Abstract
Nocardia brasiliensis is the most common cause of cutaneous nocardiosis. Nocardia pseudobrasiliensis is an emerging species responsible for invasive and disseminated disease in immunocompromised patients. We describe a case of a 67-year-old immunocompetent patient without significant past medical history diagnosed with primary cutaneous nocardiosis with N pseudobrasiliensis as the causative organism. In our opinion, we report the first case of primary cutaneous nocardiosis in an immunocompetent patient with N pseudobrasiliensis being the causative agent.
Keywords: primary cutaneous nocardiosis, Nocardia pseudobrasiliensis, immunocompetent
Introduction
In human infected with bacterial genus Nocardia there are more than 30 clinically relevant species that have been isolated.1 Nocardia asteroides is the common cause of opportunistic infections in immunocompromised patients followed closely by N brasiliensis, which is the most common cause of cutaneous nocardiosis. N pseudobrasiliensis is an emerging species of Nocardia that was first identified in 1996.2 Thus far, there have been 7 cases of N pseudobrasiliensis causing invasive and disseminated disease in immunocompromised patients.3 We present a case of an immunocompetent 67-year-old female who presented with an ulceration of her right middle finger post rose thorn-related injury, diagnosed with N pseudobrasiliensis.
Case Presentation
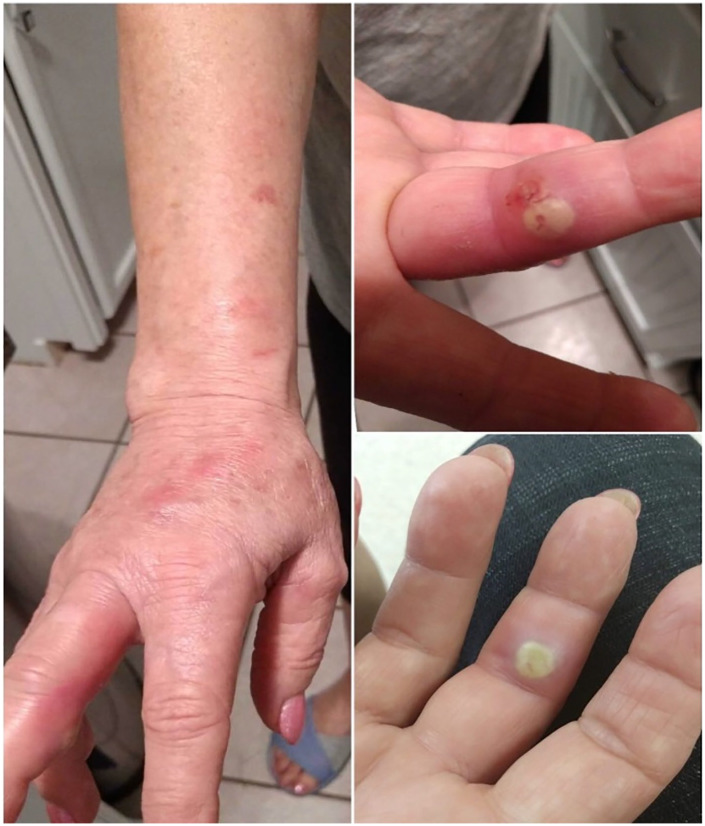
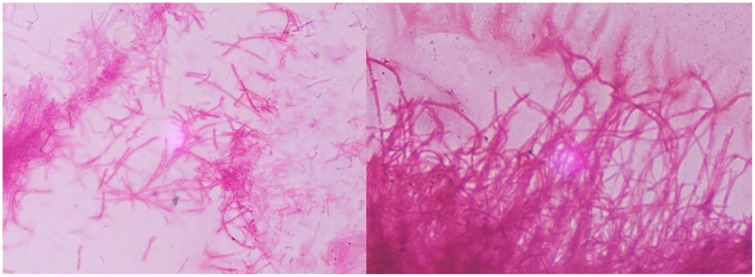
A 67-year-old female presented with an ulceration on the right middle finger post rose thorn-related injury to the site. Patient reported a papular lesion on her right middle finger (Figure 1), which was progressively enlarging with swelling, and pain, ultimately ulcerating within a week of onset. In the emergency department, surgical drainage was carried out, and purulent samples were sent for culture. As patient was afebrile with no systemic symptoms, she was discharged home on oral cephalexin and fluconazole with outpatient follow-up. Few days later, the patient noticed a second ulceration on the right middle finger, with painful swelling of the right axillary region. During the second visit to the emergency department, 2 tender ulcers on the middle phalanx of right middle finger with tender nodular lymphangitis tract leading up her forearm were found on the patient (Figure 1) with right axillary lymphadenopathy. The patient was initially started on itraconazole for suspected diagnosis of sporotrichosis. However, wound culture revealed growth of filamentous acid fast organisms (Figure 2), and medications were changed to intravenous trimethoprim-sulfamethoxazole followed by oral formulation. Bacterial cultures were sent to a reference laboratory at LabCorp Burlington, North Carolina. Eventually species identification was done by matrix-assisted laser desorption ionization-time of flight mass spectroscopy and polymerase chain reaction sequencing, which showed N pseudobrasiliensis. Susceptibility testing was not performed. In subsequent outpatient visits the patient showed significant improvement on trimethoprim-sulfamethoxazole. Thus, antibiotics were continued for an additional 3 months after improvement in skin findings.
Figure 1.
Right middle finger papule, with lymphangitis tracking up patient’s forearm.
Figure 2.
Positive acid fast filaments of Nocardia pseudobrasiliensis.
Discussion
