Abstract
Epidermal growth factor receptor (EGFR) gene-mutated non-small cell lung cancer may initially respond to EGFR tyrosine kinase inhibitors (TKIs), but may subsequently become resistant; however, the resistance mechanisms remain unclear. We report a rare case of acquired resistance to osimertinib associated with transformation to small cell lung cancer (SCLC) with cis-C797S mutation. A man with recurrent lung adenocarcinoma harboring an EGFR exon 19 deletion received erlotinib for 10 months following curative surgery and adjuvant chemotherapy. However, he switched to osimertinib after repeat biopsy showed EGFR exon 19 deletion and T790M mutation leading to erlotinib resistance. His disease progressed after 15 months and repeat biopsy showed SCLC. Next-generation sequencing of peripheral blood detected EGFR exon 19 deletion, T790M mutation, cis-C797S mutation, and RB1 inactivation. The tumor was reduced after four cycles of etoposide and cisplatin and his respiratory symptoms improved. However, computed tomography after six cycles of chemotherapy showed multiple bilateral lung lesions, and single-photon emission computed tomography showed bone metastasis. The patient received paclitaxel plus cisplatin for two cycles with partial response. Because heterogeneous genetic and phenotypic mechanisms of TKI-resistance may occur at different times and locations, histopathological and molecular testing both provide evidence to support appropriate treatment.
Keywords: Osimertinib, acquired resistance, histological transformation, C797S, epidermal growth factor receptor tyrosine kinase inhibitor, lung adenocarcinoma, small cell lung carcinoma
Introduction
Lung cancer remains the most common cancer worldwide in terms of both new cases and deaths, related to its high mortality rate.1 Non-small cell lung cancer (NSCLC) comprises 80% to 85% of all lung cancers, and most patients present with locally advanced or metastatic disease at the time of diagnosis.2 With the development of targeted therapy, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have become the first-line treatment for patients with advanced EGFR-mutated NSCLC. However, many patients acquire resistance to EGFR-TKIs within 1 year. T790M mutations account for approximately 60% of these resistant cases.3 Although third-generation TKIs, such as osimertinib, are initially effective against T790M-mutated NSCLC, with an overall response rate of approximately 60%, acquired resistance occurs within approximately 10 months.4 Understanding the mechanisms of resistance to osimertinib thus presents a new challenge.
In this study, we report a patient with pulmonary adenocarcinoma (ADC) who first acquired resistance to a first-generation EGFR-TKIs via a T790M mutation, and subsequently acquired resistance to osimertinib by transformation to small cell lung cancer (SCLC) and cis-C797S mutation. He ultimately had an EGFR exon 19 deletion, T790M mutation, cis-C797S mutation, and inactivation of RB1.
Case report A 52- year -old male nonsmoker visited our hospital because of an abnormal chest X-ray finding during routine screening. Chest computed tomography (CT) showed a mass in the right lower lung lobe. He underwent right lower lobectomy with regional lymph node dissection on December 11, 2014. The pathological diagnosis was invasive ADC with metastasis to lymph nodes in group 7 (1/1), group 9 (0/1), group 10 (0/5), and group 11 (0/1) on December 17, 2014 (Figure 1). Carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), and progastrin-releasing peptide (ProGRP) were monitored after surgery on January 6, 2015 (Table 1). EGFR analysis revealed exon 19 deletion mutation. We carried out immunohistochemical examination of the lobectomy specimen and found that RB1 and TP53 were inactivated. The patient was diagnosed with T1bN2M0, stage IIIA, and received four cycles of chemotherapy combined with antiangiogenic therapy, consisting of vinorelbine, cisplatin, and endostar. Ten months later, after completing the 4th cycle, a CT scan revealed a lesion on the bronchial stump and new mediastinal lymph node metastases. CEA, NSE and ProGRP was monitored on January 12, 2016 (Table 1). The patient started treatment with erlotinib 150 mg orally once daily on January 18, 2016 and achieved a partial response within 1 month. Regular CT examinations were conducted every 2 months. However, after 1 year of erlotinib treatment, the primary lung tumor had regrown and the patient had symptoms of cough and shortness of breath. Transbronchial biopsy of a pulmonary metastatic nodule was performed to determine the pathological type and EGFR mutation status. Histopathologic examination of the biopsy sample indicated high-grade ADC on February 17, 2017 (Figure 2). We also carried out immunohistochemical examination of the metastatic nodule and found that RB1 and TP53 were inactivated. DNA extracted from the lung biopsy sample (see below) showed EGFR T790M and exon 19 deletion. The patient was prescribed osimertinib, and his cancer remained well-controlled for 15 months.
