Abstract
Purpose:
Keloid is a poorly understood disease that is unique to humans. Hypertrophic scars are similar to keloids and may transform into keloids over time. The standard treatments for these scars are limited by inconsistent efficacy and long treatment/follow-up times. Therefore, a new treatment that is effective for all abnormal scar cases is needed. One option may be photodynamic therapy (PDT). This review assesses the current evidence regarding the safety and efficacy of PDT for keloids and hypertrophic scars.
Methods:
PubMed, Medline and Web of Science were searched from 1900 onwards for the following terms: ‘keloid and photodynamic therapy (PDT)’; ‘hypertrophic scar and photodynamic therapy (PDT)’; and ‘scar and photodynamic therapy (PDT)’. Articles were included if they reported using topical PDT to treat keloids or hypertrophic scars, the patient(s) had one or more keloids and/or hypertrophic scars, and the effect of PDT on these abnormal scars was described.
Results:
In total, 538 articles were identified. Thirteen fulfilled all inclusion criteria. Eight were laboratory studies on keloid/hypertrophic scar explants, fibroblasts or tissue-engineered skin models and five were clinical studies/case reports. The clinical results of PDT on keloids and hypertrophic scars are encouraging.
Conclusion:
PDT appears to play a promising role in keloid and hypertrophic scar therapy but additional clinical studies, particularly randomised clinical trials, are needed.
Keywords: Photodynamic therapy, keloid, hypertrophic scar, scar, light therapy, treatment
Lay summary
The absence of an animal model to study keloid scarring hinders our fully understanding of the pathogenesis and establishing optimal therapeutic strategy. Therefore, investigating novel effective treatment methods is critical in keloid management. Photodynamic therapy (PDT) is widely used to treat various skin diseases such as cutaneous malignant tumors. We conducted a systematic review of the literature to assess the effectiveness of PDT in keloids and hypertrophic scars management. Several studies provide evidence on the potential use of PDT as a safe and effective treatment for keloid and hypertrophic scars. Yet, further research is needed to determine the optimal treatment protocol.
Introduction
Keloids and hypertrophic scars develop due to abnormal chronic inflammation originating from the wound area. Keloid scars commonly grow beyond the wound boundaries while hypertrophic scars are confined to the original area of the wound. Both types of scars arise as a result of impaired fibroblastic proliferation and collagen deposition after skin injury.1–3 They can greatly affect the patient’s quality of life and emotional wellbeing by causing intense pain, itching, unappealing red appearance and inexorable spread. Interestingly, keloids and hypertrophic scars are exclusively found in humans and do not occur in animals naturally. The lack of suitable animal models that recapitulate the key processes in keloidal/hypertrophic scarring has greatly hampered our understanding and treatment of these scars.
The current treatments for keloids and hypertrophic scars include surgical resection and local corticosteroid injections. However, all monotherapies associate with high rates of recurrence. Moreover, while good results can be obtained with the careful customised use of combination therapies, these approaches require very long treatment and/or follow-up times.4,5 Thus, new treatment methods that rapidly and consistently improve keloids and hypertrophic scars are urgently needed.6
One possible option is photodynamic therapy (PDT). This non-invasive and safety treatment involves applying a photosensitiser substance onto a lesion and then subjecting it to light in the presence of molecular oxygen.7–11 The first medical use of PDT in dermatology was by von Tappeiner and Jesionek,12 who showed that skin tumours responded to a combination of topical eosin and white light. In 1978, Dougherty reported the first large-scale patient series of PDT-treated primary or secondary skin tumours: 111 of the 113 tumours responded partially or completely to topical PDT composed of hematoporphyrin derivative and red light from a xenon arc lamp.14 Today, topical PDT in the dermatology field involves a variety of light sources, including lasers, intense pulsed light, light-emitting diodes, blue light, red light and many other visible lights (including natural light). The current photosensitisers include 5-aminolevulinic acid (ALA), which is a naturally occurring intermediate in the haem biosynthetic pathway, and its methyl ester, methylaminolevulinic acid (MAL).7–9 ALA and MAL do not have intrinsic photosensitising effects: they are pro-drugs that are converted by haem pathway enzymes into the potent endogenous photosensitiser called protoporphyrin IX. When exposed to light, protoporphyrin IX becomes activated and interacts with molecular oxygen in the local tissue, thereby generating cytotoxic reactive oxygen species (ROS) and free radicals that selectively destroy rapidly growing cells.7–16
Topical PDT is now an established treatment for tumours such as actinic keratosis, Bowen’s disease and superficial basal cell carcinoma. PDT has also been shown to be an effective treatment for several non-neoplastic skin diseases, including photoaged skin, leishmaniasis, pyogenic sweatadenitis, sebaceous gland hyperplasia and acne vulgaris.17 Research into the molecular mechanisms underlying the anti-tumour activity of topical PDT suggests that it has anti-bacterial, anti-inflammatory and immunomodulatory effects on keratinocytes, fibroblasts, mast cells, sebaceous glands and hair follicles.18
There are also a number of studies that suggest that topical PDT has beneficial effects on keloids, including a case report showing that PDT effectively resolved a persistent keloid that was refractory to multiple routine therapies.19 However, the therapeutic efficacy of PDT in keloids and hypertrophic scars is not well described. The aim of the present article is to systematically review the available literature regarding the use of topical PDT to treat keloids and hypertrophic scars.
