Abstract
Background:
The predominance of neutrophils in pleural effusions of patients with different serious impairments of the pleural cavity organs is often found. The aim of this study was to identify the type of injury using the cytological-energy analysis of pleural effusions.
Methods:
We analysed 635 samples of pleural effusions with predominance of neutrophils. We compared the values of the coefficient of energy balance (KEB), lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) catalytic activities in the following subgroups of patients: with transudative effusions, purulent pneumonia, chest empyema and after chest surgery with and without purulent complications. Statistical analysis was performed using the ANOVA Kruskal–Wallis test (p < 0.05 was considered as significant).
Results:
We found the lowest KEB values in pleural effusions of patients with chest empyema and their gradual increases in patients with purulent pneumonia and with transudative effusions. We observed the highest LDH and AST enzymes activity in patients with chest empyema and their gradual decrease in patients with purulent pneumonia and with transudative effusions. LDH and AST enzymes activity was significantly higher in pleural effusions of patients after chest surgery with purulent complications compared with non-purulent cases.
Conclusion:
The most intensive inflammation and the most extensive tissue destruction in the pleural cavity were found in patients with chest empyema. Significantly better parameters were observed in patients with purulent pneumonia. The absence of serious inflammation and the absence of tissue destruction were typical for patients with transudative effusions. Finally, our results confirmed an anticipated higher tissue destruction in patients after chest surgery. Significantly worse injury was found in surgical patients with purulent complications compared with non-purulent ones.
The reviews of this paper are available via the supplemental material section.
Keywords: coefficient of energy balance, complicated exudate, cytology of pleural effusions, empyema, exudate, inflammation, neutrophils, pleural effusions, pneumonia, transudate
Introduction
Production of pleural effusion can accommodate different pathological processes in the pleural cavity. In accordance with their origin, the pleural effusions are either transudates or exudates. Imbalance between hydrostatic and oncotic pressure within the capillaries usually leads to production of transudative effusions in patients with congestive cardiac failure, renal failure and some other systemic disorders. Exudative effusions are the consequence of mesothelial and capillary permeability increasing during inflammatory response in the pleural cavity.1–5
The subject of our interest is the pleural effusions with predominance of neutrophils from patients with different impairments of the pleural cavity.
Neutrophils are considered as effector cells of innate immunity. Their key role is to phagocytose extracellular bacteria.6–12 Therefore they can be found primarily in the pleural effusions of patients suffering with bacterial infection of pleural cavity organs, for example in cases of bacterial pneumonia or chest empyema. Both these pathologies are characterized by microphagocytosis of extracellular bacteria and oxidative burst of neutrophils in the pleural cavity in the presence of purulent inflammation and tissue damage with a high risk for patients.13–21 On the other hand, the significant presence of neutrophils in pleural effusions can also be found in patients with heart failure, systemic sepsis and patients who underwent chest surgery. Our aim was to identify dangerous purulent complication using the cytological-energy analysis of pleural effusions as early as possible, in groups of our patients with different impairments of pleural cavity.22,23
The first step of the cytological-energy analysis of pleural effusion consists of the determination of the frequency of immunocompetent cells. The second step is the investigation of molar concentrations of glucose and lactate in pleural effusion and the calculation of the coefficient of energy balance (KEB):
The KEB is the theoretical average number of adenosine triphosphate molecules produced from one molecule of glucose under the conditions found in the extravascular compartment. The KEB values represent the intensity of local inflammatory response.22–25
Two energy models of the pleural compartment22,23
Oxygen is dissolved in the pleural effusion under the physiological conditions, enabling predominantly aerobic metabolism in the pleural compartment. This is associated with a relatively high production of adenosine triphosphate (ATP), which is expressed as a high KEB value (Figure 1).
Figure 1.
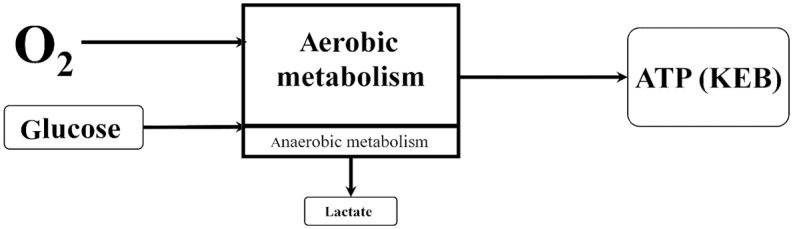
The first model of energy relationships in the pleural effusion–physiological response.
ATP, adenosine triphosphate; KEB, the coefficient of energy balance [in Czech ‘Koeficient Energetické Bilance’]; O2, oxygen
Pathological changes in the pleural compartment are usually associated with the inflammatory response (Figure 2). There is a great demand of activated immunocompetent cells in the pleural effusion for energy. Therefore, they consume higher amounts of glucose along with more oxygen utilization. This leads to the decreased level of oxygen in pleural effusion and results in anaerobic metabolism with an overproduction of lactate. Anaerobic metabolism is energetically less efficient, resulting in decreased ATP production. This is reflected as decreased KEB values.
