Abstract
Protective antigen (PA) 63 (PA63) is a protein derived from the PA83 component contained in the anthrax vaccine. The anthrax vaccine (“Biothrax”) was administered together with other vaccines to Gulf War veterans, about 35% of whom later developed a multisymptom disease (Gulf War Illness [GWI]), with prominent neurological/cognitive/mood symptoms, among others. The disease has been traditionally attributed to exposures to toxic chemicals during the war but other factors could be involved, including vaccines received. Of these, the anthrax vaccine is the most toxic. Here, we assessed directly the PA63 toxin’s harmful effects on cultured neuroblastoma 2A (N2A) cells with respect to cell spreading, process formation, apoptosis, and integrity of cell membrane, cytoskeleton, and mitochondria. We found that, when added in N2A cultures, PA63 toxin led to decreased cell spreading and cell aggregation, leading to apoptosis. The mechanisms of PA63-induced cell damage included compromised cell membrane permeability indicated by enhanced access of propidium iodide in cells. In addition, signaling pathways leading to organization of N2A cytoskeleton were negatively affected, as both actin and microtubular networks were compromised. Finally, the mitochondrial membrane potential was impaired in specific assays. Altogether, these alterations led to apoptosis as a collective toxic effect of PA63 which was substantially reduced by the concomitant addition of specific antibodies against PA63.
Keywords: Gulf War Illness, anthrax PA63, N2A cultures, apoptosis, cytoskeleton, membrane permeability, mitochondrial membrane potential
Introduction
After the Persian Gulf War of 1990-1991, about one-third (>200 000) deployed veterans complained of a variety of chronic physical and neurocognitive symptoms1-4 presently identified as Gulf War Illness (GWI). We previously described a number of functional and structural brain abnormalities in GWI, such as changes in synchronous neural communication patterns5-8 and subcortical brain atrophy in certain GWI patients.9 This atrophy was absent in veterans carrying the human leukocyte antigen (HLA) allele DRB1*13:02,10 one of 6 HLA class II alleles that we had reported previously as protective for GWI, but were lacking from GWI patients.11 The fewer of those alleles carried, the more severe the symptomatology. The function of HLA class II alleles includes specific immunity by matching to external antigens, presenting them to CD4+ lymphocytes and leading to the production of specific antibodies by B cells to neutralize the offending antigen.12 Hence, the lack of HLA class II protection observed in GWI would have allowed pathogens to persist.
Indeed, administered antigens have been reported to persist for prolonged times following immunization in lymphatic endothelial cells13 and lymphoid follicles.14 It is possible then that one or several antigens/pathogens persisted in GWI patients. We hypothesized that such persisting antigens could have originated from vaccines to which GW veterans were exposed, thus leading to cell damage, low-grade inflammation, and multisymptom chronic disease. According to the “persistent pathogen” hypothesis,10 healthy GW veterans carrying protective alleles would have specific antibodies in their blood, which could neutralize the hypothesized persistent antigens in GWI serum. As a first step in testing this prediction, (1) we assessed the effect of GWI serum on function and morphology of neural cultures (primary neuronal cells and neuroblastoma 2A [N2A] cells) in vitro, and (2) we tested serum from veterans. Indeed, we found that (1) GWI serum exerted harmful effects on neural cultures, as it compromised cell-cell communication, cell spreading, and cell survival by significantly enhancing cell apoptosis and (b) those effects were prevented by the addition of serum from healthy GW-era veterans.15 Therefore, healthy serum may contain, among others, antibodies against harmful antigens present in GWI serum; if so, such antibodies may hold promise for a successful intervention in treating GWI.
To test this hypothesis, we initially assessed the effect of pooled human antibodies in vitro, by adding to the culture pooled human immunoglobulin G (IgG), which contains antibodies against a broad range of pathogens, partially overlapping with some of those contained in the vaccines administered to GW veterans. However, rare pathogens such as anthrax antigens should not be present in pooled human IgG, the presence of which exerted a partial beneficial effect in N2A cultures.16 We then tested the effects of serum from 15 GWI patients on N2A cells, in the absence or presence of specific anti-PA63 anthrax antibodies, and observed a significant protective effect in the presence of GWI serum which was incubated with anthrax antibodies.17
In the present report, we specifically addressed the harmful effects of anthrax, a rare antigen which was administered to GW veterans in the form of bacillus proteins, mainly the protective 83 antigen (PA83) the major component in the “Biothrax” vaccine (https://www.rxlist.com/biothrax-drug.htm#indications). In vivo PA83 binds to its receptor and is cleaved by furin family and serum proteases to a 63-kDa PA63 to be active.18,19 In this study, specific anti-PA63 antibodies co-incubated with GWI serum had a pronounced protective effect on functional aspects of N2A cells involving their cell membrane, cytoskeleton, and mitochondrial integrity. Similar protective effects were observed in N2A cells exposed to PA63 which was previously co-incubated with anti-PA63 antibodies. The obtained data suggest that neutralization or removal of components of the anthrax vaccine from GWI patients could represent a useful intervention to alleviate symptoms of GWI in the future.
Materials and Methods
Anthrax PA63 toxin and serum from 2 GWI patients with substantial neurocognitive symptoms and no protective alleles were used; serum from 1 healthy GW era veteran who was free of Neuro-Cognitive-Mood (NCM) symptoms and had 2 of the 6 HLA protective alleles 11 was used as control.
Cell Culture
Neuro-2A neuroblastoma (N2A) cells were cultured in Eagle’s minimal essential medium (EMEM; ATCC, Manassas, VA, USA) containing 10% fetal bovine serum (ThermoFisher Scientific, Waltham, MA, USA) poly-d-lysine–coated, 24-well plates at a concentration of 30 000 to 50 000/well for 48 to 72 hours. The medium was then changed to Neurobasal containing N2 supplement and l-glutamine (ThermoFisher Scientific), in the absence (medium control) or presence of human serum (healthy, non-GWI veteran or GWI veteran). Moreover, protective anthrax antigen 63 (PA63) was added in N2A cultures at 0.5 μg/mL. The anthrax protein PA63 (Creative Diagnostics, Shirley, NJ, USA) was used instead of the anthrax vaccine, because the anthrax vaccine administered to GW veterans contained aluminum (“Biothrax”; https://www.rxlist.com/biothrax-drug.htm#indications), which is toxic to cells. For all experiments making use of serum, it was added in 3 combinations: control healthy (10%), GWI (10%), and GWI preincubated with anthrax antibodies, for assessing cell membrane, cytoskeletal, and mitochondrial integrity.
