HJ, Warren ND et al. (2011) Creatinine adjustment of biological monitoring results. Occup Med (Lond); 61: 349–53. [DOI] [PubMed] [Google Scholar]
- Creely KS, Hughson GW, Cocker J et al. (2006) Assessing isocyanate exposures in polyurethane industry sectors using biological and air monitoring methods. Ann Occup Hyg; 50: 609–21. [DOI] [PubMed] [Google Scholar]
- De Palma G, Cortesi I, Ghitti R et al. (2012) Biological monitoring as a valid tool to assess occupational exposure to mixtures of 2,4-:2,6-toluene diisocyanate. Med Lav; 103: 361–71. [PubMed] [Google Scholar]
- DECOS (2018) Di- and triisocyanates. Health-based recommendation on occupational exposure limits (No. 2018/20). The Hague, The Netherlands: Health Council of the Netherlands [Google Scholar]
- ECHA (2005) European Union risk assessment report. Methylenediphenyl diisocyanate (MDI). CAS No: 26447-40-5. EINECS No: 247-714-0. Available at https://echa.europa.eu/documents/10162/9f8ad2fd-9b47-4eb6-9bf9-e0fc898f874d. Accessed 4 January 2020.
- ECHA (2010) Restrictions on the manufacture, placing on the market and use of certain dangerous substances, mixtures and articles (Entry 56). Available at https://echa.europa.eu/documents/10162/46cb2ae0-ee6b-4319-8604-b5b1d4a41d53. Accessed 4 January 2020.
- ECHA (2017) Opinion on an Annex XV dossier proposing restrictions. Diisocyanates. ECHA/RAC/RES-O-0000001412-86-174/F. Available at https://echa.europa.eu/documents/10162/737bceac-35c3-77fb-ba7a-0e417a81aa4a. Accessed 4 January 2020.
- ECHA (2019a) Brief profile 4-methyl-m-phenylene diisocyanate (2,4-TDI). Available at https://echa.europa.eu/fi/brief-profile/-/briefprofile/100.008.678. Accessed 4 January 2020.
- ECHA (2019b) Brief profile m-tolylidene diisocyanate Available at https://echa.europa.eu/fi/brief-profile/-/briefprofile/100.043.369. Accessed 4 January 2020.
- ECHA (2019c) Brief profile on 4,4′-methylenediphenyl diisocyanate. Available at https://echa.europa.eu/fi/brief-profile/-/briefprofile/100.002.697. Accessed 4 January 2020.
- ECHA (2019d) Brief profile. Hexamethylene diisocyanate. Available at https://echa.europa.eu/nl/substance-information/-/substanceinfo/100.011.350. Accessed 4 January 2020.
- Fent KW, Jayaraj K, Ball LM et al. (2008) Quantitative monitoring of dermal and inhalation exposure to 1,6-hexamethylene diisocyanate monomer and oligomers. J Environ Monit; 10: 500–7. [DOI] [PubMed] [Google Scholar]
- Flack SL, Fent KW, Gaines LGT et al. (2011) Hemoglobin adducts in workers exposed to 1,6-hexamethylene diisocyanate. Biomarkers; 16: 261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flack SL, Fent KW, Trelles Gaines LG et al. (2010) Quantitative plasma biomarker analysis in HDI exposure assessment. Ann Occup Hyg; 54: 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines LG, Fent KW, Flack SL et al. (2010) Urine 1,6-hexamethylene diamine (HDA) levels among workers exposed to 1,6-hexamethylene diisocyanate (HDI). Ann Occup Hyg; 54: 678–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geens T, Dugardin S, Schockaert A et al. (2012) Air exposure assessment of TDI and biological monitoring of TDA in urine in workers in polyurethane foam industry. Occup Environ Med; 69: 93–8. [DOI] [PubMed] [Google Scholar]
- Gries W, Leng G (2013) Analytical determination of specific 4,4′-methylene diphenyl diisocyanate hemoglobin adducts in human blood. Anal Bioanal Chem; 405: 7205–13. [DOI] [PubMed] [Google Scholar]
