Abstract
Currently, there is a growing interest in Janus kinase (JAK) intracellular signalling since targeted inhibitors against these pathways are proving effective in the treatment of a range of immune-mediated diseases, such as rheumatoid arthritis (RA), psoriasis, psoriatic arthritis (PsA), inflammatory bowel disease and atopic dermatitis. In particular, post marketing experience and the increasing development of new pharmacological inhibitors of broad and increasingly selective JAK pathways provide new insights into the JAK pathway role in viral infections as well as their pathogenic role in immune-mediated inflammatory diseases. Herein we provide an overview of the biological role of JAK signalling and its role in immunity against viruses, with particular regard to herpes zoster reactivation. Thereafter, we will discuss the evidence currently available on the principal JAK inhibitors and their association with viral infections.
Keywords: AK, herpes zoster, immune-mediated diseases, inflammatory arthritis, JAK inhibitors, rheumatoid arthritis
Brief overview of the key functions of Janus kinases in the immune system concerning virus biology
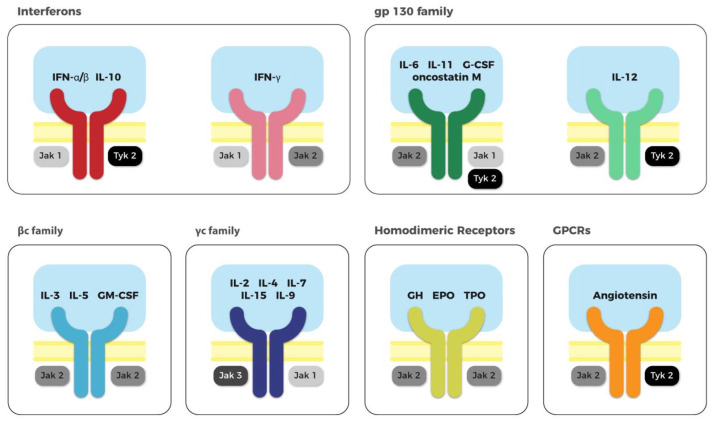
The Janus kinases (JAKs) are a family of intracellular proteins that mediate cytokine receptor signalling. The JAK family includes four tyrosine kinase members, named JAK 1, JAK 2, JAK 3 and TYK2 (Figure 1). JAKs engage the intracellular domains of distinct cytokine and growth factor receptors (Table 1), as homodimers or heterodimers. When a ligand binds its receptor, the receptor undergoes conformational change, bringing two associated JAKs closer together.1 The proximity of the JAKs permits auto-phosphorylation and trans-phosphorylation, resulting in their consequent activation. Activated JAKs phosphorylate other proteins to create a docking site for intracellular proteins containing Src Homology 2 (SH2) domain, such as the signal transducer and activator of transcription (STAT) family.2 The phosphorylation of intracellular proteins by JAK interactions engages a range of intracellular pathways, such as STATs, AKT, MAPK/ERK and phosphoinositide 3-kinase (PI3K). The result is the activation of transcription factors that regulate the transcription of selected genes. The intracellular signals transduced by the JAK pathways are vital for proliferation, differentiation, function and apoptosis of immune and haematopoietic cells. Genetic mutations of JAKs, either lack or gain of function, are implicated in several diseases, including severe haematopoietic disorders, myeloid and lymphoid proliferative syndromes, metabolic diseases and immune deficiencies.3–5 The role of JAKs in cell survival and effector functions are essential for innate and adaptive immunity as demonstrated in phylogenetic studies; the evolution of the JAK-STAT pathway occurred simultaneously with the development and diversification of the adaptive immunity suggesting that cytokine signalling is vital to drive the diversity of the immune system.6
Figure 1.
JAK family proteins are coupled with different membrane receptors.
The JAK family includes four tyrosine kinases: JAK 1, JAK 2, JAK 3 and TYK 2. Different member of the JAK family are engaged by the intracellular domains of distinct cytokines or growth factor. The receptors can be distinguished into six groups: interferon receptors, gp130 family, βc family, γc family receptors, homodimeric receptors and GPCRs. Every family can specifically bind different mediators. Successively to the ligand binding, the receptors dimerise (either as homodimers or heterodimers) allowing the activation of the associated JAK. Therefore, each mediator, cytokine or growth factor, preferentially transduce their intracellular signals throughout different JAKs, mediating different biological effects.