Cell morphology assay—process formation
The effect of anthrax on the morphology of N2A cells was examined. In experiments involving anthrax antibodies to study the effects on cell membrane, cytoskeletal, and mitochondrial integrity, anti-Anthrax Protective Antigen (polyclonal antiserum Cat. No. CPBT-66806RA) and monoclonal anti-anthrax antibody (CABT-51076MA, both from Creative Diagnostics, Shirley, NY, USA) were used in parallel with similar results. Both antibodies were titrated for effects at a series of concentrations following preincubation with either anthrax PA63 or GWI serum and used at the lowest active concentration (15% for polyclonal anti-anthrax antiserum and 5 μg/mL of monoclonal anti-anthrax). Each antibody was incubated with either anthrax PA63 at 0.5 μg/mL or 100 μL of GWI serum for 60 minutes at 37°C and then added in a final volume of 1 mL of Neurobasal medium containing N2 supplement and l-glutamine.
The N2A cells were cultured with PA63 (0.5 μg/mL) for 2 days and the cells were photographed. Images were obtained from 5 to 8 different fields per sample, from a minimum of 3 experiments using a Motic AE2000-Trinocular inverted microscope (Ted Pella, Redding, CA, USA), with a Zeiss Axiocam 105 color digital camera (Carl Zeiss Microscopy, LLC, Thornwood, NY, USA). The extent of cell spreading was then calculated with ImageJ software by measuring the number of cells with processes relative to the total cell number.
Cell apoptosis with terminal deoxynucleotidyl transferase-mediated dUTP Nick End Labeling assay
The extent of cell apoptosis of Neuro-2A cells was examined at 2 days postexposure to medium and PA63, using 4- and 8-chamber glass slides (ThermoFisher Scientific) coated with poly-d-lysine at 50 μg/mL as mentioned above. Neuroblastoma 2A cells were seeded at a concentration of 50 000 to 100 000 cells per chamber, in 1 mL of Neurobasal/N2/l-glutamine medium for 2 days. In sequence, 0.5 μg of anthrax antibodies or 15% of polyclonal anthrax antiserum were added for 2 more days. At the end of the incubation period, the cells were examined for apoptosis. Apoptotic cells were detected using the In Situ Cell Death Detection Kit (terminal deoxynucleotidyl transferase [TdT] enzyme and fluorochrome labeling solution, Click-iT Alexa Fluor488/green color assay, Thermo Fisher Scientific) according to the manufacturer’s protocol. Briefly, the cells were fixed in ice-cold methanol for 10 minutes at room temperature, rinsed with phosphate-buffered saline (PBS) and permeabilized with 0.1% Triton X-100 in PBS for 3 minutes on ice. The cells were then incubated with 150 μL of transferase-mediated dUTP Nick End Labeling (TUNEL) reaction mixture for 60 minutes at 37°C in the dark. The cells were then washed 3× with PBS and Diamond AntiFade mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) stain (ThermoFisher Scientific) was used for visualization of nuclei, using the EVOS FL Cell Imaging System (ThermoFisher Scientific). Eight-10 images were obtained from different fields from a minimum of 2 experiments with each different experimental condition. Apoptosis was then calculated with ImageJ software by measuring the number of TUNEL-labeled cells (green nuclei) relative to the total cell number (blue/DAPI-stained nuclei).
Cell membrane permeability assessed with PI/membrane repair assay
Membrane perturbations allowing abnormal membrane permeability are the result of harmful factors such as vaccine components: usually membrane damage can be repaired instantly by intracellularly available calcium in healthy cells, but in pathological conditions, the exogenous addition of calcium is required to restore plasma membrane integrity. Assessment of plasma membrane integrity in the presence and absence of CaCl2 was performed with the use of propidium iodide (PI; molecular weight: 668.39; Sigma-Aldrich, Milwaukee, WI, USA) by PI (red) staining of the nucleus through cell membrane openings, in the absence and presence of 1.8 mM CaCl2.20 A total of 20 000 N2A cells per chamber were cultured in 8-chamber slides in the presence of each of the following: healthy serum, GWI serum, GWI serum co-incubated with anti-PA63 antibody, PA63, PA63 co-incubated with PA63 antibody, for 24 hours and were then treated with DMEM (Dulbecco’s Modified Eagle medium) or DMEM containing 1.8 mM CaCl2 for 3 minutes. The cells were then exposed to 50 μg/mL PI for 5 minutes in the dark and rinsed 3× with Tris-buffered saline (TBS). In sequence, the cells were fixed in ice-cold methanol (−20°C) for 10 minutes and washed 3× with TBS; then, Diamond AntiFade mounting medium with DAPI stain (ThermoFisher Scientific) was used for visualization of nuclei; the slides were covered with coverslips and examined with an Olympus 3000 confocal microscope. Eight-10 images were obtained from different fields from a minimum of 2 experiments with each different experimental condition. Propidium iodide–stained cells/nuclei were then calculated with ImageJ software by measuring the number of PI-stained cells/nuclei (red color) relative to the total cell number (DAPI-stained nuclei, blue color).
Cytoskeletal integrity
Microtubule-associated protein 2 (MAP2) indicates microtubule stabilizing activity and regulates microtubule networks in the axons and dendrites of neurons; hence, decreased MAP2 indicates cell dysfunction and damage.21 Cofilin is a protein downstream of Rho-kinase signaling, phosphorylation of which is required for re-organization and stabilization of the actin cytoskeleton. Decreased amounts of phosphorylated cofilin (p-cofilin) indicate impaired signaling resulting in decreased stability of the actin network during the spreading of neurons and N2A cells.22 The extent of MAP2 and p-cofilin present in N2A cells in the presence of GWI serum, GWI serum plus antiPA63 antibodies, or medium and PA63 was examined as follows: 20 000 N2A cells per chamber were seeded in 4- or 8-chamber slides and were cultured in the presence of each of the following: GWI serum, GWI serum co-incubated with anti-PA63 antibodies, medium, or PA63 for 48 hours. The cells were then fixed in ice-cold methanol (−20°C) for 10 minutes and washed 3× with PBS. The cells were permeabilized with 0.3% Triton-X for 5 minutes at room temperature and washed 3× with PBS. Blocking buffer (200 μL) (PBS containing 4% bovine serum albumin [BSA] and 0.3% Triton-X) was added and the cells were incubated for 60 minutes at room temperature with shaking. MAP2 antibody labeled with Alexa Fluor 594 (EDM Millipore, Danvers, MA, USA), diluted 1:1000 was then added and incubated in the dark for 60 minutes at room temperature with shaking. The cells were subsequently washed 3× with PBS and p-cofilin antibody (Cell Signaling, Danvers, MA, USA) diluted 1:1000 was added overnight at 4°C in the dark with shaking. Next day, the cells were washed 3× with PBS and anti-rabbit IgG (Cell Signaling) was added (1:500) for 60 minutes at room temperature in the dark with shaking. The cells were then washed, and Diamond AntiFade mounting medium with DAPI stain (ThermoFisher Scientific) was used for visualization of nuclei; the slides were covered with coverslips and examined with an Olympus 3000 confocal microscope. Eight-10 images were obtained from different fields from a minimum of 2 experiments with each different experimental condition, and the intensity of MAP2 (Alexa fluor 488 nm/green) combined with p-cofilin (RFP, 588 nm/red) was then calculated with ImageJ software in 8 to 10 different fields from each condition.