Methods
Literature search
A comprehensive review of the literature was conducted from 1900 to February 2019 using PubMed, Medline and the Web of Science for all relevant published studies using the following keywords: ‘Photodynamic therapy (PDT) and keloid’; ‘Photodynamic therapy (PDT) and hypertrophic scar’; and ‘Photodynamic therapy (PDT) and scar’. All authors contributed in the screening and selection process. Case reports, case-series, clinical studies, in vitro and in vivo studies were considered for this review. The following inclusion and exclusion criteria were followed to narrow the focus on the appropriate research studies.
Inclusion criteria
The inclusion criteria were: the article reported the use of topical PDT to treat keloids or hypertrophic scars; the patient(s) had one or more keloids and/or hypertrophic scars; and the effect of PDT on these abnormal scars was described. There were no restrictions with regards to the number of patients in the clinical study or the duration of follow-up. The systematic review also aimed to include all in vivo studies in topical PDT-treated organ culture model of human skin scarring and all in vitro studies involving PDT-treated keloid- or hypertrophic scar-derived tissues/cells. The language of the article was not restricted to English.
Exclusion criteria
The exclusion criteria were articles on PDT for acute wounds, contour deformity and scars that were neither keloids nor hypertrophic scars.
Statistical analysis
A formal statistical analysis was not performed because the extensive methodological heterogeneity of the articles limited us to a qualitative analysis.
Results
In total, 538 articles were identified by our database search (Figure 1). A PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart for literature attrition is included (Figure 1). After duplicates were removed, 412 individual articles remained. After screening their titles, the abstracts of 94 articles were assessed for adherence to our inclusion criteria. Of these articles, 61 were excluded. The remaining 33 full-text articles were then reviewed in their entirety. Finally, 13 studies that satisfied all eligibility criteria were included (Figure 1).
Figure 1.
PRISMA flowchart showing literature attrition.
In vitro studies
There were no in vivo studies involving animal models. The remaining eight studies included in the systematic analysis were in vitro studies on the effect of PDT on keloid or hypertrophic scar explants, fibroblasts or tissue-engineered skin-like rafts (Table 1). The rafts were created by Chiu et al.19 as an organotypic wound-healing model. They were generated by laying keratinocytes on top of fibroblasts embedded in a collagen matrix. The study showed that when rafts created from keloid-derived keratinocytes and fibroblasts were subjected to ALA-PDT with higher light energies (10 and 20 J/cm2), the fibroblasts were killed. At a lower light energy (5 J/cm2), the fibroblasts survived but their contractile ability and collagen production dropped markedly.
Table 1.
Laboratory studies showing the effect of photodynamic therapy on keloids and hypertrophic scars.