Table 1.
JAK family is associated to the intracellular portion of different transmembrane receptors family.
| JAK 1 | JAK 2 | JAK 3 | TYK 2 | Ligands using the receptors family | |
|---|---|---|---|---|---|
| IFNs | X | X | IFN α/β, IL-10 | ||
| X | X | IFN γ | |||
| gp130 family | X | X | X | IL-6, IL-11, G-CSF, oncostatin M, | |
| X | X | IL-12 | |||
| βc family | X | IL-3, IL-5, GM-CSF | |||
| γc family | X | X | IL-2, IL-4, IL-7, IL-9, IL-15 | ||
| Homodimeric receptors | X | GH, EPO, TPO | |||
| GPCRs | X | X | Angiotensin |
EPO, erythropoietin; G-CSF, granulocyte-colony stimulating factor; GH, growth hormone; GM-CSF, granulocyte-macrophage colony-stimulating factor; GPCRs, G protein-coupled receptors; IFN, interferon; IL, interleukin; JAK, Janus kinase; TPO, thrombopoietin.
The JAK-STAT pathway is implicated in regulation and differentiation of T and B lymphocytes and the monocyte/macrophage compartment.7 This is relevant for innate and adaptive immune responses to different pathogens. Briefly, the adaptive response to intracellular pathogens, such as viruses, is directed mainly by Th1 cells. The main cytokines responsible for Th1 polarisation are IFNγ and IL-12, whose intracellular pathways are mediated by JAK 1-2/STAT1 and JAK 2-TYK2/STAT4, respectively.8,9 Similarly, differentiation towards Th2 immunity, involved in the response against parasites in particular, is driven by IL-4, IL-5 and IL-9, while Th17 immunity, involved in extracellular bacterial and fungal responses, is driven by IL-23, IL-6 and IL-21. Therefore, many important T cell fate differentiation decisions are predicated on cytokines that act via JAK-STAT pathways.10,11 Interestingly, differentiation of Th2 and Th17 cells is possible without JAK signalling, whereas the immune response to intracellular pathogens is obligatorily dependent on JAK-STAT pathways.12 Additionally, CD8 T cells, essential in viral immune responses, require activation of JAK pathways to optimally exert their anti-viral functions.13,14 Similarly, both B cells and innate immune cells rely on JAK pathways to mature, differentiate and survive.15 Antibody production is also dependent on JAK/STAT intracellular signalling; accordingly, the inhibition of JAK3 and JAK1 can significantly inhibit the effective production of antibodies and the differentiation of B cells.16 Both innate and acquired immunity are essential to coordinate the response to viral infections, such as herpes zoster primary infection and reactivation. The key role of JAKs in immune responses to viruses, particularly herpes viruses, thus warrants specific attention.
Life cycle and pathogenesis of herpes virus/varicella zoster virus
There are eight herpes viruses that infect humans, namely herpes simplex virus (HSV) types 1 and 2, varicella-zoster virus (VZV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), human herpesvirus 6 (variants A and B), human herpesvirus 7 and Kaposi's sarcoma virus or human herpesvirus 8. Focusing on VZV, the virus almost exclusively infects humans; the primary infection usually affects children as varicella or chicken pox. A latent period usually follows primary infection, during which the VZV localises in sensory neurons of the cranial nerves and/or the dorsal root ganglia. Later in life, particularly in the setting of immune suppression, the infection can reactivate as zoster or shingles in the area innervated by the infected neurons.