Mitochondrial membrane potential assay
Many exogenous toxic factors reduce mitochondrial membrane potential (MMP) by perturbing a variety of macromolecules in the mitochondria, and therefore affecting different mitochondrial functions. A decrease in the MMP may also be linked to apoptosis; hence, these organelles are an ideal target for in vitro toxicity studies.23 To assess MMP, 5000 N2A cells per well were seeded in 96-well plates for 34 hours. The cells were then treated with medium, PA63 at 0.5 μg/mL, PA63+ anti-anthrax antibody, healthy serum, GWI serum, or GWI serum preincubated with anthrax antibodies for 60 minutes at 37°C for 24 hours. The cells were then processed as follows: as a positive control, FCCP (mesoxalonitrile 4-trifluoromethoxyphenylhydrazone), a mitochondrial oxidative phosphorylation uncoupler (ab120081; Abcam, Cambridge, MA, USA) was used at 20 μM for 10 minutes; FCCP depolarizes MMP. Τetramethyl rhodamine ethyl ester (Abcam) in neurobasal medium was then added at 1500 nM to the wells after removing the media, and the cells were incubated for 30 minutes and 37°C in the dark. The cells were then washed with PBS containing 0.2% BSA and the plates were read (549/575 nm) with a Molecular Devices Spectramax M5 microplate reader (Sunnyvale, CA, USA).
Statistical analysis
For statistics, the paired t test was used in all instances.
Results
Cell spreading and apoptosis
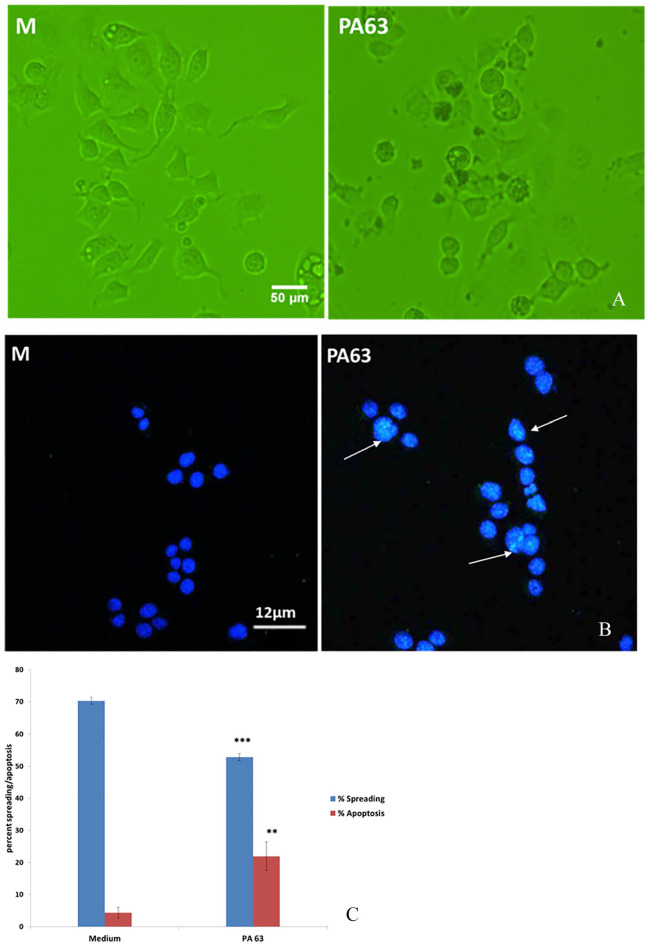
In the presence of PA63, cell spreading was suppressed and N2A were mostly spherical, aggregated, and devoid of processes, similar to cells exposed to GWI serum, as described previously.11,17 Percent spreading of N2A cells in the presence of 0.5 μg/mL PA63 was approximately 15% less, and apoptosis was increased almost 4× more compared with medium (Figure 1A to C).
Figure 1.
(A) N2A cell morphology in medium and in the presence of PA63. Cell apoptosis in medium and in the presence of PA63. (B) Intact nuclei stain blue with DAPI; apoptotic nuclei have green areas stained with TUNEL/green (arrows). (C) Percent spreading of N2A cultures in the presence and absence of PA63 (**P < .01; ***P < .001). DAPI indicates 4′,6-diamidino-2-phenylindole; N2A cells, neuroblastoma 2A cells; PA63, anthrax protective antigen 63; TUNEL, Terminal deoxynucleotidyl transferase-mediated dUTP Nick End Labeling assay.
Cell membrane permeability to PI/membrane repair assay
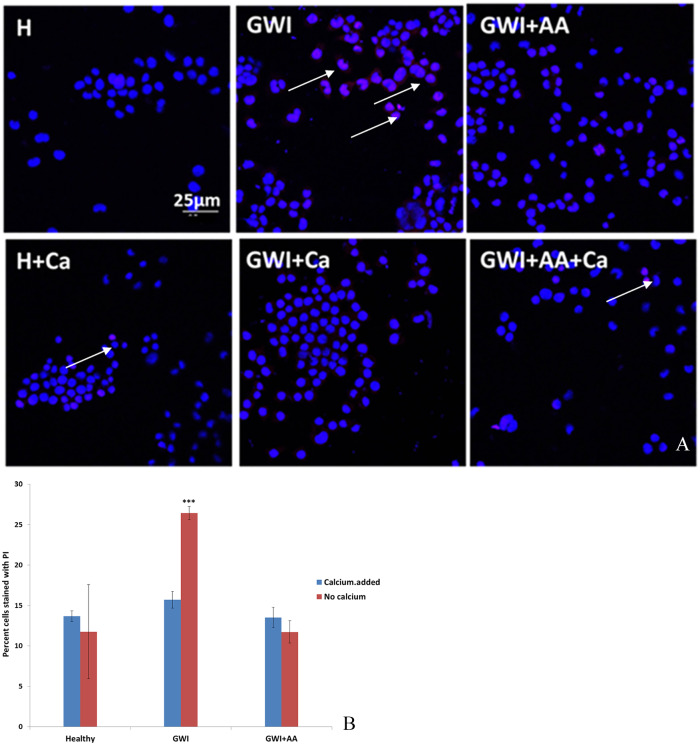
Neuroblastoma 2A cells cultured with healthy serum had 15% cells permeable to PI in the presence or absence of calcium. In the presence of GWI, more than 25% cells became permeable to PI; when calcium was added in the cultures, there was no difference in PI permeability in healthy or GWI-treated cultures. Moreover, PI permeability of N2A cells treated with GWI serum which was previously incubated in the presence of antibodies to anthrax was similar to healthy serum, in the presence or absence of calcium (Figure 2A and B).
Figure 2.
N2A cells cultured in the presence of healthy serum (H) or GWI serum (GWI) with and without CaCl2 (Ca) in the presence or absence of CaCl2. (A) More cells (intact nuclei stained blue with DAPI) became permeable to PI (red stain, arrows) in the presence of GWI serum compared with healthy indicating compromised ability to reseal their membranes; the addition of exogenous CaCl2 had a protective effect. (B) GWI increased the percentage of cells permeable to PI almost by 2× (***P < .001). The addition of CaCl2 prevented increased permeability to PI, as did the addition of antibodies to anthrax (AA) (B). DAPI indicates 4′,6-diamidino-2-phenylindole; GWI, Gulf War Illness; N2A cells, neuroblastoma 2A cells; PI, propidium iodide.