- Henriks-Eckerman ML, Mäkelä EA, Laitinen J et al. (2015) Role of dermal exposure in systemic intake of methylenediphenyl diisocyanate (MDI) among construction and boat building workers. Toxicol Lett; 232: 595–600. [DOI] [PubMed] [Google Scholar]
- Health and Safety Laboratory (HSL) (2005) Biological monitoring for isocyanates. In Analysis of urine to assess exposure to isocyanates. Guidance for workers, employers, and occupational health professionals. Buxton, UK: Health Sciences Group, Health and Safety Laboratory; p. 5. [Google Scholar]
- Hu J, Cantrell P, Nand A (2017) Comprehensive biological monitoring to assess isocyanates and solvents exposure in the NSW Australia motor vehicle repair industry. Ann Work Expo Health; 61: 1015–23. [DOI] [PubMed] [Google Scholar]
- IARC (1999) IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 71 Available at https://monographs.iarc.fr/wp-content/uploads/2018/06/mono71.pdf. Accessed 20 January 2020. [Google Scholar]
- Jones K, Cocker J, Piney M (2013) Isocyanate exposure control in motor vehicle paint spraying: evidence from biological monitoring. Ann Occup Hyg; 57: 200–9. [DOI] [PubMed] [Google Scholar]
- Jones K, Johnson PD, Baldwin PEJ et al. (2017) Exposure to diisocyanates and their corresponding diamines in seven different workplaces. Ann Work Expo Health; 61: 383–93. [DOI] [PubMed] [Google Scholar]
- Kääriä K, Hirvonen A, Norppa H et al. (2001) Exposure to 2,4- and 2,6-toluene diisocyanate (TDI) during production of flexible foam: determination of airborne TDI and urinary 2,4- and 2,6-toluenediamine (TDA). Analyst; 126: 1025–31. [DOI] [PubMed] [Google Scholar]
- Keen C, Coldwell M, McNally K et al. (2012) A follow up study of occupational exposure to 4,4′-methylene-bis(2-chloroaniline) (MbOCA) and isocyanates in polyurethane manufacture in the UK. Toxicol Lett; 213: 3–8. [DOI] [PubMed] [Google Scholar]
- LaKind JS, Sobus JR, Goodman M et al. (2014) A proposal for assessing study quality: biomonitoring, environmental epidemiology, and short-lived chemicals (BEES-C) instrument. Environ Int; 73: 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littorin M, Axmon A, Broberg K et al. (2007) Eye and airway symptoms in low occupational exposure to toluene diisocyanate. Scand J Work Environ Health; 33: 280–5. [DOI] [PubMed] [Google Scholar]
- Littorin M, Rylander L, Skarping G et al. (2000) Exposure biomarkers and risk from gluing and heating of polyurethane: a cross sectional study of respiratory symptoms. Occup Environ Med; 57: 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JC, Chen Y, Zekveld C et al. (2005) Incidence by occupation and industry of acute work related respiratory diseases in the UK, 1992–2001. Occup Environ Med; 62: 836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J et al. ; PRISMA Group. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piney M. (2015) Reducing isocyanate exposure and asthma risk in motor vehicle repair. Int J Workplace Health Manag; 8: 272–83. [Google Scholar]
- Pronk A, Yu F, Vlaanderen J et al. (2006) Dermal, inhalation, and internal exposure to 1,6-HDI and its oligomers in car body repair shop workers and industrial spray painters. Occup Environ Med; 63: 624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAC/SEAC. (2017) Opinion on an Annex XV dossier proposing restrictions DIISOCYANATES. ECHA/RAC/RES-O-0000001412-86-174/F Available at https://echa.europa.eu/documents/10162/737bceac-35c3-77fb-ba7a-0e417a81aa4a Accessed 3 March 2020. [Google Scholar]