The VZV life-cycle starts with entry into the host cell, initiated by the fusion of the virus envelop and cell membrane. The viral genome is transported to, and released into, the cell nucleus where it will be sequentially transcribed. Initially, the ‘immediate early’ and ‘early’ genes regulating transcription and DNA replication, are transcribed, followed by the ‘late’ genes, which have structural functions, for example, creating the viral capsid. Virus assembly occurs in the nucleus around the newly synthesised viral DNA, before egressing through the cell membrane.17 VZV can directly target T lymphocytes, epithelial cells and neurons in the ganglia, with associated clinical symptoms.18
After the primary infection, varicella virions probably reach the ganglia sensory neurons by retrograde axonal transport from affected skin areas. Herein, VZV becomes latent in nerve cell bodies; this latency can last for many years. The immune system has a key role in controlling herpes virus infection and in maintaining virus latency after the primary infection; cytokines such as the IFNs, TNF and IL-12, produced by monocytes and natural killer (NK) cells, are responsible for the early response to VZV infection by inducing VZV-specific T cells maturation, essential for the resolution of the primary infection and to control reactivations.19 In more detail, the VZV is able to inhibit STAT1 pathways, induced by IFN alpha and beta signalling, and upregulate the STAT3 transcription factor, which helps the replication and survival of the virus in host tissues. Unsurprisingly, STAT3 inhibition by small-molecule drugs were demonstrated to worsen clinical infection in animal models.20 In the event of immune suppression, such as in elderly people, or during the initiation of immune suppressive treatments, VZV can reactivate and target the skin dermatome(s) linked to the afferent nerve fibres from a single dorsal root of the affected ganglion spinal nerve where the virus started its latency. Both during primary infection and reactivation, DNA transcription and translation systems of infected keratinocytes are used by the virus to replicate its genome; the infected epithelial cells are finally lysed, allowing viral diffusion into the tissues of the infected host and, potentially, infection of other individuals. Infected keratinocytes are distressed, and in consequence produce IL-6, which induces the immune response and autophagy, and initiates mechanisms of tissue repair.21 VZV can interact with the host immune system, suppressing antigen presentation and the innate immune response.22,23 JAK family dependent functions are implicated in numerous steps in this viral life cycle pathway. Accordingly, there has been concern that inhibition of JAK-associated intracellular pathways could be associated with an increased susceptibility to primary infections or reactivation of latent viral infections, such as those caused by VZV.
JAKs inhibitors – overview
In light of their key role in innate and adaptive immunity, inhibitors of JAKs (JAKinibs) have been developed and are currently used to treat a range of advanced solid tumours, such as non-small cell lung carcinoma, renal cell carcinoma, melanoma, thyroid carcinoma and different gastrointestinal solid tumours, as well as several myeloproliferative disorders including chronic and acute myeloid leukaemia, chronic lymphocytic leukaemia and acute lymphoblastic leukaemia, mantle cell lymphoma, Waldenstrӧm’s macroglobulinaemia, mastocytosis, mast cells leukaemia and polycythaemia vera. Currently, JAKinibs are also indicated to treat chronic immune-mediated inflammatory diseases, such as rheumatoid arthritis (RA), psoriasis, psoriatic arthritis (PsA) and inflammatory bowel disease. The JAKinibs have a modulatory effect on cytokine signalling and thus on effector cell functions. The JAKinibs discussed here are summarised in Table 2.
Table 2.
Small molecules targeting JAK family in RA and their selectivity for different member of the family.
| Drug | Commercial Name | Target | IC50 |
|---|---|---|---|
| Tofacitinib | Xeljanz | JAK 3, JAK 1 > JAK 2 | 0.16 nM, 0.68 nM and 0.7 nM |
| Baricitinib | Olumiant | JAK 1 and JAK 2 | 0.8 nM and 0.99 nM |
| Upadacitinib | Jak 1 | 43 nM | |
| Filgotinib | Jak 1 | 10 nM | |
| Decernotinib | Jak 3 | 1.2 nM | |
| Peficitinib | Smyraf | Jak 3 >> Jak 1 | 0.7 nM and 3.9 nM |
IC50, inhibitory concentration 50%.
JAK, Janus kinase; RA, rheumatoid arthritis.