Figure 2.
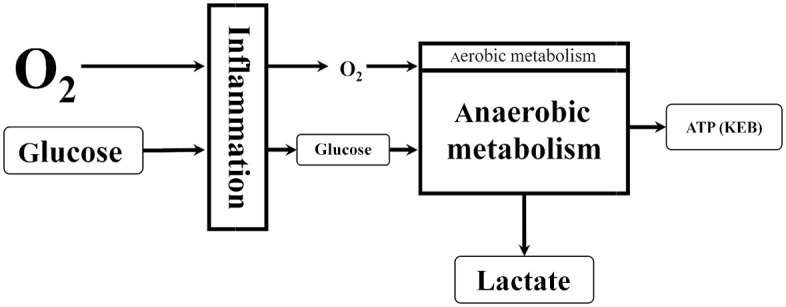
The second model of energy relationships in the pleural effusion–local inflammatory response.
ATP, adenosine triphosphate; KEB, the coefficient of energy balance [in Czech ‘Koeficient Energetické Bilance’]; O2, oxygen
Materials and methods
This retrospective study was approved by the local Ethics Committee of the Masaryk Hospital Usti nad Labem (reference number: 279/1). No informed consent was required for this study. The work did not involve any human experiments and did not require the collection of data out of common routine investigation. All patient records and information were anonymized and deidentified.
We have collected data from all investigated patients and their pleural effusions for a few years. After the finish of the diagnostic process patients were sorted in accordance to the type of pleural cavity impairment. In this study we focused only on causes with predominance of neutrophils in pleural effusions of patients with heart failure (33 samples), systemic sepsis (26 samples), after chest surgery without purulent complication (128), after chest surgery with purulent complication (69 samples), with bacterial pneumonia (96 samples) and chest empyema (283 samples). Every specimen of patients after chest surgery was collected during the first 9 days after operation. Patients with heart failure and systemic sepsis were assessed together in a subgroup with transudative pleural effusions.
The pleural effusion samples were collected via pleural cavity drainage in a test tube without anticoagulants and immediately transported to our laboratory. In all cases, the total number of elements in the pleural effusion was calculated under the optical microscope using a Fuchs-Rosenthal chamber and microscopic smear using cytocentrifuge method was prepared immediately after receiving the sample. Permanent cytological smears were stained using Hemacolor (Merck Co., Germany). Microscopic analyses were performed using Olympus BX40 microscope (Olympus, Japan) to determine cellular composition of pleural effusions.
Another aliquot of the sample was centrifuged and the molar concentrations of glucose and lactate and catalytic activities of lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) were determined. We measured the molar concentrations of glucose using the hexokinase method and molar concentrations of lactate using the lactate-oxidase and peroxidase method on a Cobas 6000 analyzer (Roche Diagnostics, Switzerland). The KEB values were calculated for all samples.
The catalytic activities concentrations of LDH and AST in pleural effusions were measured using the IFCC method on a Cobas 6000 analyzer (Roche Diagnostics, Switzerland).
The rest of the supernatant was transiently stored at +4°C to +8°C for potential future analysis.
Relative frequencies of neutrophils, KEB values and catalytic activities concentrations of LDH and AST in the pleural effusions are presented as a median and the first and the third quartile. Statistical analysis was performed using the ANOVA Kruskal-Wallis test (p < 0.05 was considered as significant) via STATISTICA 13.3 software (StatSoft Inc., USA) (Table 1).
Table 1.
Cytological-energy analysis of pleural effusions and concentrations of LDH and AST catalytic activities in pleural effusions.
| Median (1st–3rd quartile) |
Transudates n = 59 |
Post surgery without purulent
complication n = 128 |
Post surgery with purulent
complication n = 69 |
Exudates |
Complicated exudates |
|---|---|---|---|---|---|
| Purulent
pneumonia n = 96 |
Chest empyema n = 283 |
||||
| Neutrophils, % | A | B | C | C | C |
|
58.0
(49.5–73.5) |
80.5
(63.8–88.0) |
87.0
(81.0–93.0) |
84.5
(75.0–91.3) |
88.0
(78.0–94.0) |
|
| KEB | A | A | B | C | D |
|
32.7
(30.5–34.2) |
24.5
(18.6–28.1) |
−14.8
(−91.4–3.4) |
−169.6
(−1771.1 to −5.1) |
−2334.4
(−5701.8 to −50.8) |
|
| LDH, IU/L | A | B | C | C | D |
|
150.6
(110.1–290.7) |
822.6
(501.8–1734.6) |
1842.0
(1168.2–3109.0) |
1430.4
(869.2–3212.1) |
4170.9
(1495.8–13,980.0) |
|
| AST, IU/L | A | B | C | B | C |
|
15.6
(9.6–27.6) |
127.2
(65.9–259.2) |
208.2
(110.4–561.0) |
69.9
(46.0–142.6) |
236.4
(76.8–570.0) |
Groups sharing capital letters (A, B, C, D) are not significantly different as analysed by ANOVA Kruskal–Wallis test for multiple comparisons (family wise α = 0.05).