| Study | Design | Treatment | Outcome |
|---|---|---|---|
| Liu et al., 2019 26 | In vitro | ALA PDT 633-nm red LED |
PDT beneficial ALA-PDT induced superoxide anion-dependent autophagic cell death |
| Hu et al., 2017 24 | In vitro | Hypocrellin B PDT yellow light from LED |
HB-LED PDT treatment induced significant keloid fibroblast apoptosis and decreased cell viability |
| Mendoza-Garcia et al., 2015 20 | Ex vivo | ALA or MALA PDT 633-nm red LED |
Post-PDT, matrix components were found to be reorganised in both hypertrophic and keloid scars. |
| Zheng et al., 2015 25 | In vitro | Pheophorbide a-based PDT 664-nm LED |
RUNX3 expression was detected more often in keloid tissues than in the dermis of normal skin. Significant differences were found after pheophorbide a-based PDT in RUNX3-expressing keloid fibroblasts |
| Mendoza et al., 2012 21 | In vitro | ALA or MALA PDT 633-nm red LED |
Cytotoxicity post-PDT in keloid fibroblasts is dependent on the lesional site, photosensitiser pro-drug and fluence |
| Sebastian et al., 2011 22 | In vitro | PDT + DW | Combination treatment is more effective than PDT on its own |
| Chiu et al., 2005 19 | In vitro | ALA PDT 633-nm Red LED |
The study established a PDT dosimetry range that reduces tissue contraction and collagen density while minimising injury to fibroblasts |
| Li et al. 2012 23 |
In vitro | ALA PDT 633-nm Red LED |
Hypertrophic scar-derived fibroblasts efficiently accumulate protoporphrin IX after ALA treatment and can be eliminated via apoptosis by red light |
ALA, 5-aminolevulinic acid; DW, degenerate wave; LED, light-emitting diode; MAL, methylaminolevulinate.
The Bayat group cultured skin explants from normal human skin (n = 4) and skin that had increasing degrees of fibrosis, namely, striae alba, fine line scars, hypertrophic scars and keloids (n = 3–6 patients/tissue). Treatment with ALA-PDT or MAL-PDT induced fibrotic tissue degradation and apoptosis that increased with the severity of skin fibrosis: thus, it was most pronounced in keloids. The inverse pattern was observed for proliferation and expression of the replication-related PCNA gene. Moreover, PDT decreased collagen I and III gene expression, increased matrix metalloproteinase-3 and tropoelastin expression, and improved matrix component organisation: these effects were particularly notable in hypertrophic scars and keloids.20 Another study from the same group21 evaluated the ability of ALA-PDT and MAL-PDT to kill cultured fibroblasts that had been harvested from the top, middle and margin of a keloid. They showed that various combinations with different photosensitiser pro-drugs and light fluences influenced the killing efficacy of PDT. The location in the lesion from which the fibroblasts originated also shaped PDT efficacy: for example, ALA-PDT killed fibroblasts from the middle of the keloid better than fibroblasts from the top and margin.21 The Bayat group22 also examined whether adding electrical stimulation with non-invasive degenerate wave to ALA/MAL-PDT improved the cytotoxic effect of PDT on keloid fibroblasts from 10 patients. Indeed, the combination treatment was significantly better than PDT alone in terms of keloid fibroblast killing, ROS production and apoptosis (caspase-3 activation). It also decreased proliferation and collagen I and III production better than PDT alone.
Li et al.23 reported a study on the effect of ALA-PDT on hypertrophic scar fibroblasts from five patients. They showed that the treated hypertrophic-scar fibroblasts effectively accumulated protoporphyrin IX after treatment. Moreover, like PDT-treated keloid fibroblasts, the hypertrophic-scar fibroblasts underwent apoptosis, as measured by TUNEL and annexin analysis.
Several in vitro studies have also examined the effect on keloid fibroblasts of PDT employing new photosensitisers. Hu et al.24 found that PDT with the natural perylenequinone photosensitiser hypocrellin B and yellow light significantly reduced keloid fibroblast viability by inducing apoptosis (as shown by upregulated BAX expression and caspase-3 activation and downregulated BCL-2 expression). Moreover, Zheng et al.25 explored the role of RUNX3 in the response of keloid fibroblasts to pheophorbide a-based PDT (Pa-PDT). RUNX3 is a transcription factor whose expression may predict responsiveness of cancer cells to PDT. Immunohistochemical analysis showed that on average, 32 keloid tissues expressed higher levels of RUNX3 than six normal skin samples. RUNX3 expression in the keloids associated with pain and pruritis and a higher proliferative index. When cultured keloid fibroblasts that did and did not express RUNX3 were treated with Pa-PDT, the RUNX3-expressing fibroblasts exhibited significantly less viability, proliferation and collagen I expression and significantly more apoptotic cell death. Moreover, when RUNX3 expression in two keloid-fibroblast lines was silenced with an siRNA, they became less responsive to Pa-PDT. This suggests that RUNX3 may contribute to keloid pathogenesis and is a biomarker of keloid responsiveness to PDT.