However, a CT scan then showed disease progression, and histologic analysis of a repeat transbronchial lung biopsy showed SCLC on June 23, 2018 (Figure 3). CEA, NSE and ProGRP was monitored on June 28, 2018 (Table 1). Next-generation sequencing (NGS) of peripheral blood revealed EGFR exon 19 deletion, T790M mutation, cis-C797S mutation, and a loss of RB1. Based on the transformation from NSCLC to SCLC, the patient was treated with six cycles of etoposide and cisplatin therapy. CT examination after the second and fourth cycles showed reduced pulmonary lesions, but no partial response. The patient’s disease progressed quickly after the sixth cycle, and he was administered two cycles of paclitaxel plus cisplatin chemotherapy. CT examination then showed partial response. The patient received further treatment and was followed-up.
Peripheral blood lymphocytes and plasma were separated by centrifugation at 1600 × g for 10 minutes. The supernatant was then transferred to a new 2-mL centrifuge tube and centrifuged at 16,000 × g for 10 minutes. Plasma cell-free DNA was extracted using a MagMAXTM Cell-Free DNA isolation kit (Life Technologies, CA, USA) according to the manufacturer’s instructions, and DNA was extracted from peripheral blood lymphocytes using a Whole Blood DNA kit (Tiangen) according to the manufacturer’s instructions. The DNA concentration was measured using a Qubit dsDNA HS Assay kit or Qubit dsDNA BR Assay kit (Life Technologies) according to the manufacturer’s recommended protocol.
Genomic DNA was sheared into 150 to 200 base pair fragments using a Covaris M220 Focused Ultrasonicator (Covaris, MA, USA) and fragmented DNA libraries were constructed with a KAPA HTP Library Preparation Kit (Illumina Platform) (KAPA Biosystems, MA, USA) according the manufacturer’s instruction. DNA libraries were captured with a designed 543-gene panel, NimbleGen SeqCap EZ Library (Roche, WI, USA), which includes major tumor-related genes. The captured samples were then subjected to a NovaSeq 6000 System for paired-end sequencing.
The generated sequencing reads were subjected to adapter trimming and low-quality bases were filtered out using Trimmomatic. Obtained reads were then aligned to the HG19 human genome reference using BWA aligner v 0.7.12, and duplicate reads were removed from the aligned and sorted BAM files using novesort 3.08.00. Local realignment around potential small indels and base recalibration for next-step mutation calling procedures were carried out using GATK v3.7. Single nucleotide variations and small indels were detected using verdict v1.5.1, and complex mutations were detected using FreeBayes v1.1.0-44. Paired tumor–normal sample calling was processed during the mutation calling procedure to filter out personal germline mutations. DNA translocation analysis was performed using FusionMap v8.0.2.32.
Discussion
Resistance to osimertinib is induced by EGFR-dependent resistance mechanisms (such as EGFR T790M mutation, EGFR amplification, MET gene amplification, or PIK3CA mutation) and EGFR-independent resistance mechanisms (such as alternative kinase activation, histological transformation, and phenotypic changes).5,6 Histologic transformation to SCLC occurs in 3% to 14% of patients with EGFR-mutant NSCLC associated with acquired resistance to first- or second-generation EGFR-TKIs.7 However, transformation to SCLC after treatment with a third-generation EGFR-TKI, such as osimertinib, is rare, with only nine previously reported cases.8–11 It may be difficult to determine if a SCLC arose by transformation from NSCLC, rather than being a new tumor or being present simultaneously with the NSCLC from the start. In the current case, the disease progressed after 15 months of osimertinib treatment and a repeat tissue biopsy showed SCLC. SCLC grows quickly and is not controlled by EGFR-TKIs. If the tumor had been a mixed histological type initially, it would therefore have been expected to recur in a short time; however, this patient initially responded well to EGFR-TKIs for approximately 2.5 years, suggesting that the SCLC was not part of the initial presentation. The origin of the SCLC by transformation from NSCLC, rather than as a new tumor, was also supported by the tumor harboring the original activating EGFR mutations after acquiring resistance to osimertinib. Furthermore, CEA, NSE, and ProGRP were all monitored routinely throughout the patient’s treatment course (Table 1). After transformation to SCLC, ProGRP and NSE levels remained normal but CEA levels were elevated, as a typical tumor marker of ADC. RB1 was also inactivated after transformation. Some researchers believe that inactivation of RB1 and TP53 play critical roles in the histologic transformation of EGFR mutant NSCLC into SCLC.12–15 We carried out immunohistochemical examination of the initial lobectomy specimen and subsequent pulmonary metastatic nodule (resistance to erlotinib), and confirmed that RB1 and TP53 were inactivated in both these specimens. The reason for the TP53 mutation apparently disappearing after histological transformation is unclear; however, NGS to detect mutation status was conducted using peripheral blood because of insufficient tissue, which might have given less-accurate results. All the above results suggest that the SCLC in the current patient was homologous to the original ADC, which had transformed from an NSCLC. The evaluation of RB1 and TP53 status in patients with EGFR-TKI-treated ADC can help to predict SCLC transformation. A similar situation has been reported in the transformation of ADC to squamous cell carcinoma, and Park et al. found that at least one gene mutation involving the PIK3CA pathway was already present before squamous cell carcinoma transformation.16 It is therefore necessary to be aware of genetic mutations that might predict histological transformation at an early stage, as well as emphasizing the importance of re-biopsy in light of tumor heterogeneity and pathological transformation.