AST, aspartate aminotransferase; KEB, coefficient of energy balance; LDH, concentrations of lactate dehydrogenase catalytic activities in pleural effusions; n, number of patients
Light’s criteria are considered as the gold standard to differentiate transudates from exudates26,27 and have evolved over time. Therefore, their usage is not uniform. We compared specificities, sensitivities and diagnostic efficiency of some Light’s criteria parameters and KEB values in patients with typical transudates (heart failure and systemic sepsis), exudates (bacterial pneumonia) and complicated exudates (chest empyema) (Table 2).
Table 2.
Specificities, sensitivities and diagnostic efficiencies of some traditional Light’s criteria parameters and KEB values in selected groups of patients.
| Parameters | Transudates | Exudates |
Complicated exudates |
|
|---|---|---|---|---|
| Purulent pneumonia | Chest empyema | |||
| Glucose ⩾3.4 mmol/L |
Specificity (%) | 64.9 | ND | ND |
| Sensitivity (%) | 100.0 | ND | ND | |
| Dg. efficiency (%) | 80.6 | ND | ND | |
| Lactate ⩽5.0 mmol/L |
Specificity (%) | 82.3 | ND | ND |
| Sensitivity (%) | 91.5 | ND | ND | |
| Dg. efficiency (%) | 86.8 | ND | ND | |
| LDH ⩽1000.0 IU/L |
Specificity (%) | 71.2 | ND | ND |
| Sensitivity (%) | 98.3 | ND | ND | |
| Dg. efficiency (%) | 83.7 | ND | ND | |
| Total protein ⩽30.0 g/L |
Specificity (%) | 64.9 | ND | ND |
| Sensitivity (%) | 72.9 | ND | ND | |
| Dg. efficiency (%) | 68.8 | ND | ND | |
| Glucose <3.4 mmol/L |
Specificity (%) | ND | 45.1 | 65.6 |
| Sensitivity (%) | ND | 82.3 | 89.8 | |
| Dg. efficiency (%) | ND | 60.9 | 76.8 | |
| Lactate >5.0 mmol/L |
Specificity (%) | ND | 27.5 | 39.5 |
| Sensitivity (%) | ND | 91.7 | 94.0 | |
| Dg. efficiency (%) | ND | 50.2 | 60.9 | |
| LDH >1000.0 IU/L |
Specificity (%) | ND | 35.6 | 51.4 |
| Sensitivity (%) | ND | 65.6 | 84.5 | |
| Dg. efficiency (%) | ND | 48.4 | 65.9 | |
| Total protein >30.0 mg/L |
Specificity (%) | ND | 40.6 | 44.3 |
| Sensitivity (%) | ND | 70.8 | 68.0 | |
| Dg. efficiency (%) | ND | 53.6 | 54.9 | |
| KEB >20.0 | Specificity (%) | 83.2 | ND | ND |
| Sensitivity (%) | 96.6 | ND | ND | |
| Dg. efficiency (%) | 89.6 | ND | ND | |
| KEB <0.0 | Specificity (%) | ND | 43.2 | ND |
| Sensitivity (%) | ND | 80.2 | ND | |
| Dg. efficiency (%) | ND | 58.9 | ND | |
| KEB <−100.0 | Specificity (%) | ND | ND | 80.7 |
| Sensitivity (%) | ND | ND | 79.5 | |
| Dg. efficiency (%) | ND | ND | 80.1 | |
Diagnostic efficiency = .
Dg., diagnostic; KEB, coefficient of energy balance; LDH, concentrations of lactate dehydrogenase catalytic activities in pleural effusions; ND, not done
We accepted the following values of pleural effusion parameters for the determination of complicated exudates: the low concentrations of glucose <3.4 mmol/L,5,28–31 the high concentrations of lactate >5.0 mmol/L,32 the high value of lactate dehydrogenase catalytic activities >1000.0 IU/L4,26,31 and the high concentration of total protein >30.0 g/L26–28 (Table 2).
We plotted the receiver operator characteristic (ROC) curves for comparison of glucose concentrations, LDH catalytic activities and KEB values in pleural effusions of patients with purulent (positive group; n = 448) and non-purulent (negative group; n = 187) pleural effusions (MedCalc® v19.2.1, MedCalc Software Ltd, Belgium) (Figure 3; Table 3).
Figure 3.