Some of the preceding studies suggest that PDT induces apoptotic cell death.20,22–25 By contrast, the study by Liu et al.21 suggests that PDT may induce autophagic cell death that is dependent on superoxide anions. Thus, when cultured fibroblasts isolated from the excised keloids of six patients were treated with ALA-PDT, the fibroblasts died. This effect was prevented by co-treatment with an autophagy inhibitor but not by treatment with an apoptosis inhibitor. The PDT-induced autophagic cell death may have been mediated by the sirtuin SIRT1: treatment with a SIRT1 inhibitor decreased ALA-PDT-induced keloid fibroblast death while a SIRT1 activator had the opposite effect.26
Clinical studies
Of the 13 articles, three and two were clinical studies that examined the effect of topical PDT on keloids and hypertrophic scars inpatients, respectively (Table 2).
Table 2.
Clinical studies demonstrating the effect of photodynamic therapy on keloids and hypertrophic scars.
| Study | Study type | Outcome measures | Intervention methods and parameters | Follow-up period (months) | Efficacy/Conclusion | Recurrence rate |
|---|---|---|---|---|---|---|
| Nie et al., 2010 27 | Case report |
Clinical assessment of size and erythema | Topical MAL applied to keloid for 3 h; irradiated with 633-nm LED. Five sessions over 5 months | 12 | Overall reduction in volume | No recurrence |
| Tosa et al., 2007 29 | Case series |
Clinical assessment | Topical ALA applied to keloid for 3 h; irradiated with 633-nm LED. Five sessions over 5 months | Not stated | 50% of patients showed complete resolution; 10% of patients showed > 50% improvement; 40% of patients showed < 50% improvement | N/A |
| Ud-Din et al., 2012 28 | Case series |
Clinical assessment of recurrence, pain and pruritus scores | Topical MAL applied to keloid for 3 h; irradiated with 630-nm LED. Each patient received three treatments at weekly intervals | 9 | Pain, pruritus, haemoglobin and collagen levels were all decreased. Pliability increased | 5% (1/20) |
| Bruscino et al., 2011 32 | Case report |
Clinical assessment of size and cosmetic result | Topical MAL applied to hypertrophic scar on the cheek for 3 h; irradiated with 633-nm LED. Three sessions over 2 weeks | 12 | Overall reduction in size and became an unremarkable scar | No recurrence |
| Campbell et al., 2010 31 | Case report | Clinical and histological assessment | Topical ALA applied to hypertrophic scars for 4 h; irradiated with 635-nm LED. Three sessions at 6-weekly intervals | Not stated | Hypertrophic scars softened significantly and became more flexible clinically and histologically | N/A |
ALA, 5-aminolevulinic acid; LED, light-emitting diode; MAL, methylaminolevulinate; N/A, not applicable.
With regard to the keloid studies, one was a case report of a patient whose chin keloid persisted despite multiple conservative and surgical treatments over four years: five sessions of PDT with MAL gradually reduced the area and volume of the keloid. One year later, recurrence was not observed.27 The second study was a case-series study that evaluated the effect of PDT in 20 patients who either had small (⩽ 2 mm high) existing keloids (n =10), keloids that had been debulked surgically to a height of ⩽ 2 mm (n = 6) and keloids that had been excised completely (n = 4): three topical MAL-PDT treatments at weekly intervals significantly improved the itching, pain and pliability of the keloids. The treatments also markedly decreased the collagen and haemoglobin levels in the lesions (as measured by spectrophotometric intracutaneous analysis). These changes associated with a significant reduction in keloid volume. Only one case of recurrence was observed at the nine-month follow-up.28 The third study was another case-series study on 15 keloids in 14 patients that were refractory to ⩾ 3 injections of triamcinolone acetonide: after monthly treatments with ALA-PDT for three months, 50% of the patients showed complete keloid clearance.29
The two clinical PDT studies on hypertrophic scars were case reports. Campbell et al.30 showed when two patients with long-standing chest hypertrophic scars due to thermal burns underwent three sessions of MAL-PDT at six-weekly intervals (each session consisted of two treatments one week apart), the scars softened and became more pliable. Biopsies taken before treatment started and six weeks after the last PDT showed that PDT significantly increased the elastin fiber numbers. Similarly, Bruschino et al.31 showed that when a refractory hypertrophic scar on the cheek that was caused by a dog bite was treated four times at two-week intervals with MAL-PDT, it softened and became unnoticeable. The patient was very satisfied with the clinical and cosmetic outcome.