Tofacitinib (Xeljanz) was the first JAKinib approved by the United States (US) Food and Drug Administration (FDA) for the treatment of moderate to severe RA. Tofacitinib preferentially inhibits JAK 3, JAK 1 and, to a lesser extent, JAK 2, in a reversible manner. Tofacitinib demonstrated efficacy in reducing clinical symptoms and radiographic progression in RA, both combined with a conventional synthetic (cs) disease-modifying antirheumatic drug (DMARD) and as monotherapy.24–27 Tofacitinib is currently approved for the treatment of RA, PsA and severe ulcerative colitis in Europe. Baricitinib (Olumiant) is another reversible inhibitor of JAK/STAT intracellular signalling that is selective for JAK 1 and JAK 2. Baricitinib demonstrated clinical improvement and inhibition of radiographic progression in patients with RA who were non-responders or intolerant of csDMARDs or biological DMARDs.28,29 Moreover, baricitinib demonstrated equivalent efficacy to adalimumab in patients with active RA in non-responders to csDMARDs.30 Baricitinib appears promising in treating other immune mediated conditions, including psoriasis, atopic dermatitis and systemic lupus erythematosus (SLE).31–33
Next-generation agents are now emerging, with increased JAK selectivity. Upadacitinib (Rinvoq) is a selective JAK1 inhibitor. Recently, it has shown to be effective, in association with methotrexate (MTX) and as monotherapy, in patients with moderate-severe RA with inadequate response to csDMARDs and biologics.34–36 Furthermore, superiority of upadacitinib compared with adalimumab has been demonstrated in biologic-naïve patients with RA.37 Currently, the efficacy of upadacitinib in treating other immune disease, such as atopic dermatitis, ulcerative colitis, ankylosing spondylitis, Crohn‘s disease, SLE, vasculitis and PsA, is under investigation. Filgotinib is another selective inhibitor of JAK1. In patients with active RA, filgotinib demonstrated efficacy, as monotherapy and in combination with MTX, with a rapid onset of action and a good safety profile.38,39 Recently, the efficacy of filgotinib in treating RA patients after the failure of one or more biologic DMARDs has been demonstrated.40 Additionally, phase II clinical trials in PsA and ankylosing spondylitis demonstrated efficacy of filgotinib.41,42 Similarly, filgotinib demonstrated efficacy in active Crohn’s disease, with a satisfactory safety profile.43 Currently, active clinical trials are evaluating the efficacy of filgotinib in other autoimmune diseases including SLE, uveitis and other inflammatory bowel disease, such as ulcerative colitis and small bowel Crohn’s disease.
Decernotinib and peficitinib are selective inhibitors of JAK 3. Decernotinib was evaluated in RA as monotherapy and in combination with csDMARDs; despite demonstrating efficacy in controlling clinical symptoms and reducing inflammatory MRI findings, safety issues related to infections, lipid levels and transaminase alterations arose, as did a disadvantageous pharmacologic interaction.44–46 Peficitinib, which has a preferential selectivity for JAK 3 and JAK 1, was evaluated in patients with RA and ulcerative colitis. Despite a satisfactory response, a dose-dependent effect was not demonstrated in ulcerative colitis.47 In RA patients, peficitinib was effective48; however, no clear dose-dependent responses to peficitinib in combination with MTX emerged.49,50 In Japan, peficitinib (Smyraf) was approved for RA in 2019 as it demonstrated efficacy in reducing symptoms and radiographic damage in two phase III clinical trials.51,52 In patients with moderate-severe psoriasis, peficitinib demonstrated efficacy with an acceptable safety profile.53