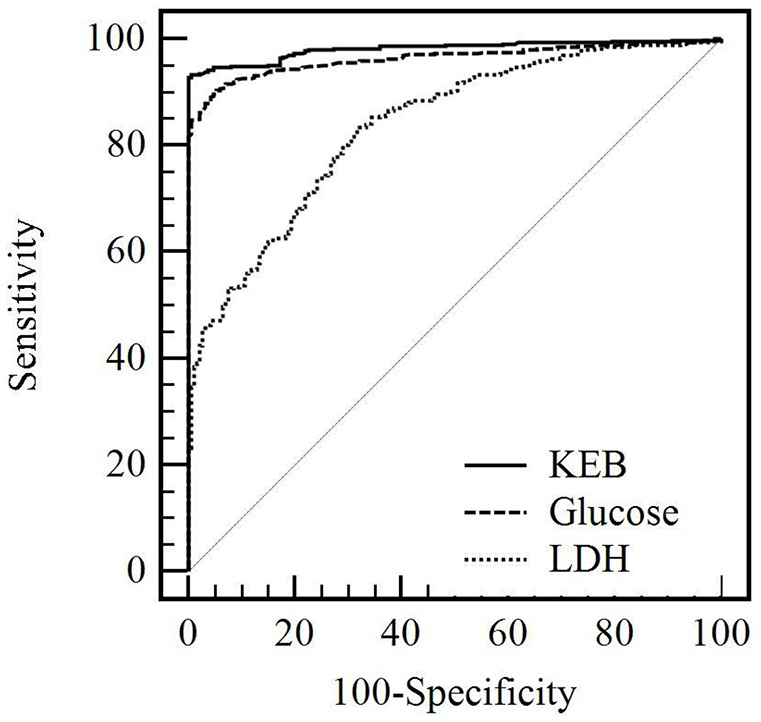
ROC curves for KEB value, glucose concentration and catalytic activity of LDH in the pleural effusions.
Glucose, glucose concentrations in the pleural effusions; KEB, coefficient of energy balance; LDH, lactate dehydrogenase catalytic activities in the pleural effusions; ROC, receiver operator characteristic
Table 3.
Comparison of the ROC curves for KEB values, glucose concentrations and LDH catalytic activities in purulent and non-purulent pleural effusions of our patients (p < 0.05 was considered as significant).
| Parameters | AUC | SE | 95% CI | Glucose |
LDH |
|---|---|---|---|---|---|
| p-values | |||||
| KEB | 0.981 | 0.005 | 0.968–0.990 | <0.001 | <0.001 |
| Glucose | 0.963 | 0.007 | 0.946–0.977 | ND | <0.001 |
| LDH | 0.837 | 0.016 | 0.806–0.865 | ||
AUC, area under ROC curve; 95% CI, 95% confidence interval (binomial exact); Glucose, glucose concentrations in the pleural effusions; KEB, coefficient of energy balance; LDH, lactate dehydrogenase catalytic activities in the pleural effusions; ND, not done; ROC, receiver operator characteristic; SE, standard error (method of De Long et al.33).
Results
We found the highest frequency of neutrophils in pleural effusions of patients with purulent inflammation in the presence of chest empyema, pneumonia and after chest surgery. Significantly lower frequency of neutrophils was found in pleural effusions of patients without purulent inflammation after chest surgery. The lowest frequency of neutrophils was observed in transudative pleural effusions of patients with heart failure and systemic sepsis (Table 1).
The lowest KEB values were found in pleural effusions of patients with chest empyema. Significantly higher KEB values were found in pleural effusions of patients with pneumonia. There was a further significant increase of KEB values in pleural effusions of patients after chest surgery with a purulent complication. The highest KEB values were found in the subgroups of patients without purulent inflammation after chest surgery and with transudative pleural effusions (Table 1).
The highest concentrations of LDH catalytic activities were determined in pleural effusions of patients with chest empyema. Significantly lower values were found in patients with pneumonia and in patients after chest surgery with a purulent complication. The significantly decreased catalytic activities of LDH in pleural effusions were found in patients after chest surgery without purulent complications. The lowest values of LDH catalytic activities were found in patients with transudative pleural effusions (Table 1).
We found the highest concentrations of AST catalytic activities in pleural effusions of patients with chest empyema and in patients who underwent chest surgery with the subsequent purulent complications. Significantly lower values of AST catalytic activities were found in pleural effusions of patients suffering with pneumonia and in patients after chest surgery without purulent complications. The lowest values of AST catalytic activities were observed in pleural effusions of patients with transudative pleural effusions (Table 1).
We found the high diagnostic efficiency of high glucose concentrations and low lactate and LDH catalytic activity concentrations in patients with transudative pleural effusions. The best resolution was reached when the KEB values were over 20.0. Low concentrations of total protein in pleural effusions also confer acceptable diagnostic efficiency in this subgroup of our patients (Table 2).
The low glucose concentrations and KEB values under 0.0 in pleural effusions of patients with purulent pneumonia give us similarly acceptable diagnostic efficiencies. Diagnostic efficiencies for high concentrations of lactate, total protein and LDH catalytic activity in pleural effusions of the same subgroup of patients were low (Table 2).
The best diagnostic efficiency for the KEB values under −100.0 and high diagnostic efficiency for low glucose concentrations in the pleural effusions of patients with chest empyema were determined. Furthermore, we found acceptable diagnostic efficiencies for high concentrations of lactate and LDH catalytic activity in pleural effusions of the same subgroup of patients. Diagnostic efficiency for high concentrations of total protein in pleural effusions is very low in these patients (Table 2).