According to the Centers for Disease Control and Prevention, 500 to 1000 cases of nocardiosis occur in the United States every year, and are grouped into systemic versus cutaneous group. Approximately 60% of the cases are seen in immunocompromised patients.4 Systemic nocardiosis is most often seen in immunocompromised patients following inhalation of N asteroides and progression to pulmonary infection.5 Out of all cases of nocardiosis, 7.8% to 10% of cases are cutaneous in nature, but exact prevalence of primary cutaneous nocardiosis is unknown. Traumatic inoculation of skin progresses to cellulitis and/or lymphangitis in immunocompetent patients.6,7 N brasiliensisis is the causative agent in almost 80% of the cases of cutaneous nocardiosis.6 In 1996, Ruimy et al2 confirmed that N pseudobrasiliensis, which was thought to belong to N brasiliensis phenotypically, is actually genotypically a new taxon constituting a new species of Nocardia. In 2015, Kandasamy et al3 reported a total of 7 published case reports on N pseudobrasiliensis to date. Among all those 7 cases, all except 2 cases had predisposing condition causing immunosuppression and none was reported to cause cutaneous infection. To the best of our knowledge, this is the first reported case of primary cutaneous infection caused by N pseudobrasiliensis in an immunocompetent patient.
Manifestation of primary cutaneous nocardiosis can be varied. Mostly seen clinical presentation include localized superficial, lymphocutaneous, mycetoma-like, or cutaneous involvement in disseminated infection.7 Similar to our patient’s presentation, lymphocutaneous nocardiosis usually starts from the site of inoculation and spreads proximally along lymphatic vessels giving rise to inflammatory nodules like picture.1 Clinical presentation of lymphocutaneous nocardiosis is clinically similar to sporotrichosis, staphylococcal, or streptococcal soft tissue infections, as well as ulceroglandular tularemia.8 Coccidioides immitis along with Histoplasma capsulatum, Cryptococcus neoformans, Pseudoallescheria boydii, Blastomyces dermatitidis, and viruses like cowpox or herpes simplex are few of the less common causes of lymphangitis.9
Diagnosing nocardiosis is challenging as clinical presentation is similar to other diseases mentioned above. Commercial availability as well as reliability of serological test is not good.7 Therefore, gram stain and culture is used for the diagnosis of Nocardia.7 Nocardia species are gram-positive, rod-shaped bacteria, which are partially acid-fast beaded branching filaments that grow under aerobic conditions.10 N brasiliensis and N pseudobrasiliensis can be distinguished from the other species by adenine hydrolysis and nitrate reductases, but these tests are not commonly available in most microbiology laboratories. Therefore, matrix-assisted laser desorption ionization-time of flight mass spectroscopy and gene sequence analysis are used for species identification as was seen in our case.11 N pseudobrasiliensis is thought to be associated with more invasive and disseminated disease, as well as less favorable antibiotic susceptibility patterns.2
Due to lack of prospective controlled trials, optimal treatment for nocardiosis has been debated. Appropriate surgical drainage followed by susceptible antibiotics is the preferred treatment for cutaneous nocardiosis. Various factors like infection site, disease extension, and host factors influence clinical outcome and duration of the therapy.9 Trimethoprim-sulfamethoxazole is the first-line antibiotic therapy for nocardiosis. Immunocompetent patient with cutaneous nocardiosis should be ideally treated with trimethoprim-sulfamethoxazole for 2 to 4 months, which may need to be extended further for some immunocompromised patients to prevent recurrence.10
Conclusion
This is the first case of culture proven primary cutaneous infection due to N pseudobrasiliensis. Primary cutaneous nocardiosis is a rare infection, but presents similarly to other bacterial skin infections. Due to the potential for this infection to rapidly disseminate early recognition and prompt treatment ensures better prognosis.