- Robbins Z, Bodnar W, Zhang Z et al. (2018) Trisaminohexyl isocyanurate, a urinary biomarker of HDI isocyanurate exposure. J Chromatogr B Analyt Technol Biomed Life Sci; 1076: 117–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A, Ducos P, Francin JM et al. (2007) Biological monitoring of workers exposed to 4,4′-methylenediphenyl diisocyanate (MDI) in 19 French polyurethane industries. Int Arch Occup Environ Health; 80: 412–22. [DOI] [PubMed] [Google Scholar]
- Rosenberg C, Nikkilä K, Henriks-Eckerman ML et al. (2002) Biological monitoring of aromatic diisocyanates in workers exposed to thermal degradation products of polyurethanes. J Environ Monit; 4: 711–6. [DOI] [PubMed] [Google Scholar]
- Rosenberg C, Savolainen H (1986) Determination in urine of diisocyanate-derived amines from occupational exposure by gas chromatography-mass fragmentography. Analyst; 111: 1069–71. [DOI] [PubMed] [Google Scholar]
- Sabbioni G, Dongari N, Kumar A (2010) Determination of a new biomarker in subjects exposed to 4,4′-methylenediphenyl diisocyanate. Biomarkers; 15: 508–15. [DOI] [PubMed] [Google Scholar]
- Sabbioni G, Vanimireddy LR, Lummus ZL et al. (2017) Comparison of biological effects with albumin adducts of 4,4′-methylenediphenyl diisocyanate in workers. Arch Toxicol; 91: 1809–14. [DOI] [PubMed] [Google Scholar]
- Sabbioni G, Wesp H, Lewalter J et al. (2007) Determination of isocyanate biomarkers in construction site workers. Biomarkers; 12: 468–83. [DOI] [PubMed] [Google Scholar]
- Sakai T, Morita Y, Roh J et al. (2005) Improvement in the GC-MS method for determining urinary toluene-diamine and its application to the biological monitoring of workers exposed to toluene-diisocyanate. Int Arch Occup Environ Health; 78: 459–66. [DOI] [PubMed] [Google Scholar]
- Säkkinen K, Tornaeus J, Hesso A et al. (2011) Protein adducts as biomarkers of exposure to aromatic diisocyanates in workers manufacturing polyurethane (PUR) foam. J Environ Monit; 13: 957–65. [DOI] [PubMed] [Google Scholar]
- Sennbro CJ, Lindh CH, Mattsson C et al. (2006) Biological monitoring of exposure to 1,5-naphthalene diisocyanate and 4,4’-methylenediphenyl diisocyanate. Int Arch Occup Environ Health; 79: 647–53. [DOI] [PubMed] [Google Scholar]
- Sennbro CJ, Lindh CH, Tinnerberg H et al. (2003) Development, validation and characterization of an analytical method for the quantification of hydrolysable urinary metabolites and plasma protein adducts of 2,4- and 2,6-toluene diisocyanate, 1,5-naphthalene diisocyanate and 4,4′-methylenediphenyl diisocyanate. Biomarkers; 8: 204–17. [DOI] [PubMed] [Google Scholar]
- Sennbro CJ, Lindh CH, Tinnerberg H et al. (2004) Biological monitoring of exposure to toluene diisocyanate. Scand J Work Environ Health; 30: 371–8. [DOI] [PubMed] [Google Scholar]
- Swierczynska-Machura D, Brzeznicki S, Nowakowska-Swirta E et al. (2015) Occupational exposure to diisocyanates in polyurethane foam factory workers. Int J Occup Med Environ Health; 28. doi: 10.13075/ijomeh.1896.00284 [DOI] [PubMed] [Google Scholar]
- Tinnerberg H, Broberg K, Lindh CH et al. (2014) Biomarkers of exposure in Monday morning urine samples as a long-term measure of exposure to aromatic diisocyanates. Int Arch Occup Environ Health; 87: 365–72. [DOI] [PubMed] [Google Scholar]
- Tinnerberg H, Mattsson C (2008) Usage of air monitoring and biomarkers of isocyanate exposure to assess the effect of a control intervention. Ann Occup Hyg; 52: 187–94. [DOI] [PubMed] [Google Scholar]
- Wisnewski AV, Liu J, Redlich CA (2013) Connecting glutathione with immune responses to occupational methylene diphenyl diisocyanate exposure. Chem Biol Interact; 205: 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]