We found the highest area under the ROC curve (AUC) for KEB values, lower AUC for glucose concentrations and the lowest AUC for LDH catalytic activities in the pleural effusions. There are highly significant differences between all AUC values (p < 0.001). Assessments of KEB values and glucose concentrations were classified as excellent diagnostic tools for detection of the purulent inflammation in the pleural cavity. Assessment of LDH catalytic activities was classified as a very good diagnostic tool for detection of the purulent inflammation in the pleural cavity34 (Figure 3; Table 3).
Discussion
We often found a predominance of neutrophils in the cytological analysis of pleural effusions from patients with different pathologies in the pleural cavity. The activation of neutrophils in this compartment represents a relevant type of local immune response. We observed the absence of or mild local inflammatory response in some patients. Contrarily, we found very intense inflammation with oxidative burst of neutrophils as the sign of purulent inflammation in other patients.8,22,35–38
Energy assessment of pleural effusions, contrary to their similar cellular composition, allows to distinguish between different immunity patterns in the pleural cavity. One aim of this study was to delineate between non-purulent immunity response and damaging purulent inflammation in the pleural cavity using the KEB values. The ROC analysis confirmed an excellent diagnostic ability of this parameter for detection of the purulent inflammation in the pleural cavity. We also found very good results for glucose concentrations in pleural effusions (Figure 3; Table 3). In comparison with glucose concentrations the KEB values allow better assessment of the local inflammation dynamics. The glucose concentration in pleural effusion is directly dependent on actual concentration in the patient plasma. For example, diabetic, alimentary or stress hyperglycaemia can mask the local glucose depletion in the pleural cavity during inflammation. Similarly, the systemic hypoglycaemia can lead to decrease of glucose concentrations in the pleural effusions without inflammation in the pleural cavity. The KEB eliminates these disadvantages successfully.22,24,25 In addition, its continuous progression allows reliable monitoring of efficacy of the therapy.
We compared the intensity of different local inflammatory response in the pleural cavity using the KEB values in transudative (from patients with heart failure and systemic sepsis), exudative (from patients with purulent pneumonia) and complicated exudative (from patients with chest empyema) pleural effusions with predominance of neutrophils values.4,22,24,27,36,39–42 Subsequently, we determined the degree of tissue injury in the pleural cavity using the LDH and AST catalytic activities in the transudative, exudative and complicated exudative pleural effusions (Table 1). We identified the considerable differences comparing transudative, exudative and complicated exudative pleural effusions. Exudates and complicated exudates were characterized by significantly lower KEB values and higher concentrations of LDH and AST catalytic activities when compared with transudates (Table 1). These results evidenced in patients with purulent inflammation higher intensity of anaerobic metabolism and tissue injury in the pleural cavity caused by oxidative burst of neutrophils which is usually induced by the invasion of extracellular bacteria.43,44 In addition, the significant difference in KEB values when we compared patients with chest empyema (complicated exudates) and purulent pneumonia (exudates) could delineate intensities of these different purulent inflammatory impairments’ pathologies.
Furthermore, our results confirmed the correlation between KEB values and concentrations of LDH and AST catalytic activities in pleural effusions (Table 1). KEB values were significantly lower and concentrations of LDH and AST catalytic activity were significantly higher in pleural effusions of patients with chest empyema (complicated exudates) compared with patients with purulent pneumonia (exudates). The increased tissue injury in relation to the increase in the intensity of destructive purulent inflammation is our explanation for this phenomenon.
The very specific aim of this study is to reveal the different immune response in the pleural cavity of patients after chest surgery without or with local purulent complications. The cytological-energy analysis of pleural effusions using KEB value determination is very good method to distinguish between these two pathologies. Our long-term experiences and recent studies have revealed as an optimal cut-off a KEB value of 10.0, to distinguish between ‘purulent’ and ‘non-purulent’ extravascular body fluids.22–25 The intensity of local immunity response in the pleural effusions of patients without purulent complications, represented by KEB values, is similar to those in patients with transudative pleural effusions. Concentrations of LDH and AST catalytic activities in pleural effusions of these patients compared with patients with purulent complications are significantly lower (Table 1). We explain this phenomenon as the absence of local destructive purulent inflammation in the pleural cavity. On the other hand, concentrations of LDH and AST catalytic activities in pleural effusions in these non-purulent cases after chest surgery compared with transudative effusions are significantly higher. This is the consequence of mechanical tissue destruction after surgical intervention in our opinion.
Conclusion
A cytological-energy analysis of pleural effusions with predominance of neutrophils is a useful method to differentiate between transudative effusions and purulent inflammatory response in the pleural cavity. In addition, a cytological-energy analysis using the KEB values enables to distinguish between relatively mild inflammatory response in purulent pneumonia and more intensive purulent inflammation in patients with chest empyema. Furthermore, we observed a direct relationship between the intensity of local immunity response represented by KEB values and the extent of tissue injury in the pleural cavity represented by LDH and AST catalytic activities in pleural effusions. While high KEB values in transudative effusions were associated with low LDH and AST catalytic activities, higher catalytic activities of these enzymes were typically linked to low KEB values in patients with purulent pneumonia. The highest catalytic activities of LDH and AST were found in patients with chest empyema, who were characterized by the lowest KEB values.