Table 1.
Typical tumor markers during treatment.
| Tumor marker | After surgery | Before erlotinib | After transformation to SCLC |
|---|---|---|---|
| CEA <3 µg/L | 0.55 | 2.12 | 12.55 |
| NSE <12 µg/L | 2.73 | 5.44 | 4.65 |
| ProGRP <45 pg/mL | 18 | 33.68 | 5.90 |
CEA, carcinoembryonic antigen; NSE, neuron-specific enolase; ProGRP, progastrin-releasing peptide.
Notably, the current patient’s circulating tumor DNA revealed three coexisting mutations, T790M, cis-C797S, and EGFR 19 del, as well as inactivation of RB1, at disease progression after the administration of osimertinib. EGFR T790M and C797S were located in cis, which is a rarely reported configuration, but was observed in a previous study17 in which C797S mutation was reported as a mechanism of acquired resistance to third-generation TKIs. The prevalence of the C797S mutation ranges from 22% to 40%, and it was detected in 22 of 99 NSCLC patients who progressed on osimertinib in one study,18 and in 6 of 15 patients in another study.19 Osimertinib specifically binds irreversibly to EGFR by targeting the cysteine-797 residue in the ATP-binding site via covalent bond formation, potently inhibiting its phosphorylation.20 However, C797S mutant EGFR cannot form a covalent bond with osimertinib because serine fails to form a covalent bond under physiological conditions, leading to resistance.21 The choice of treatment strategy depends on the relationship between C797S and T790M. If the EGFR T790M mutation is lost when resistance emerges, the EGFR C797S resistance mutation is sensitive to first-generation TKIs,22 while if C797S emerges in trans of the T790M allele, the tumor remains sensitive to first- and third-generation EGFR-TKI combinations. However, tumors remain broadly resistant if C797S emerges in the cis position of the T790M allele.23 Fourth-generation EGFR-TKIs, such as EAI045, have been designed to deal with C797S-related resistance but are still in the research stage. Unfortunately, in the current case, the patient harbored the cis-C797S mutation with T790M.
To the best of our knowledge, this is the first reported case of resistance to osimertinib caused by SCLC transformation and cis-C797S mutation in a patient harboring EGFR exon 19 deletion and T790M mutation, as well as inactivation of RB1. Re-biopsy may be necessary to guide the appropriate treatment, in light of tumor heterogeneity and histologic transformation. The status of gene mutations that might predict histological transformation should also be evaluated during treatment, especially in the early stage, to provide valuable evidence to support appropriate treatment
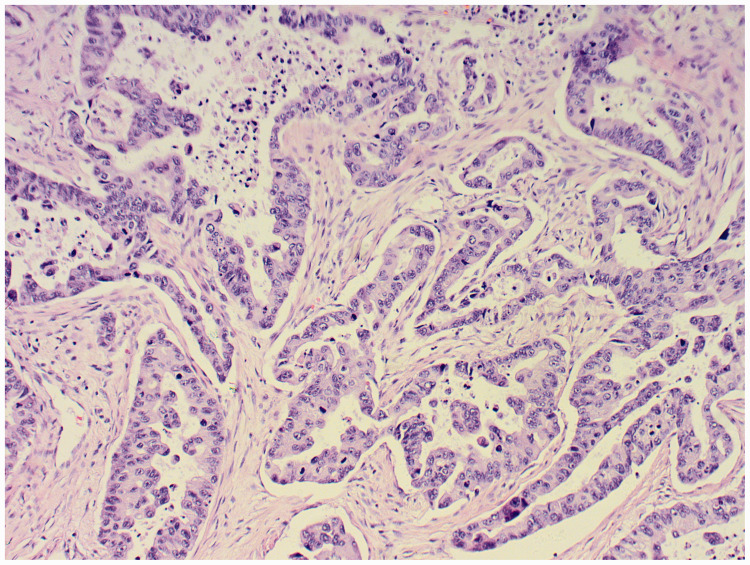
Figure 1.