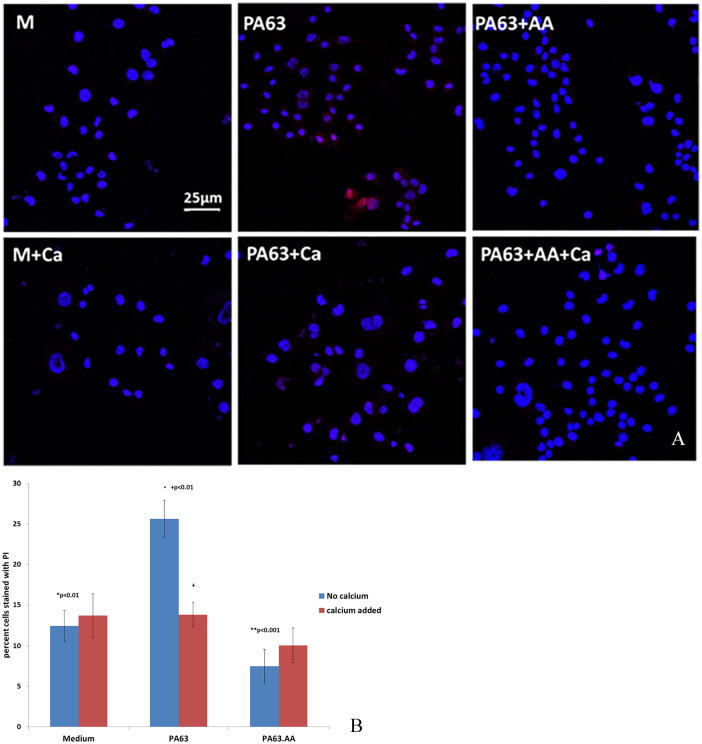
Moreover, when N2A cells were cultured in the presence of PA63, increased permeability to PI compared with cells cultured in medium was observed, similar to the presence of GWI, which was prevented by the addition of exogenous calcium. Neuroblastoma 2A cells cultured in medium had 15% cells permeable to PI in the presence or absence of calcium. In the presence of PA63, more than 25% cells became permeable to PI; when calcium was added in the cultures, there was no difference in PI permeability in medium or PA-treated cultures. Moreover, PI permeability of N2A cells treated with PA63 which was previously incubated in the presence of antibodies to anthrax was similar to medium, in the presence or absence of calcium (Figure 3A and B).
Figure 3.
N2A cells in the presence of medium (M) with and without CaCl2 (Ca) or PA63 in the presence or absence of CaCl2. (A) More cells (intact nuclei stained blue with DAPI) became permeable to PI (red stain) in the presence of PA63 compared with cells in medium indicating compromised ability to reseal their membranes; the addition of exogenous CaCl2 had a protective effect. PA63 increased percent cells permeable to PI by almost 2× (*P < .05). (B) The addition of CaCl2 prevented increased permeability to PI, as did the addition of antibodies to anthrax (AA) (**P < .01). DAPI indicates 4′,6-diamidino-2-phenylindole; N2A cells, neuroblastoma 2A cells; PA63, anthrax protective antigen 63; PI, propidium iodide.
Cytoskeletal organization: MAP2 and phospho-cofilin (p-cofilin) staining
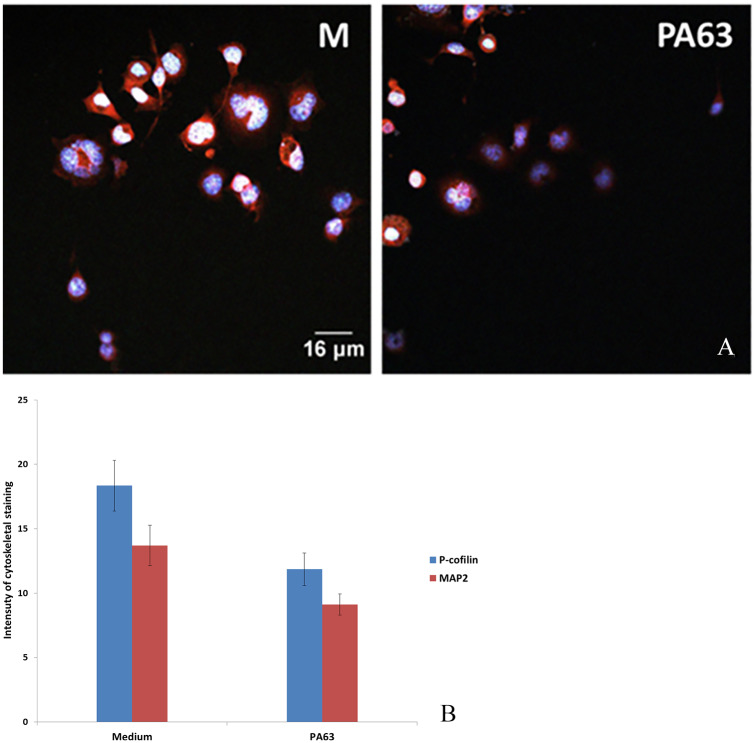
The organization of microtubular and actin networks was examined with anti-MAP2 (Alexa fluor green 488 nm) for microtubules and p-cofilin for actin (RFP red 588 nm). Neuroblastoma 2A cells in medium had approximately 30% staining intensity for MAP2 and p-cofilin combined, compared with cells in the presence of PA63 (Figure 4A and B).
Figure 4.
(A) N2A cells cultured in medium (M) and with PA63. Intensity of staining of MAP2 and p-cofilin combined. Cell nuclei are stained with DAPI (blue). (B) Intensity of staining for MAP2 and p-cofilin was ranked arbitrarily from 0 to 25. Intensity of staining for combined microtubular and actin networks was approximately 30% less in the presence of PA63 (B) (*P < .05). DAPI indicates 4′,6-diamidino-2-phenylindole; MAP2, microtubule-associated protein-2; N2A cells, neuroblastoma 2A cells; PA63, anthrax protective antigen 63.