The specific is the application of cytological-energy analysis of pleural effusions of patients after chest surgery. Low KEB values (usually under 10.0) are associated with purulent complications in the pleural cavity. The increase in the LDH and AST catalytic activities is not exclusively caused by a local immunity response in the pleural cavity. The tissue destruction during surgical intervention also contributes to this increase.
Supplemental Material
Supplemental material, Author_Response_1 for Cytological-energy analysis of pleural effusions with predominance of neutrophils by Inka Matuchova, Petr Kelbich, Jan Kubalik, Eva Hanuljakova, Ivan Stanek, Vilem Maly, Ondrej Karpjuk and Jan Krejsek in Therapeutic Advances in Respiratory Disease
Supplemental material, Author_Response_2 for Cytological-energy analysis of pleural effusions with predominance of neutrophils by Inka Matuchova, Petr Kelbich, Jan Kubalik, Eva Hanuljakova, Ivan Stanek, Vilem Maly, Ondrej Karpjuk and Jan Krejsek in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Cytological-energy analysis of pleural effusions with predominance of neutrophils by Inka Matuchova, Petr Kelbich, Jan Kubalik, Eva Hanuljakova, Ivan Stanek, Vilem Maly, Ondrej Karpjuk and Jan Krejsek in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.2 for Cytological-energy analysis of pleural effusions with predominance of neutrophils by Inka Matuchova, Petr Kelbich, Jan Kubalik, Eva Hanuljakova, Ivan Stanek, Vilem Maly, Ondrej Karpjuk and Jan Krejsek in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.3 for Cytological-energy analysis of pleural effusions with predominance of neutrophils by Inka Matuchova, Petr Kelbich, Jan Kubalik, Eva Hanuljakova, Ivan Stanek, Vilem Maly, Ondrej Karpjuk and Jan Krejsek in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Cytological-energy analysis of pleural effusions with predominance of neutrophils by Inka Matuchova, Petr Kelbich, Jan Kubalik, Eva Hanuljakova, Ivan Stanek, Vilem Maly, Ondrej Karpjuk and Jan Krejsek in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.2 for Cytological-energy analysis of pleural effusions with predominance of neutrophils by Inka Matuchova, Petr Kelbich, Jan Kubalik, Eva Hanuljakova, Ivan Stanek, Vilem Maly, Ondrej Karpjuk and Jan Krejsek in Therapeutic Advances in Respiratory Disease
Acknowledgments
We thank Ms Kvetoslava Sykorova for preparation of the patient database and Mr Gregory Jeffrey Evans for critical proofreading of the manuscript.
Footnotes
Author contribution(s): Inka Matuchova: Conceptualization; Formal analysis; Investigation; Methodology; Software; Validation; Visualization; Writing-original draft.
Petr Kelbich: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Validation; Visualization; Writing-original draft; Writing-review & editing.
Jan Kubalik: Conceptualization; Investigation; Resources; Visualization; Writing-original draft.
Eva Hanuljakova: Conceptualization; Data Curation; Investigation; Resources; Software; Writing-original draft.
Ivan Stanek: Conceptualization; Formal analysis; Investigation; Resources; Validation; Writing-review & editing.
Vilem Maly: Conceptualization; Formal analysis; Investigation; Resources; Validation; Writing-review & editing.
Ondrej Karpjuk: Conceptualization; Investigation; Resources; Writing-review & editing.
Jan Krejsek: Conceptualization; Formal analysis; Supervision; Validation; Writing-review & editing.
Conflict of interest: The authors declare that there is no conflict of interest.
All relevant data are within the paper.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by the Internal Grant of the Krajska zdravotni, a.s. in Usti nad Labem, Czech Republic ‘IGA-KZ-2017-1-5’ and by Charles University in Prague, Faculty of Medicine in Hradec Kralove, Czech Republic, project ‘PROGRES Q40/10’.
ORCID iD: Petr Kelbich  https://orcid.org/0000-0002-9261-6827
https://orcid.org/0000-0002-9261-6827
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Inka Matuchova, Biomedical Centre, Masaryk Hospital in Usti nad Labem, Usti nad Labem, Czech Republic; Faculty of Medicine and University Hospital in Hradec Kralove, Department of Clinical Immunology and Allergology, Charles University in Prague, Hradec Kralove, Czech Republic; Laboratory for Cerebrospinal Fluid, Neuroimmunology, Pathology and Special Diagnostics Topelex, Prague, Czech Republic.
Petr Kelbich, Biomedical Centre, Masaryk Hospital in Usti nad Labem, Socialni pece 3316/12A, 401 13, Usti nad Labem, Czech Republic; Faculty of Medicine and University Hospital in Hradec Kralove, Department of Clinical Immunology and Allergology, Charles University in Prague, Hradec Kralove, Czech Republic; Laboratory for Cerebrospinal Fluid, Neuroimmunology, Pathology and Special Diagnostics Topelex, Prague, Czech Republic.