Thus, ALA/MAL-PDT may be a useful primary or adjunctive treatment for keloids and hypertrophic scars.
Discussion
Keloids are the result of abnormal wound healing and are red, hard, raised lesions that are characterised by chronic inflammation and excessive fibrous tissue formation.3 They grow gradually and associate with strong pain and itching and aesthetic problems. Hypertrophic scars are similar to keloids except that their growth is confined to the original wound bed. They may progress into keloids over time.
The cause of keloid or hypertrophic scar onset is unknown. Both involve over-synthesis of extracellular matrix components, particularly collagen: for example, keloids synthesise approximately 20- and 4-fold more collagen (particularly collagen I and III) and fibronectin, respectively, than normal skin.32 The fibroblasts in these scars are also activated: for example, keloid fibroblasts express high levels of growth factors such as vascular endothelial growth factor, transforming growth factor-β1 and -β2, and platelet-derived growth factor-α1. These cells themselves also respond strongly to these growth factors.32
It is believed that 5%–15% of all wounds develop into keloids and hypertrophic scars, particularly in ethnic Asian or African people. Since animal models of these scars are lacking, there are still no specific drugs and treatments for them.32 Various treatments have been identified by empirical trial and error. The more successful treatments that are currently in use are topical-steroid injection, steroid-containing tape,33 surgical treatment, radiotherapy, silicone-gel sheeting, imiquimod, fluorouracil cream and intralesional injection with bleomycin.33 However, all have a number of limitations: the drugs are not specific for keloids and hypertrophic scars; steroid injection and radiotherapy can cause side effects such as skin atrophy and telangiectasia; and keloid surgery alone associates with a high rate of recurrences—many of which are even worse than the original scar. Moreover, combination therapies inevitably involve long treatment or follow-up times. Therefore, it is desirable to establish an effective new treatment for keloids and hypertrophic scars that rapidly and consistently resolves them.32,34
Focal PDT is widely used to treat superficial basal cell carcinoma, actinic keratosis and Bowen’s disease. It involves topically applying the pro-drug MAL or ALA. After the pro-drug is absorbed by the lesion, it is converted by the haem biosynthetic pathway into the photosensitiser protoporphyrin IX. Application of light then activates protoporphyrin IX, which turns endogenous molecular oxygen into cytotoxic ROS, particularly singlet oxygen. PDT is also known to be an effective treatment for photoaged skin35 and acne.37 Photodynamic therapy (PDT) uses light to activate photosensitizers that are localized in the diseased tissue. LED phototherapy is a completely different treatment from PDT because it does not use a photosensitizer and only emits light.37 The present review describes the five clinical and eight in vitro studies on the treatment potential of PDT for keloids and hypertrophic scars that have been published to date. One of the five clinical studies was by our group: we reported that when 15 keloids that were refractory to steroid injections were treated with ALA-PDT, half were completely resolved. An example is shown in Figure 2.21
Figure 2.
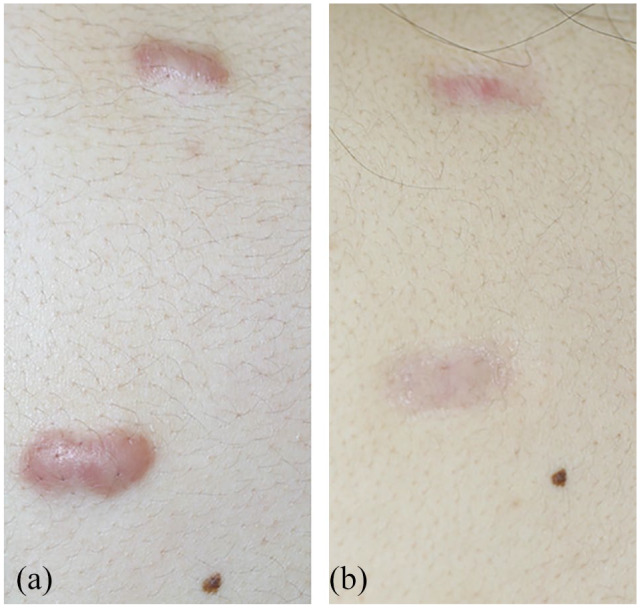
A pre-sternal acne keloid in a 21-year-old man who was treated with ALA photodynamic therapy. (a) Before starting treatment; (b) 6 months after initiating treatment.