Acknowledgments
The authors gratefully acknowledge and thank our patient for the kind support.
Footnotes
Author Contribution: Shraddhadevi Makadia and Ishan Patel wrote the original draft of the article. Shraddhadevi Makadia participated in gathering the data for the case. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received financial support for the research, authorship, and/or publication of this article: This research was supported (in whole or in part) by HCA Healthcare and/or HCA Healthcare affiliated entity. The views expressed in this publication represent those of author(s) and do not necessarily the official views if HCA Healthcare or any of its affiliated entities.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Verbal informed consent was obtained from the patient for their anonymized information to be published in this article
References
- 1. Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ., Jr. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruimy R, Riegel P, Carlotti A, et al. Nocardia pseudobrasiliensis sp nov, a new species of Nocardia which groups bacterial strains previously identified as Nocardia brasiliensis and associated with invasive diseases. Int J Syst Bacteriol. 1996;46:259-264. [DOI] [PubMed] [Google Scholar]
- 3. Kandasamy VV, Nagabandi A, Horowitz EA, Vivekanandan R. Multidrug-resistant Nocardia pseudobrasiliensis presenting as multiple muscle abscesses. BMJ Case Rep. 2015;2015:bcr2014205262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers of Disease Control and Prevention. Risk of infection. Accessed June 12, 2019 https://www.cdc.gov/nocardiosis/infection/index.html
- 5. Saubolle MA, Sussland D. Nocardiosis: review of clinical and laboratory experience. J Clin Microbiol. 2003;41:4497-4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fukuda H, Saotome A, Usami N, Urushuibata O, Mukai H. Lymphocutaneous type of nocardiosis caused by Nocardia brasiliensis: a case report and review of primary cutaneous nocardiosis caused by N brasiliensis reported in Japan. J Dermatol. 2008;35:346-353. [DOI] [PubMed] [Google Scholar]
- 7. Maraki S, Chochlidakis S, Nioti E, Tselentis Y. Primary lymphocutaneous nocardiosis in a immunocompetent patient. Ann Clin Microbiol Antimicrob. 2004;3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smego RA, Jr, Castiglia M, Asperilla MO. Lymphocutaneous syndrome. A review of non-sporothrix causes. Medicine (Baltimore). 1999;78:38-63. [DOI] [PubMed] [Google Scholar]
- 9. Palmieri JR, Santo A, Johnson SE. Soil-acquired cutaneous nocardiosis on the forearm of a healthy male contracted in a swamp in rural eastern Virginia. Int Med Case Rep J. 2014;7:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Secchin P, Trope BM, Fernandes LA, Barreiros G, Ramos-E-Silva M. Cutaneous nocardiosis simulating cutaneous lymphatic sporotrichosis. Case Rep Dermatol. 2017;9:119-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Conville PS, Brown-Elliot BA, Smith T, Zelazny AM. The complexities of Nocardia taxonomy and identification. J Clin Microbiol. 2018;56:e01419-17. doi: 10.1128/JCM.01419-17 [DOI] [PMC free article] [PubMed] [Google Scholar]