Jan Kubalik, Faculty of Medicine and University Hospital in Hradec Kralove, Department of Clinical Immunology and Allergology, Charles University in Prague, Hradec Kralove, Czech Republic; Department of Thoracic Surgery, Masaryk Hospital in Usti nad Labem, Usti nad Labem, Czech Republic.
Eva Hanuljakova, Biomedical Centre, Masaryk Hospital in Usti nad Labem, Usti nad Labem, Czech Republic; Laboratory for Cerebrospinal Fluid, Neuroimmunology, Pathology and Special Diagnostics Topelex, Prague, Czech Republic.
Ivan Stanek, Department of Thoracic Surgery, Masaryk Hospital in Usti nad Labem, Usti nad Labem, Czech Republic.
Vilem Maly, Department of Thoracic Surgery, Masaryk Hospital in Usti nad Labem, Usti nad Labem, Czech Republic.
Ondrej Karpjuk, Department of Thoracic Surgery, Masaryk Hospital in Usti nad Labem, Usti nad Labem, Czech Republic.
Jan Krejsek, Faculty of Medicine and University Hospital in Hradec Kralove, Department of Clinical Immunology and Allergology, Charles University in Prague, Hradec Kralove, Czech Republic.
References
- 1. Batra H, Antony VB. Pleural mesothelial cells in pleural and lung diseases. J Thorac Dis 2015; 7: 964–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Charalampidis C, Youroukou A, Lazaridis G, et al. Physiology of the pleural space. J Thorac Dis 2015; 7(Suppl. 1): S33–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chinchkar NJ, Talwar D, Jain SK. A stepwise approach to the etiologic diagnosis of pleural effusion in respiratory intensive care unit and short-term evaluation of treatment. Lung India 2015; 32: 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karkhanis VS, Joshi JM. Pleural effusion: diagnosis, treatment, and management. Open Access Emerg Med 2012; 4: 31–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Light RW. Pleural effusion. N Engl J Med 2002; 346: 1971–1977. [DOI] [PubMed] [Google Scholar]
- 6. Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Front Physiol 2018; 9: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Teng TS, Ji AL, Ji XY, et al. Neutrophils and immunity: from bactericidal action to being conquered. J Immunol Res 2017; 2017: 9671604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosales C, Demaurex N, Lowell CA, et al. Neutrophils: their role in innate and adaptive immunity. J Immunol Res 2016; 2016: 1469780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Filias A, Theodorou GL, Mouzopoulou S, et al. Phagocytic ability of neutrophils and monocytes in neonates. BMC Pediatr 2011; 11: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Turvey SE, Broide DH. Innate immunity. J Allergy Clin Immunol 2010; 125(2 Suppl. 2): S24–S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Segal AW. How neutrophils kill microbes. Annu Rev Immunol 2005; 23: 197–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee WL, Harrison RE, Grinstein S. Phagocytosis by neutrophils. Microbes Infect 2003; 5: 1299–1306. [DOI] [PubMed] [Google Scholar]
- 13. Sattar SBA, Sharma S. Bacterial pneumonia, https://www.ncbi.nlm.nih.gov/books/NBK513321 (2019, accessed 20 December 2019).
- 14. Chen L, Deng H, Cui H, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017; 9: 7204–7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nguyen GT, Green ER, Mecsas J. Neutrophils to the ROScue: mechanisms of NADPH oxidase activation and bacterial resistance. Front Cell Infect Microbiol 2017; 7: 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kruger P, Saffarzadeh M, Weber ANR, et al. Neutrophils: between host defence, immune modulation, and tissue injury. PLoS Pathog 2015; 11: e1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahmed O, Zangan S. Emergent management of empyema. Semin Intervent Radiol 2012; 29: 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosenstengel A. Pleural infection-current diagnosis and management. J Thorac Dis 2012; 4: 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006; 3: 75–80. [DOI] [PubMed] [Google Scholar]
- 20. Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 2006; 6: 173–182. [DOI] [PubMed] [Google Scholar]
- 21. Dahlgren C, Karlsson A. Respiratory burst in human neutrophils. J Immunol Methods 1999; 232: 3–14. [DOI] [PubMed] [Google Scholar]
- 22. Kelbich P, Malý V, Matuchová I, et al. Cytological-energy analysis of pleural effusions. Ann Clin Biochem 2019; 56: 630–637. [DOI] [PubMed] [Google Scholar]
- 23. Kelbich P, Hejčl A, Staněk I, et al. Principles of the cytological-energy analysis of the extravascular body fluids. Biochem Mol Biol J 2017; 3: 1–3. [Google Scholar]
- 24. Kelbich P, Hejčl A, Selke Krulichová I, et al. Coefficient of energy balance, a new parameter for basic investigation of the cerebrospinal fluid. Clin Chem Lab Med 2014; 52: 1009–1017. [DOI] [PubMed] [Google Scholar]