The mechanisms by which topical PDT improves abnormal scars are largely unknown. However, they probably involve downstream responses to the ROS produced by the photodynamic reactions: the ROS induce membrane and mitochondrial damage, which in turn activates signalling molecules such as TNF-α and interleukins 1 and 6 and cell death.38 The cell death may be via apoptosis, necrosis and/or autophagy.24 These changes may alter growth factor and cytokine expression in the lesion, thereby modulating collagen production and extracellular matrix organisation. This is supported by the fact that ALA-PDT on cultured normal fibroblasts significantly reduces their type I collagen synthesis and upregulates their collagenolytic enzymes.38 This may also explain the finding of the Bayat group, namely, that PDT reorganised the extracellular matrix components in both hypertrophic scars and keloids.25 Heckenkamp et al.38 also showed that the photodynamic reaction reduces the proliferation and migration of normal dermal fibroblasts grown in a gel matrix. In addition, they found that PDT markedly limits fibroblast expression of TGF-β1 and basic fibroblast growth factor mRNA.39
It should be noted that PDT is a relatively superficial treatment due to the penetration limits of both the pro-drug and red light. This may be problematic for keloids, many of which are at least 3 mm deep. However, there have been reports of successful PDT treatment for nodular basal cell carcinoma at depths greater than 3 mm for instance when combined with carbon laser therapy.40,41 Moreover, the efficacy of PDT on more superficial layers of abnormal scars suggests that multiple PDT treatments could eventually resolve even thick lesions. Nevertheless, further improvements in pro-drugs and light-delivery methods may be needed to assure deep treatment and a more rapid response to PDT. Randomised clinical trials will also be needed to establish standard guidelines for optimal photosensitiser concentration, incubation period and light source parameters.
Notably, the shallowness of PDT suggests that it may be useful as an adjunct treatment of the wound area after keloid resection: in this setting, PDT may help to prevent postoperative keloid recurrence.
Given the limitations in the literature to date, it is evident that further clinical and laboratory research is needed to determine the efficacy and underlying mechanisms of PDT in patients with keloids and hypertrophic scars. In particular, good quality randomised controlled trials are needed. There is also an urgent need to establish a suitable animal model of keloids, as this will greatly facilitate the development of specific new drugs and treatments.42
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Mamiko Tosa  https://orcid.org/0000-0002-6826-9028
https://orcid.org/0000-0002-6826-9028
References
- 1. Mrowietz U, Seifert O. Keloid scarring: new treatments ahead. Actas Dermosifiliogr 2009; 100: 75–83. [DOI] [PubMed] [Google Scholar]
- 2. Murray JC. Keloids and hypertrophic scars. J Clin Dermatol 1994; 12: 27. [DOI] [PubMed] [Google Scholar]
- 3. Tosa M, Watanabe A, Ghazizadeh M. IL-6 Polymorphism and Susceptibility to Keloid Formation in a Japanese Population. J Invest Dermatol 2016; 136(5): 1069–1072. [DOI] [PubMed] [Google Scholar]
- 4. Froelich K, Staudenmaier R, Kleinsasser N, et al. Therapy of auricular keloids: a review of different treatment modalities and proposal for a therapeutic algorithm. Eur Arch Otorhino 2007; 264: 1497–1508. [DOI] [PubMed] [Google Scholar]
- 5. Gauglitz GG, Korting HC, Pavicic T, et al. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med 2011; 17: 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bayat A, McGrouther DA. Clinical management of skin scarring. Skinmed 2005; 4: 165–173. [DOI] [PubMed] [Google Scholar]