Histopathologic examination of surgical sample of lung showed invasive adenocarcinoma. (17 December 2014). Hematoxylin and eosin, magnification ×100.
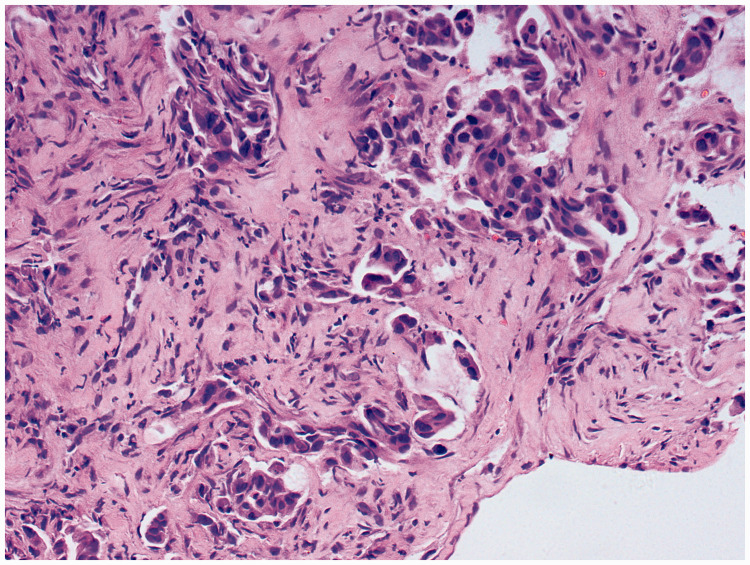
Figure 2.
Histopathologic examination of transbronchial lung biopsy sample showed high-grade adenocarcinoma. (17 February 2017). Hematoxylin and eosin, magnification ×200.
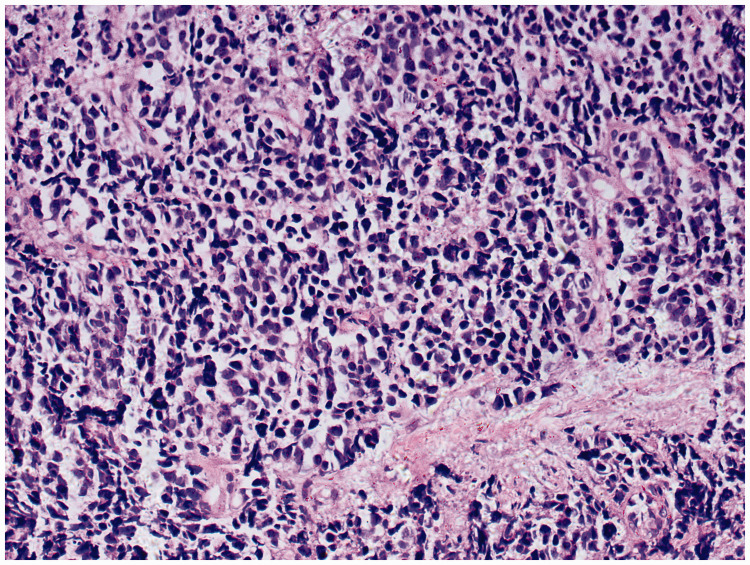
Figure 3.
Histopathologic examination of transbronchial lung biopsy sample showed small cell lung carcinoma. (23 June 23 2018). Hematoxylin and eosin, magnification ×200.
Acknowledgements
We would like to express our gratitude to the members of the Second Department of Respiratory Medicine, Shanxi Provincial Cancer Hospital for their helpful discussions.