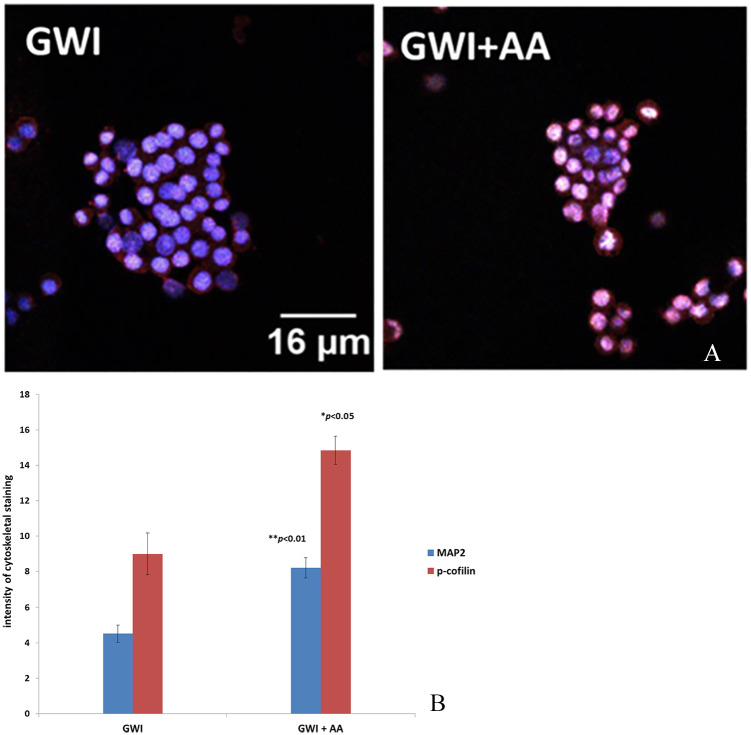
Moreover, microtubular and actin cytoskeletal staining was also significantly decreased in N2A cells exposed to of GWI serum but increased substantially in the presence of GWI preincubated with antibodies to anthrax (AA). Staining for MAP2 in the presence of GWI + anthrax antibodies increased about 2× for MAP2 and about 1.6× for p-cofilin compared with anthrax (Figure 5A and B).
Figure 5.
N2A cells cultured in the presence of GWI serum and GWI serum preincubated with anthrax antibodies (GWI + AA). (A) Intensity of staining of MAP2 (green) and p-cofilin (red) combined. Cell nuclei are stained with DAPI (blue). (B) Intensity of staining for MAP2 and p-cofilin was ranked arbitrarily from 0 to 18 (*P < .05). DAPI indicates 4′,6-diamidino-2-phenylindole; GWI, Gulf War Illness; MAP2, microtubule-associated protein 2; N2A cells, neuroblastoma 2A cells.
MMP assay
Neuroblastoma 2A cells in the presence of medium (M), healthy serum (H), GWI serum (GWI), and GWI serum preincubated with anthrax antibodies (GWI + AA) were examined for depolarization of their mitochondrial membrane, by assessing the mitochondrial potential. Membrane mitochondrial potential was decreased approximately 40% in the presence of GWI serum compared with the healthy serum; the presence of antibodies to anthrax had a protective effect, preventing loss of MMP (Figure 6).
Figure 6.
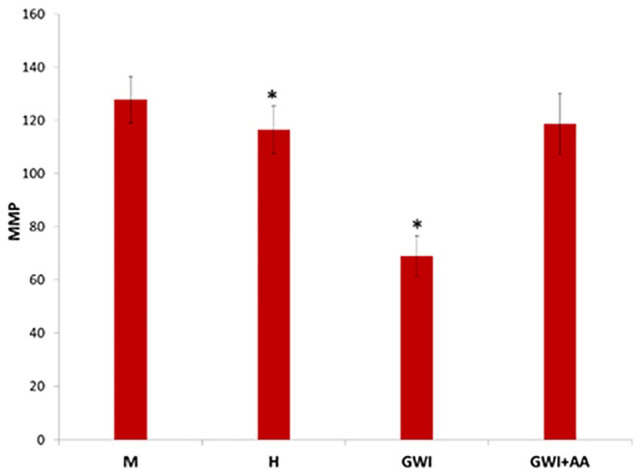
Mitochondrial membrane potential (MMP) assessed in N2A cells incubated for 24 hours in the presence of medium (M), 10% healthy serum (H) or GWI serum (GWI), and GWI serum incubated in the presence of anthrax antibodies. Mitochondrial membrane potential is shown as an arbitrary scale (*P < .01). GWI indicates Gulf War Illness; N2A cells, neuroblastoma 2A cells.
In addition, N2A cells cultured in the presence of PA63 had approximately 30% decrease of their MMP, compared with the control (medium). In the simultaneous presence of PA63 and anthrax antibodies, MMP was similar to control cells in medium (Figure 7).
Figure 7.
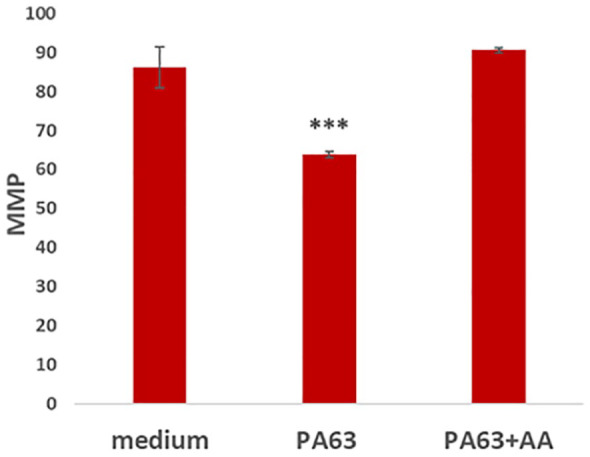
Mitochondrial membrane potential (MMP) of N2A cells cultured with medium, PA63, and PA63 plus anthrax antibodies. Mitochondrial membrane potential is shown with an arbitrary scale (*P < .0001). Neuroblastoma 2A cells indicates neuroblastoma 2A cells; PA63, anthrax protective antigen 63.
Discussion
Gulf War Illness is estimated to have affected 175 000 to 250 000 of the nearly 700 000 troops deployed to the 1990-1991 GW operations. The disease is characterized by a cluster of medically unexplained chronic symptoms that can include fatigue, headaches, joint pain, indigestion, insomnia, dizziness, respiratory disorders, and memory problems.3 Proposed causes have originally included exposure to chemical and microbiological agents and psychological factors. No definitive evidence has emerged to link chemical exposures to these symptoms, although epidemiological studies and animal research suggest some causal relationship with development of the disease.3 Although exposure to toxicants including toxic warfare chemicals and pesticides have been attributed to play a major role based on epidemiological and animal studies,24,25 recent reviews contemplated additional, multifactorial causes including genetic factors and the administration of multiple vaccines to Gulf War Veterans.26
It becomes apparent then that vaccines and genetics cannot be ignored as contributors to this chronic multisymptom disease. In our previous work, it was demonstrated that a cohort of 82 GWI patients lacked one or more 6 protective HLA alleles11 which are of paramount importance for the production of antibodies to various pathogens12; the severity of GWI symptoms was related to the number of missing alleles, in that patients with fewer protective alleles had more severe symptoms,11 thus strongly suggesting a genetic component in the development of GWI.
To explore mechanisms of GWI, we previously examined the effect of serum obtained from GWI patients lacking all 6 protective HLA class II alleles on N2A cultures. Differentiated N2A cells resembling neural cells in culture were selected as neurocognitive symptoms are a main symptom of GWI patients accompanied by structural9 and functional5 brain abnormalities in GWI patients.