- 7. Calzavara-Pinton PG, Rossi MT, Aronson E, et al. Italian Group for Photodynamic Therapy: A retrospective analysis of real-life practice of off-label photodynamic therapy using methyl aminolevulinate (MAL-PDT) in 20 Italian dermatology departments. 1. Inflammatory and aesthetic indications. Photochem Photobiol Sci 2013; 12: 148–157. [DOI] [PubMed] [Google Scholar]
- 8. Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol 2014; 7: 145–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rkein AM, Ozog DM. The scientific basis for PDT has been recognized since 1900. Oscar Raab and Herman von Tappeiner first reported the concept of cell death caused by the interaction of light and chemicals. Photodynamic therapy. Dermatol Clin 2014; 32: 415–425. [DOI] [PubMed] [Google Scholar]
- 10. Raab O. Uber die Wirkung fluoreszierender Stoffe auf Infusorien. Z Biol 1900; 39: 524–546. [Google Scholar]
- 11. Von Tappeiner H. Uber die Wirkung fluoreszierender Stoffe auf Infusorien nach Versuchen von O. Raab. Muench Med Wochenschr 1900; 47: 5. [Google Scholar]
- 12. Von Tappeiner H, Jesionek A. Therapeutische Versuche mit fluoreszierenden Stoffen. Muench Med Wochenschr 1903; 47: 2042–2044. [Google Scholar]
- 13. Ackroyd R, Kelty C, Brown N, et al. The history of photodetection and photodynamic therapy. Photochem Photobiol 2001; 74: 656–669. [DOI] [PubMed] [Google Scholar]
- 14. Dougherty TJ. Photoradiation therapy for the treatment of malignant tumors. Cancer Res 1978; 36: 2628–2635. [PubMed] [Google Scholar]
- 15. Gold MH. Therapeutic and aesthetic uses of photodynamic therapy part five of a five-part series: ALA-PDT and MAL-PDT what makes them different. J Clin Aesthet Dermatol 2009; 2:44–47. [PMC free article] [PubMed] [Google Scholar]
- 16. Fonda-Pascual P, Moreno-Arrones OM, Alegre-Sanchez A, et al. In situ production of ROS in the skin by photodynamic therapy as a powerful tool in clinical dermatology. Methods 2016; 109: 190–202. [DOI] [PubMed] [Google Scholar]
- 17. Morton C, Szeimies RM, Sidoroff A, et al. European Dermatology Forum: European Dermatology Forum Guidelines on topical photodynamic therapy. Eur J Dermatol 2015; 25: 296–311. [DOI] [PubMed] [Google Scholar]
- 18. Ortel B, Shea CR, Calzavara-Pinton PG: Molecular mechanisms of photodynamic therapy. Front Biosci 2009; 14: 4157–4172. [DOI] [PubMed] [Google Scholar]
- 19. Chiu LL, Sun CH, Yeh AT, et al. Photodynamic therapy on keloid fibroblasts in tissue-engineered keratinocyte-fibroblast co-culture. Lasers Surg 2005; 37: 231–244. [DOI] [PubMed] [Google Scholar]
- 20. Mendoza-Garcia J, Sebastian A, Alonso-Rasgado T, et al. Ex vivo evaluation of the effect of photodynamic therapy on skin scars and striae distensae. Photodermatol Photoimmunol Photomed 2015; 31: 239–251. [DOI] [PubMed] [Google Scholar]
- 21. Mendoza J, Sebastian A, Allan E, et al. Differential cytotoxic response in keloid fibroblasts exposed to photodynamic therapy is dependent on photosensitiser precursor, fluence and location of fibroblasts within the lesion. Arch Dermatol Res 2012; 304: 549–562. [DOI] [PubMed] [Google Scholar]
- 22. Sebastian A, Allan E, Allan D, et al. Additional of novel degenerate electrical waveform stimulation with photodynamic therapy significantly enhances its cytotoxic effect in keloid fibroblasts: First report of a potential combination therapy. J Dermatol Sci 2011; 64: 174–184. [DOI] [PubMed] [Google Scholar]
- 23. Li X, Zhou ZP, Hu L, et al. Apoptotic cell death induced by 5-aminolevulinic acid-mediated photodynamic therapy of hypertrophic scar-derived fibroblasts. J Dermatolog Treat 2014; 25(5): 428–433. [DOI] [PubMed] [Google Scholar]
- 24. Hu Y, Zhang C, Li S, et al. Effects of Photodynamic Therapy Using Yellow LED-light with Concomitant Hypocrellin B on Apoptotic Signaling in Keloid Fibroblasts. Int J Biol Sci 2017; 13(3): 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zheng Z, Zhu L, Zhang X, et al. RUNX3 expression is associated with sensitivity to pheophorbide a-based photodynamic therapy in keloids. Lasers Med Sci 2015; 30: 67–75. [DOI] [PubMed] [Google Scholar]