Overall, JAKinibs appear to have an acceptable safety profile, with the most commonly reported adverse side effects being infections, such as laryngopharyngeal, respiratory and urinary tract infections, as well as mild hematologic and hepatic disorders, altered levels of cholesterol, and a probable increased risk of thrombosis. Inhibition of JAK2 may be associated with increased risk of anaemia, which was less frequently observed with more selective second generation JAKinibs. The latter may also be associated with fewer abnormalities in other laboratory values, particularly lipid profile and transaminases levels.54 However, comparison between first and second generation JAKinibs is still premature as clinical trials investigate different doses, head-to-head studies are still awaited and sufficient robust real-world data are not currently available for these drugs. Consequently, a direct comparison of the safety implications of different JAK selectivity is currently lacking. We can postulate that the risk of viral infections, such as varicella or zoster reactivation, might be considered mainly associated with JAK 1 and JAK 3 because they mediate the intracellular signalling and survival of immune cells and cytokines relevant to VZV control. The immune response requires the coordination of several specialised players, including Th1, CD8+ cells and B cells. All cells need the JAK/STAT intracellular signalling to mature and differentiate, but also to exert their activity. JAK 3 is expressed specifically in hematopoietic cells, and is mandatory for the differentiation of lymphocytes and also for transducing the signal different cytokines, that is, IL-2, IL-4, IL-7, IL-15, and IL-21. While JAK 1 and JAK 2 coordinate the response of Th1 cells associated with the receptors of several cytokines, such as IL-6, IL-12 and IFNs. Particularly important is IFN signalling, which, via JAK 1 (and JAK 2 for IFN-gamma only) and mostly STAT1, inhibits the intracellular virus replication directly, increases antigen presentation and, lastly, induces the differentiation CD8+ effector cells. Therefore, JAK 1 and JAK 3 signalling is essential for maturation and survival of Th1, CD8+ cells and B cells – key cells in the immune response against virus. Surely, the combined inhibition of more JAK signals is more likely to interfere with the lymphocyte function. The role of JAKs in the VZV response was particularly highlighted for tofacitinib, a preferential inhibitor of both JAK 1 and 3; however, clinical data, especially in real-life settings, are required to confirm or refute this hypothesis and will determine differences around safety and efficacy regarding the selective inhibition of JAKs.
HSV and JAKinibs in inflammatory rheumatic diseases
The data currently available from the phase II/III trial programmes and emerging long-term follow-up studies and observational reports suggest that JAKinibs are relatively safe and well tolerated overall. Consistent with other immunomodulatory drugs and the biologic mechanism of their targets, the JAKinibs are associated with an increased risk of infections compared with placebo.24,55,56 There is particular interest in herpes virus infections in light of the role of JAKs in the immune response to these viruses. The risk of zoster reactivation is already slightly increased in patients with immune-mediated rheumatic musculoskeletal diseases (RMDs), such as RA.57,58 Moreover, the risk of infection in these patients is also increased in association with immunosuppressive treatments, particularly corticosteroids, as well as age, ethnicity, diabetes, smoking, etc.59 Tofacitinib was the first licensed JAKinib in RA, so offers a good opportunity to study the long-term incidence of zoster and other infections.
In RA patients receiving tofacitinib, upper respiratory tract and urinary tract infections were the most commonly reported adverse events associated with tofacitinib, although serious opportunistic infections were also reported.60 In clinical trials, there was an increased risk of serious infection events and especially of HSV reactivation. In two clinical trials evaluating the efficacy of tofacitinib in RA patients, there was an increased rate of serious infections with tofacitinib compared with placebo; the most common of these adverse events were upper respiratory tract infections. Furthermore, 6 (out of 321; 1.9%) and 11 (out of 316; 3.5%) cases of localised herpes zoster infections were reported during 6–12 months treatment with tofacitinib 5 mg and 10 mg, respectively, in combination with MTX.24,25 Tofacitinib monotherapy showed a higher zoster infection rates compared with MTX alone: 13 of 373 patients (3.5%) and 18 of 397 patients (4.5%), with tofacitinib 5 mg and 10 mg, respectively, developed herpes zoster reactivation compared with only 2 out of 186 (1.1%) patients who received only MTX. Only in patients receiving tofacitinib 10 mg, were some cases of disseminated herpes zoster reported.26 