We previously reported a compromising effect of GWI serum on primary cortical and N2A cells, which when exposed to serum from different GWI subjects, had increased network variability in electrophysiological readings, became aggregated, could not develop, and/or retracted processes and underwent apoptosis; these changes were not observed in N2A cultures exposed to healthy serum from a veteran with all 6 protective alleles. Moreover, the adverse effects of GWI serum were neutralized in the simultaneous presence of healthy serum.15 This finding suggests the presence of protective factors in the healthy serum.
The observed adverse effects of GWI serum on neural cells could be due to a number of reasons, including toxic or inflammatory factors. In a study by Johnson et al,27 500 patients with GWI were used to examine by proteomic analysis, inflammatory, and other factors associated with the disease. Despite a small albeit significant increase in C-reactive protein as an indicator of inflammation, none of the tested inflammatory cytokines were increased. Metalloproteinases MMP-2 and MMP-9 were also moderately increased, as in many inflammatory diseases28 indicating the presence of low-grade inflammation in GWI.
Gulf War Illness veterans, other than having been exposed to toxic environmental factors, had been administered 20 different vaccines4; hence, an inflammatory response could be anticipated. The ability to mount antibody titers in response to vaccines depends on genetic and other factors.29 A number of studies have correlated HLA class II alleles with the ability to mount antibodies to different vaccines, including the influenza30-32 and hepatitis B vaccines.33 The observed lack of protective HLA class II alleles in GWI patients11 might have prevented the formation of vaccine-neutralizing antibodies resulting in persisting pathogens.6,15
In this report, we addressed the effects of the anthrax vaccine which was administered to GW veterans and was reported to be toxic in several instances (“Biothrax”; https://www.rxlist.com/biothrax-drug.htm#indications and https://www.fda.gov/media/71954/download).34 This vaccine contains anthrax bacillus components, mainly the protective anthrax antigen 83 (PA83). In vivo PA83 is readily cleaved by cellular furin or serum proteases to generate the active 63 kDa fragment (PA63). Accordingly, this vaccine fragment should persist in GWI patients given their lack of ability to form neutralizing PA63 antibodies. Hence, we previously examined the effects of serum from 15 GWI patients lacking all 6 protective HLA class I alleles on N2A cells and observed that each of the tested GWI serum samples led to decreased cell spreading with loss of processes and greatly enhanced apoptosis compared with the healthy control; the addition of exogenous PA63 antibodies to each GWI serum led to remarkable protection by preventing morphological changes and apoptosis.17 These data implied the persistence of PA63 from the anthrax vaccine in the tested GWI serum samples.
Consequently, in this report, we examined the effects of exogenous PA63 added to N2A cultures instead of the anthrax vaccine which contained toxic aluminum.35 As expected, the presence of PA63 led to substantially compromised morphology and enhanced apoptosis of N2A cells, similar to cells exposed to GWI serum (Figure 1).
Active PA63 formed by enzymatic cleavage of PA83, an 83-kDa precursor polypeptide, after the latter binds to 2 cellular anthrax toxin receptors, ANTXR1 (TEM8, tumor endothelial marker 8) and ANTXR2 (CMG2, capillary morphogenesis protein 2). The 63-kDa fragment then self-associates to form a heptameric prepore which binds the other 2 anthrax toxins, edema factor (EF) and lethal factor (LF), and inserts into the cell membrane to create a channel for translocation of LF and EF into the cytoplasm by enzymatically disrupting the host cell.18,36 Although PA63 was considered to lack toxicity and only mediate the entry of the toxic EF and LF, it was found to be toxic to several cell types in culture, including Chinese hamster ovary cells. In cultures of these cells which express the TEM8 receptor, exogenously added PA63 made the plasma membrane permeable leading to apoptosis.37
This apoptotic mechanism should also apply to N2A cells binding PA63 through their integrin receptors.38,39 The TEM8 receptor also has a von Willebrand factor/integrin binding domain.40,41
In an animal model of GWI, loss of neurons has also been observed along with neuroinflammation in rats exposed to GW-related chemicals42; neuroinflammation markers in this animal model of GWI were also present in neurons, in addition to astrocytes and microglia, and could be detected in the blood, in neuron-derived extracellular vesicles.43 It appears then that irrespectively of the cause of brain cell damage, neuronal cells are affected resulting in cell apoptosis and neuronal loss.
Persistence of PA63 from the anthrax vaccine in GWI patients is a prerequisite for the apoptotic PA63-cell membrane interaction observed in vitro, possibly mirroring neuronal damage in vivo.
Indeed, antigens have been described to persist for prolonged times following immunization in lymphatic endothelial cells13 and lymphoid follicles.14 Persisting antigens could circulate in the blood stream of GWI patients for a prolonged time due to defective antibody formation and lead to compromised permeability of the blood-brain barrier44; the anthrax PA63 represents a persisting pathogen possibly present in these patients. In support of this hypothesis, our previous reports demonstrated (1) neutralization of GWI-induced adverse effects on neural cells in the simultaneous presence of GWI and healthy serum15; this protective effect suggested the presence in healthy serum of antivaccine antibodies which could neutralize antigens present in GWI patients; (2) a partial protective effect of pooled human IgG on N2A cell cultures exposed to GWI serum16; and (3) a remarkable protective effect on N2A cultures of GWI serum preincubated with anthrax antibodies.17
Although the exact mechanism of damage observed in N2A cell cultures from bacterial, viral, and other pathogens in GWI serum is not completely understood, a number of studies suggest that, among others, binding of PA63 on the cell membrane could occur, causing disruption and resulting in cell apoptosis.
Protective antigen was previously mentioned to form pores in membranes, as a cholesterol-dependent cytolysin (CDC) because of the requirement for cholesterol for pore formation45-47,50; these pores promote release of K+ from unilamellar phospholipid vesicles.46 In general, in healthy cells, perturbation of the cell membrane results in instant membrane repair and resealing; however, CDCs and other microbial toxins can result in more generalized cell toxicity by perturbing endoplasmic reticulum and mitochondrial membranes, disturbing electrolyte balance, and leading to cell apoptosis.45 Indeed, CDCs are capable of the lysis of a wide variety of nucleated cell types in vitro.48
Perturbation of the plasma membrane was indicated in our experiments as N2A cells exposed to PA63 became permeable to PI due to loss of ability to release calcium and instantly reseal their membrane. When exogenous calcium was supplied simultaneously with PA63, plasma membrane permeability was restored to control levels, confirming the compromised ability to provide calcium from lysosomal fusion for repairing the damaged membrane.20 Moreover, when PA63 was preincubated with anti-PA63 antibodies, PI access to N2A cells was similar to that of control cells cultured in the presence of medium.
Similarly, N2A cells exposed to GWI had substantially increased PI access intracellularly compared with healthy serum, and antibodies against PA63 incubated with GWI serum maintained normal membrane function (Figures 2 and 3).