Ethics and consent
The study protocol was approved by the Peking Union Medical College Hospital institutional review board (ID:JS-1410) and written informed consent for publication was obtained from the patient.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research was part of the CAPTRA-Lung study (NCT03334864). This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016; 66: 271–289. [DOI] [PubMed] [Google Scholar]
- 3.Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013; 19: 2240–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015; 372: 1689–1699. [DOI] [PubMed] [Google Scholar]
- 5.Tang ZH, Lu JJ. Osimertinib resistance in non-small cell lung cancer: mechanisms and therapeutic strategies. Cancer Lett 2018; 420: 242–246. [DOI] [PubMed] [Google Scholar]
- 6.Planchard D, Loriot Y, Andre F, et al. EGFR-independent mechanisms of acquired resistance to AZD9291 in EGFR T790M-positive NSCLC patients. Ann Oncol 2015; 26: 2073–2078. [DOI] [PubMed] [Google Scholar]
- 7.Kim TM, Song A, Kim DW, et al. Mechanisms of acquired resistance to AZD9291: a mutation-selective, irreversible EGFR inhibitor. J Thorac Oncol 2015; 10: 1736–1744. [DOI] [PubMed] [Google Scholar]
- 8.Taniguchi Y, Horiuchi H, Morikawa T, et al. Small-cell carcinoma transformation of pulmonary adenocarcinoma after osimertinib treatment: a case report. Case Rep Oncol 2018; 11: 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Wang H, Li C, et al. Transformation to small-cell carcinoma as an acquired resistance mechanism to AZD9291: a case report. Oncotarget 2017; 8: 18609–18614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minari R, Bordi P, Del Re M, et al. Primary resistance to osimertinib due to SCLC transformation: Issue of T790M determination on liquid re-biopsy. Lung Cancer 2018; 115: 21–27. [DOI] [PubMed] [Google Scholar]
- 11.Ham JS, Kim S, Kim HK, et al. Two cases of small cell lung cancer transformation from EGFR mutant adenocarcinoma during AZD9291 treatment. J Thorac Oncol 2016; 11: e1–e4. [DOI] [PubMed] [Google Scholar]
- 12.Lee JK, Lee J, Kim S, et al. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J Clin Oncol 2017; 35: 3065–3074. [DOI] [PubMed] [Google Scholar]
- 13.Lin MW, Su KY, Su TJ, et al. Clinicopathological and genomic comparisons between different histologic components in combined small cell lung cancer and non-small cell lung cancer. Lung Cancer 2018; 125: 282–290. [DOI] [PubMed] [Google Scholar]
- 14.Niederst MJ, Sequist LV, Poirier JT, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun 2015; 6: 6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oser MG, Niederst MJ, Sequist LV, et al. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol 2015; 16: e165–e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park S, Shim JH, Lee B, et al. Paired genomic analysis of squamous cell carcinoma transformed from EGFR-mutated lung adenocarcinoma. Lung Cancer 2019; 134: 7–15. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Yang JJ, Huang J, et al. Lung adenocarcinoma harboring EGFR T790M and in trans C797S responds to combination therapy of first- and third-generation EGFR TKIs and shifts allelic configuration at resistance. J Thorac Oncol 2017; 12: 1723–1727. [DOI] [PubMed] [Google Scholar]
- 18.Oxnard GR, Thress K, Paweletz C, et al. ORAL17.07 mechanisms of acquired resistance to AZD9291 in EGFR T790M positive lung cancer. J of Thoracic Oncol 2015; 10: ORAL 17.07. [Google Scholar]
- 19.Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015; 21: 560–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Remon J, Planchard D. AZD9291 in EGFR-mutant advanced non-small-cell lung cancer patients. Future Oncol 2015; 11: 3069–3081. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Fu W, Zheng L, et al. Recent progress of small-molecule epidermal growth factor receptor (EGFR) inhibitors against C797S resistance in non-small-cell lung cancer. J Med Chem 2018; 61: 4290–4300. [DOI] [PubMed] [Google Scholar]
- 22.Ricordel C, Friboulet L, Facchinetti F, et al. Molecular mechanisms of acquired resistance to third-generation EGFR-TKIs in EGFR T790M-mutant lung cancer. Ann Oncol 2018; 29: i28–i37. [DOI] [PubMed] [Google Scholar]
- 23.Niederst MJ, Hu H, Mulvey HE, et al. The allelic context of the C797S mutation acquired upon treatment with third-generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies. Clin Cancer Res 2015; 21: 3924–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]