In the ORAL study, comparing tofacitinib, as monotherapy or in combination with MTX, with adalimumab plus MTX, herpes zoster reactivation was reported in a total of 18 (2%) patients: 4 (1%) were receiving tofacitinib monotherapy, 8 (2%) tofacitinib in combination with MTX, and 6 (2%) were receiving adalimumab. In patients who received live attenuated zoster vaccination (Zostavax) (n = 216), the zoster reactivation rate was less that 1% of cases, with overall milder manifestations, compared with the rate of 2% in the patients who had not received vaccination (n = 930). The herpes zoster cases were mostly mild and localised. Note that in the tofacitinib monotherapy group, two patients developed multi-dermatomal disease and in one, serious varicella zoster course was reported, whereas, when combined with MTX, one case of serious herpes zoster was present; in the adalimumab and MTX group, one case of disseminated herpes zoster and one serious herpes zoster event occurred.27
Clinical trials data on the other key first generation JAKinib, baricitinib, reported a comparable risk for herpes zoster reactivation in RA patients. In a phase III clinical trial, baricitinib 2 mg and 4 mg in RA patients, both in combination or in monotherapy, was associated with localised zoster reactivation frequency of 2% (4/229) and 1% (3/227), respectively, by week 24, compared with none in the placebo group.28 In this study, none of the patients had received vaccination for zoster. In a similar study of baricitinib 2 mg and 4 mg in patients with RA, herpes zoster infections were reported more frequently in the 4 mg dose group (n = 7; 4%) compared with placebo and the 2 mg dose groups, which both had similar reactivation rates (both n = 2; 1%). Most of the patients who experienced a zoster infection had previously received at least three csDMARDs or biologic treatments. To note, all the cases were mild, with no disseminated infection.61 Additionally, in a cohort of patients with early RA naïve to biologics DMARDs, herpes zoster infection was reported in two (<1%), four (3%) and five (2%) for baricitinib 4 mg alone, MTX alone, or a combination of baricitinib and MTX, respectively; a higher incidence was observed in Japanese patients in the same cohort (in two (<1%), three (2%), and three (1.4%) patients, respectively).62 Notably in this study, there were no disseminated infections. In a study directly comparing baricitinib and adalimumab (both in combination with MTX) at 52 weeks, the herpes zoster infection rates were 2% in both groups, with most of the reported cases occurring in Asia. Only one case of zoster within the baricitinib group was multi-dermatomal.30
The efficacy and safety of more selective JAKinibs, such as upadacitinib, filgotinib, and also of the broader spectrum, peficitinib, is currently under investigation. In RA non-responders to MTX receiving different doses of upadacitinib (3, 6, 12, 18, 24 mg) up to 12 weeks, non-disseminated cases of zoster were reported in 2% (1 out of 50) and 4% (2 out of 49) of patients receiving 3 mg and 24 mg doses, respectively, compared with less than 1% of patient receiving placebo63; no cases of zoster infection were reported in the patients receiving intermediate doses of upadacitinib. In a larger clinical trial evaluating the efficacy of upadacitinib 15 and 30 mg in association with csDMARDs in RA patients with inadequate response to biologics, herpes zoster reactivation rates were 1% in both groups at 24 weeks (out of 156 and 148 patients, respectively), with two serious cases of zoster in the upadacitinib 30 mg arm. To note, none of the patients who had received zoster vaccination before the treatment initiation developed zoster infection.35 When administered as monotherapy, both upadacitinib 15 mg and 30 mg, demonstrated higher zoster reactivation rates, respectively 3 out of 217 (1%) and 6 out of 215 (3%), than MTX alone (1 out of 216; <1%); 2 cases in the 30 mg group involved 3 or more dermatomes; however, none of the cases was classified as serious.36 Filgotinib monotherapy was associated with a low rate of herpes zoster infection, with 1 mild case out 211 patients (0.5%) treated up to 24 weeks.38 A low rate of mild herpes zoster infection, 4 cases out of 508 (<1%) patients, was reported in patients treated with various doses of filgotinib