Compromised membrane permeability results in cell damage often leading to apoptosis. In addition, the fact that N2A cells exposed to PA63 or GWI had few processes indicated impaired ability to form and stabilize microtubular and actin networks. This was confirmed by decreased MAP2 in N2A cells incubated with either PA63 or GWI serum, suggesting compromised microtubule stabilizing activity and damage to the microtubular network.21 Moreover, actin network stability was decreased as phosphorylation of cofilin (p-cofilin) was decreased suggesting impaired spreading process and impaired signaling from Rho kinase to the actin cytoskeleton through cofilin phosphorylation.22
Indeed, both MAP2 and p-cofilin were reduced in N2A cells exposed to PA63 or GWI; this decrease in cytoskeletal networks was prevented by antibodies to anthrax in both instances (Figures 4 and 5).
Finally, severe impairment of mitochondrial function was indicated by reduced MMP of N2A cells exposed to either GWI serum or PA63 (Figures 6 and 7), a defect linked to apoptosis.23 The presence of anthrax antibodies effectively protected the loss of mitochondrial integrity (Figures 6 and 7). Mitochondrial dysfunction observed in our in vitro system was corroborated in vivo by studies with peripheral blood mononuclear cells from veterans with GWI, as both mitochondrial DNA (mtDNA) lesion frequency and mtDNA copy number were elevated in these patients relative to controls. Moreover, greater mtDNA lesion frequency was associated with reduced enzyme activity for mitochondrial complex I.49
In summary, the obtained data strongly suggest the persistence of PA63 in the serum of GWI patients following vaccination with the anthrax vaccine. This possibility is reinforced by the observed substantial protective effect of specific polyclonal or monoclonal anthrax PA63 antibodies in structural and functional components of N2A cells. The mechanisms of damage include membrane perturbation resulting from interactions with PA63 and leading to abnormal membrane permeability, impaired MMP, and compromised signaling required for the organization and stability of actin and microtubule networks which impair the ability for process formation. These structural and functional changes which are induced by GWI serum eventually led to cell apoptosis. In vivo, this chain of events could be anticipated to lead to neuronal cell loss, as indicated by the previously described subcortical brain atrophy.9
As antibodies to anthrax PA could substantially protect from abnormal neural function in vitro, additional studies are warranted to establish whether these in vitro observations may provide strategies for in vivo intervention with GWI. The specific target is anthrax-persisting antigen(s) which could be neutralized or removed as a means to alleviate symptoms of the disease.
Acknowledgments
We gratefully acknowledge the support by the Minnesota American Legion Family Brain Sciences Foundation.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Partial funding for this study was provided by the University of Minnesota American Legion Family Brain Sciences Chair. The sponsors had no role in the current study design, analysis or interpretation, or in the writing of this paper.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ Note: The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Author Contributions: Conceptualization: A.P.G. and E.-P.C.T.; data curation: E.-P.C.T., E.P.S., M.K., and L.M.J.; formal analysis: E.-P.C.T. and A.P.G.; investigation: E.P.S., L.M.J., and B.E.E.; methodology: E.-P.C.T., E.P.S., M.K., and A.P.G.; project administration: E.-P.C.T.; visualization: E.-P.C.T.; writing-original draft: E.-P.C.T.; writing-review and editing: E.-P.C.T., E.P.S., M.K., L.M.J., B.E.E., and A.P.G. All authors have read and agreed to the published version of the manuscript.
ORCID iD: Apostolos P Georgopoulos  https://orcid.org/0000-0003-4412-725X
https://orcid.org/0000-0003-4412-725X
References
- 1. Fukuda K, Nisenbaum R, Stewart G, et al. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA. 1998;280:981-988. [DOI] [PubMed] [Google Scholar]
- 2. Kang HK, Li B, Mahan CM, Eisen SA, Engel CC. Health of US veterans of 1991 Gulf War: a follow-up survey in 10 years. J Occup Environ Med. 2009;51:401-410. [DOI] [PubMed] [Google Scholar]
- 3. Steele L. Prevalence and patterns of Gulf War illness in Kansas veterans: association of symptoms with characteristics of person, place, and time of military service. Am. J. Epidemiol. 2000;152:992-1002. [DOI] [PubMed] [Google Scholar]
- 4. Institute of Medicine, National Research Council. Gulf War and Health: Volume 1. Depleted Uranium, Pyridostigmine Bromide, Sarin, and Vaccines. Washington, DC: The National Academies Press; 2000. [PubMed] [Google Scholar]
- 5. Engdahl BE, James LM, Miller RD, et al. A magnetoencephalographic (MEG) study of Gulf War Illness (GWI). EBioMedicine. 2016;12:127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. James LM, Engdahl BE, Leuthold AC, Georgopoulos AP. Brain correlates of Human Leukocyte Antigen (HLA) protection in Gulf War Illness (GWI). EBioMedicine. 2016;13:72-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Georgopoulos AP, James LM, Carpenter AF, Engdahl BE, Leuthold AC, Lewis SM. Gulf War illness (GWI) as a neuroimmune disease. Exp Brain Res. 2017;235:3217-3225. [DOI] [PubMed] [Google Scholar]
- 8. Engdahl BE, James LM, Miller RD, et al. Brain function in Gulf War Illness (GWI) and associated mental health comorbidities. J Neurol Neuromedicine. 2018;3:24-34. [PMC free article] [PubMed] [Google Scholar]
- 9. Christova P, James LM, Engdahl BE, Lewis SM, Carpenter AF, Georgopoulos AP. Subcortical brain atrophy in Gulf War Illness. Exp Brain Res. 2017;235:2777-2786. [DOI] [PubMed] [Google Scholar]
- 10. James LM, Christova P, Engdahl BE, Lewis SM, Carpenter AF, Georgopoulos AP. Human leukocyte antigen (HLA) and Gulf War Illness (GWI): HLA-DRB1*13:02 spares subcortical atrophy in Gulf War veterans. EBioMedicine. 2017;26:126-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Georgopoulos AP, James LM, Mahan MY, Joseph J, Georgopoulos A, Engdahl BE. Reduced human leukocyte antigen (HLA) protection in Gulf War Illness (GWI). EBioMedicine. 2015;3:79-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mosaad YM. Clinical role of human leukocyte antigen in health and disease. Scand J Immunol. 2015;82:283-306. [DOI] [PubMed] [Google Scholar]
- 13. Tamburini BA, Burchill MA, Kedl RM. Antigen capture and archiving by lymphatic endothelial cells following vaccination or viral infection. Nat Commun. 2014;5:3989. doi: 10.1038/ncomms4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tew JG, Mandel TE. Prolonged antigen half-life in the lymphoid follicles of specifically immunized mice. Immunol. 1979;37:69-76. [PMC free article] [PubMed] [Google Scholar]