- 25. Kelbich P, Slavík S, Jasanská J, et al. Evaluations of the energy relations in the CSF compartment by investigation of selected parameters of the glucose metabolism in the CSF. Klin Biochem Metab 1998; 6: 213–225. [Google Scholar]
- 26. Na MJ. Diagnostic tools of pleural effusion. Tuberc Respir Dis 2014; 76: 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Light RW, MacGregor MI, Luchsinger PC, et al. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77: 507–513. [DOI] [PubMed] [Google Scholar]
- 28. Hooper C, Lee YCG, Maskell N. Investigation of a unilateral pleural effusion in adults: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65(Suppl. 2): ii4–ii17. [DOI] [PubMed] [Google Scholar]
- 29. Porcel JM, Light RW. Diagnostic approach to pleural effusion in adults. Am Fam Physician 2006; 73: 1211–1220. [PubMed] [Google Scholar]
- 30. Dixit R, Agarwal KC, Gokhroo A, et al. Diagnosis and management options in malignant pleural effusions. Lung India 2017; 34: 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Villena GV, Cases VE, Fernández VA, et al. Recommendations of diagnosis and treatment of pleural effusion: update. Arch Bronchoneumol 2014; 50: 235–249. [DOI] [PubMed] [Google Scholar]
- 32. Brook I. Measurement of lactic acid in pleural fluid. Respiration 1980; 40: 344–348. [DOI] [PubMed] [Google Scholar]
- 33. De Long ER, De Long DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–845. [PubMed] [Google Scholar]
- 34. Šimundić AM. Measures of diagnostic accuracy: basic definitions. EJIFCC 2009; 19: 203–211. [PMC free article] [PubMed] [Google Scholar]
- 35. Yang W, Zhang B, Zhang ZM. Infectious pleural effusion status and treatment progress. J Thorac Dis 2017; 9: 4690–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCauley L, Dean N. Pneumonia and empyema: causal, casual or unknown. J Thorac Dis 2015; 7: 992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol 2014; 9: 181–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miller EJ, Idell S. Interleukin-8: an important neutrophil chemotaxin in some cases of exudative pleural effusions. Exp Lung Res 1993; 19: 589–601. [DOI] [PubMed] [Google Scholar]
- 39. Gómez H, Kellum JA. Sepsis-induced acute kidney injury. Curr Opin Crit Care 2016; 22: 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ekpe EE, Essien IO, Idongesit U. Significant pleural effusion in congestive heart failure necessitating pleural drainage. Nig J Cardiol 2015; 12: 106–110. [Google Scholar]
- 41. Ahmed AE, Yacoub TE. Empyema thoracis. Clin Med Insights Circ Respir Pulm Med 2010; 4: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hoste EAJ, De Corte W. Clinical consequences of acute kidney injury. Contrib Nephrol 2011; 174: 56–64. [DOI] [PubMed] [Google Scholar]
- 43. Klebanoff SJ, Kettle AJ, Rosen H, et al. Myeloperoxidase: a front-line defender against phagocytosed microorganisms. J Leukoc Biol 2013; 93: 185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen Y, Junger WG. Measurement of oxidative burst in neutrophils. Methods Mol Biol 2012; 844: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response_1 for Cytological-energy analysis of pleural effusions with predominance of neutrophils by Inka Matuchova, Petr Kelbich, Jan Kubalik, Eva Hanuljakova, Ivan Stanek, Vilem Maly, Ondrej Karpjuk and Jan Krejsek in Therapeutic Advances in Respiratory Disease
Supplemental material, Author_Response_2 for Cytological-energy analysis of pleural effusions with predominance of neutrophils by Inka Matuchova, Petr Kelbich, Jan Kubalik, Eva Hanuljakova, Ivan Stanek, Vilem Maly, Ondrej Karpjuk and Jan Krejsek in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Cytological-energy analysis of pleural effusions with predominance of neutrophils by Inka Matuchova, Petr Kelbich, Jan Kubalik, Eva Hanuljakova, Ivan Stanek, Vilem Maly, Ondrej Karpjuk and Jan Krejsek in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.2 for Cytological-energy analysis of pleural effusions with predominance of neutrophils by Inka Matuchova, Petr Kelbich, Jan Kubalik, Eva Hanuljakova, Ivan Stanek, Vilem Maly, Ondrej Karpjuk and Jan Krejsek in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.3 for Cytological-energy analysis of pleural effusions with predominance of neutrophils by Inka Matuchova, Petr Kelbich, Jan Kubalik, Eva Hanuljakova, Ivan Stanek, Vilem Maly, Ondrej Karpjuk and Jan Krejsek in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Cytological-energy analysis of pleural effusions with predominance of neutrophils by Inka Matuchova, Petr Kelbich, Jan Kubalik, Eva Hanuljakova, Ivan Stanek, Vilem Maly, Ondrej Karpjuk and Jan Krejsek in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.2 for Cytological-energy analysis of pleural effusions with predominance of neutrophils by Inka Matuchova, Petr Kelbich, Jan Kubalik, Eva Hanuljakova, Ivan Stanek, Vilem Maly, Ondrej Karpjuk and Jan Krejsek in Therapeutic Advances in Respiratory Disease