- 26. Liu T, M X, Ouyang T, et al. Efficacy of 5-aminolevulinicacid–based photodynamic therapy against keloid compromised by downregulation of SIRT1-SIRT3-SOD2-mROS dependent autophagy pathway. Redox Biol 2019; 20: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nie Z, Bayat A, Behzad F, et al. Positive response of a recurrent keloid scar to topical methyl aminolevulinate-photodynamic therapy. Photodermatol Photoimmunol Photomed 2010; 26:330–332. [DOI] [PubMed] [Google Scholar]
- 28. Ud-Din S, Thomas G, Morris J, et al. Photodynamic therapy: an innovative approach to the treatment of keloid disease evaluated using subjective and objective non-invasive tools. Arch Dermatol Res 2012; 305: 205–214. [DOI] [PubMed] [Google Scholar]
- 29. Tosa M, Murakami M, Iwakiri I, et al. Treatment of keloids with photodynamic therapy using 5-aminolevulinic acid (5-ALAPDT): a preliminary study. The Second Japan Scar Workshop 2007. Conference abstract P.2 [Google Scholar]
- 30. Campbell SM, Tyrrell J, Marshall R, et al. Effect of MAL-photodynamic therapy on hypertrophic scarring. Photodiagnosis Photodyn Ther 2010; 7: 183–188. [DOI] [PubMed] [Google Scholar]
- 31. Bruscino N, Lotti T, Rossi R. Photodynamic therapy for a hypertrophic scarring: a promising choice. Photodermatol Photoimmunol Photomed 2011; 27: 334–335. [DOI] [PubMed] [Google Scholar]
- 32. Wolfram D, Tzankov A, Pulzl P, et al. Hypertrophic scars and keloids: a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg 2009; 35: 171–181. [DOI] [PubMed] [Google Scholar]
- 33. Goutos I, Ogawa R. Steroid tape: A promising adjunct to scar management. Scars Burns Heal 2017; 3: 2059513117690937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Durani P, Bayat A. Levels of evidence for the treatment of keloid disease. J Plast Reconstr Aesthet Surg 2008; 61: 4–17. [DOI] [PubMed] [Google Scholar]
- 35. Jang YH, Koo GB, Kim JY, et al. Prolonged Activation of ERK Contributes to the Photorejuvenation Effect in Photodynamic Therapy in Human Dermal Fibroblasts. J Invest Dermatol 2013; 133: 2265–2275. [DOI] [PubMed] [Google Scholar]
- 36. Keyal U, Bhatta AK, Wang XL. Photodynamic therapy for the treatment of different severity of acne: A systematic review. Photodiagnosis Photodyn Ther 2016; 14: 191–199. [DOI] [PubMed] [Google Scholar]
- 37. Mamalis AD, Lev-Tov H, Nguyen DH, et al. Laser and light-based treatment of Keloids–a review. J Eur Acad Dermatol Venereol 2014; 28(6): 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Karrrer S, Bosserhoff AK, Weiderer P, et al. Influence of 5-aminolevulinic acid and red light on collagen metabolism of human dermal fibroblasts. J Invest Dermatol 2003; 120: 325–331. [DOI] [PubMed] [Google Scholar]
- 39. Heckenkamp J, Aleksic M, Gawenda M, et al. Modulation of human adventitial fibroblast function by photodynamic therapy of collagen matrix. Eur J Vasc Endovasc Surg 2004; 28: 651–659. [DOI] [PubMed] [Google Scholar]
- 40. Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol 2008; 159: 1245–1266. [DOI] [PubMed] [Google Scholar]
- 41. Shokrollahi K, Javed M, Aeuyung K, et al. Combined carbon dioxide laser with photodynamic therapy for nodular and superficial basal cell carcinoma. Ann Plast Surg 2014; 73(5): 552–558. [DOI] [PubMed] [Google Scholar]
- 42. Ud-Din S, Bayat A. Strategic management of keloid disease in ethnic skin: a structured approach supported by the emerging literature. Br J Dermatol 2013; 169 (Suppl. 3): 71–81. [DOI] [PubMed] [Google Scholar]
How to cite this article
- Tosa M, Ogawa R. Photodynamic therapy for keloids and hypertrophic scars: a review. Scars, Burns & Healing, Volume 6, 2020. DOI: 10.1177/2059513118932059. [DOI] [PMC free article] [PubMed] [Google Scholar]