- 15. Georgopoulos AP, Tsilibary EP, Souto EP, James LM, Engdahl BE, Georgopoulos A. Adverse effects of Gulf War Illness (GWI) serum on neural cultures and their prevention by healthy serum. J Neurol Neuromedicine. 2018;3:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsilibary EPC, Souto EP, James LM, et al. Human immunoglobulin G (IgG) neutralizes adverse effects of Gulf War Illness (GWI) serum in neural cultures: paving the way to immunotherapy for GWI. J Neurol Neuromedicine. 2018;3:23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsilibary EPC, Souto EP, Kratzke M, James LM, Engdahl BE, Georgopoulos AP. Anthrax and Gulf War Illness (GWI): evidence for the presence of harmful anthrax antigen PA63 in the serum of veterans with GWI. J Neurol Neuromedicine. 2019;4:1-9. [Google Scholar]
- 18. Kurzchalia T. Anthrax toxin rafts into cells. J. Cell Biol. 2003;160:295-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moayeri M, Wiggins JF, Leppla SH. Anthrax protective antigen cleavage and clearance from the blood of mice and rats. Infect Immun. 2007;75:5175-5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Corrotte M, Castro-Gomes T, Koushik AB, Andrews NW. Approaches for plasma membrane wounding and assessment of lysosome-mediated repair responses. Methods Cell Biol. 2015;126:139-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dehmelt L, Halpain S. The MAP2/Tau family of microtubule-associated proteins. Genome Biology. 2004;6:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maekawa M, Ishizaki T, Boku S, et al. Signaling from rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895-898. [DOI] [PubMed] [Google Scholar]
- 23. Sakamuru S, Attene-Ramos MS, Xia M. Mitochondrial membrane potential assay. Methods Mol Biol. 2016;1473:17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. White RF, Steele L, O’Callaghan JP, et al. Recent research on Gulf War Illness and other health problems in veterans of the 1991 Gulf War: effects of toxicant exposures during deployment. Cortex. 2016;74:449-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Janulewicz P, Krengel M, Quinn E, et al. The multiple hit hypothesis for Gulf War Illness: self-reported chemical/biological weapons exposure and mild traumatic brain injury. Brain Sci. 2018;8:198. doi: 10.3390/brainsci8110198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mawson AR, Croft AM. Gulf War Illness: unifying hypothesis for a continuing health problem. Int J Environ Res Public Health. 2019;16:111. doi: 10.3390/ijerph16010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson GJ, Slater BC, Leis LA, Rector TS, Bach RR. Blood biomarkers of chronic inflammation in Gulf War Illness. PLoS ONE. 2016;11:e0157855. doi: 10.1371/journal.pone.0157855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nissinen L, Kähäri VM. Matrix metalloproteinases in inflammation. Biochim Biophys Acta. 2014;1840:2571-2580. [DOI] [PubMed] [Google Scholar]
- 29. Wiedermann U, Garner-Spitzer E, Wagner A. Primary vaccine failure to routine vaccines: why and what to do. Hum Vaccin Immunother. 2016;12:239-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lambkin R, Novelli P, Oxford J, Gelder C. Human genetics and responses to influenza vaccination. Am J Pharmacogenomics. 2004;4:293-298. [DOI] [PubMed] [Google Scholar]
- 31. Moss AJ, Gaughran FP, Karasu A, et al. Correlation between human leukocyte antigen class II alleles and HAI titers detected post-influenza vaccination. PLoS ONE. 2013;98:e71376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O E, Lee YT, Ko EJ, et al. Roles of major histocompatibility complex class II in inducing protective immune responses to influenza vaccination. J Virol. 2014;88:7764-7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Caillat-Zucman S, Gimenez JJ, Wambergue F, et al. Distinct HLA class II alleles determine antibody response to vaccination with hepatitis B surface antigen. Kidney Int. 1998;53:1626-1630. [DOI] [PubMed] [Google Scholar]
- 34. Little SF, Novak JM, Lowe JR, et al. Characterization of lethal factor binding and cell receptor binding domains of protective antigen of Bacillus anthracis using monoclonal antibodies. Microbiology. 1996;142:707-715. [DOI] [PubMed] [Google Scholar]
- 35. Müller JP, Bruinink A. Neurotoxic effects of aluminium on embryonic chick brain cultures. Acta Neuropathol. 1994;88:359-366. [DOI] [PubMed] [Google Scholar]
- 36. Goel AK. Anthrax: a disease of biowarfare and public health importance. World J Clin Cases. 2015;3:20-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taft SC, Weiss AA. Toxicity of anthrax toxin is influenced by receptor expression. Clin Vaccine Immunol. 2008;15:1330-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kadmon G, Imhof BA, Altevogt P, Schachner M. Adhesive hierarchy involving the cell adhesion molecules L1, CD24, and alpha 6 integrin in murine neuroblastoma N2A cells. Biochem Biophys Res Commun. 1995;214:94-101. [DOI] [PubMed] [Google Scholar]
- 39. Shigeta M, Shibukawa Y, Ihara H, Miyoshi E, Taniguchi N, Gu J. beta1,4-N-Acetylglucosaminyltransferase III potentiates beta1 integrin-mediated neuritogenesis induced by serum deprivation in Neuro2a cells. Glycobiology. 2006;16:564-571. [DOI] [PubMed] [Google Scholar]
- 40. Scobie HM, Rainey GJ, Bradley KA, Young JA. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci U S A. 2003;100:5170-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lacy DB, Wigelsworth DJ, Scobie HM, Young JA, Collier RJ. Crystal structure of the von Willebrand factor A domain of human capillary morphogenesis protein 2: an anthrax toxin receptor. Proc Natl Acad Sci U S A. 2004;101:6367-6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Parihar VK, Hattiangady B, Shuai B, Shetty AK. Mood and memory deficits in a model of Gulf War Illness are linked with reduced neurogenesis, partial neuron loss, and mild inflammation in the hippocampus. Neuropsychopharmacology. 2013;38:2348-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Madhu LN, Attaluri S, Kodali M, et al. Neuroinflammation in Gulf War Illness is linked with HMGB1 and complement activation, which can be discerned from brain-derived extracellular vesicles in the blood. Brain Behav Immun. 2019;81:430-443. [DOI] [PubMed] [Google Scholar]
- 44. Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cassidy SKB, O’Riordan MXD. More than a pore: the cellular response to cholesterol-dependent cytolysins. Toxins. 2013;5:618-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Collier RJ, Young JA. Anthrax toxin. Annu Rev Cell Dev Biol. 2003;19:45-70. [DOI] [PubMed] [Google Scholar]
- 47. Finkelstein A. The channel formed in planar lipid bilayers by the protective antigen component of anthrax toxin. Toxicology. 1994;87:29-41. [DOI] [PubMed] [Google Scholar]
- 48. Tweten RK. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect Immun. 2005;73:6199-6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen Y, Meyer JN, Hill HZ, et al. Role of mitochondrial DNA damage and dysfunction in veterans with Gulf War Illness. PLoS ONE. 2017;12:e0184832. doi: 10.1371/journal.pone.0184832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang S, Udho E, Wu Z, Collier RJ, Finkelstein A. Protein translocation through anthrax toxin channels formed in planar lipid bilayers. Biophys J. 2004;87:3842-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]