in combination with MTX for 24 weeks.39 Filgotinib 100 mg and 200 mg in combination with csDMARDs for 24 weeks were associated with zoster infection rates of 1.4% (2/147) and 1.3% (2/153), respectively; all cases were uncomplicated and occurred in patients older than 55 years old.40 Recently, a study evaluating the efficacy of filgotinib in MTX naïve RA patients reported similar rate of herpes zoster infection with filgotinib monotherapy (0.5%, 1/210 patients) compared with MTX alone and combination therapy with filgotinib and MTX (0.5%, 2/416 in both groups).64 For the selective inhibitor of JAK3, decernotinib, herpes zoster infection was reported in 3 out of 163 patients (2.2%) who received decernotinib monotherapy up to 12 weeks.44 Decernotinib at various doses, in combination with MTX, for active RA for 24 weeks, was associated with a herpes zoster reactivation rate of 3.1% (6 cases), all the cases involved no more than one dermatome.45 Furthermore, peficitinib 100 mg and 150 mg, in combination with MTX, was associated with zoster reactivation in 2.4% and 1.3% of patients, respectively.47 In another study evaluating the efficacy of different doses of peficitinib in combination with selected csDMARDs, there were no reported cases of herpes zoster infection.46 In contrast, in two Japanese cohorts, peficitinib was associated with higher zoster infection rates (3.6–5.5%) compared with placebo (0–1.2%), although no dose-dependent association was observed and no multiple dermatomes or diffuse zoster infections were observed.51,52 The incidence rate of herpes zoster in the discussed clinical trials is summarised in Table 3.Long-term extension data from clinical trials further highlighted herpes zoster infection as an adverse event that deserves attention as it represents a frequent cause of drug discontinuation. A recent long-term extension study, ORAL Sequel, confirmed the long-term efficacy and safety profile of tofacitinib (5 and 10 mg) up to 9.5 years.65 The estimated incidence rate of herpes zoster infection in a total tofacitinib exposure of 16,291 person-years (PY) was 2.4/100 PY [95% confidence interval (CI) 1.9–2.8; n = 106/1298] for tofacitinib 5 mg and 10 mg monotherapy and, slightly higher, 3.6/100 PY (95% CI 3.2–4.0; n = 285/2464) for tofacitinib in combination with csDMARDs. The incidence rate of zoster reactivation in all patients was higher with tofacitinib 10 mg (3.7; 95% CI: 3.4–4.1) compared with the 5 mg dose (2.3; 95% CI: 2.3–3.3). The overall incidence rate for tofacitinib 10 mg in combination with csDMARDs was 4.1 (95% CI: 3.6–4.7). Most (96%) cases were mild, with few recurrent episodes. The risk of VZV infection associated with tofacitinib in combination with csDMARDs in patients with RA is higher in eastern Asian countries.66 In a previous long-term extension study in Japan, patients with RA treated with tofacitinib were reported to have a higher rate of herpes zoster infection compared with the global population; the overall incidence rate of serious and mild herpes zoster reactivation was 7.4 (95% CI: 6.0–9.1; n = 94/486), with higher rates with tofacitinib at 10 mg (8.6, 95% CI: 5.6–12.7).67 Another long-term extension study with a particular focus on Chinese patients revealed a herpes zoster incidence rate of 1.72 (95% CI: 0.74–3.39) for tofacitinib 5 mg and 1.51 (95% CI: 0.18–5.44) for the 10 mg dose; overall, these data showed a lower incidence compared with the pooled data from clinical trials and compared with the Japanese population.68
Table 3.
Summarised incidence and patients/100years exposure of herpes zoster infections from phase II and III clinical trials up to 24 months of treatment.
| Inhibitor and dose (+/– MTX and other csDMARDs) |
Patients tot n | Zoster cases n (%) |
Disseminated/serious
zoster n (%) |
Cases/100 patient-years | Ref. |
|---|---|---|---|---|---|
| Tofacitinib 10 mg | 713 | 29 (4.1) | NS | 2.6 | 23,24 |
| Tofacitinib 5 mg | 1,454 | 31 (2.1) | 4 (0.4) | 1.7 | 24,25 |
| PBO | 160 | 0 | 0 | 23 | |
| Methotrexate | 186 | 2 (1.1) | 0 | 0.54 | 24 |
| Adalimumab | 386 | 6 (1.6) | 2 (0.5) | 1.5 | 25 |
| Baricitinib 4 mg | 1,265 | 30 (2.4) | 1 (<0.1)$ | 2.6 | 26,28,60,61 |
| Baricitinib 2 mg | 403 | 6 (1.5) | 0 | 2.9 | 26,60 |
| PBO | 404 | 0 | 26,60 | ||
| Methotrexate | 689 | 4 (0.6) | 0 | 0.8 | 28,61 |
| Adalimumab | 330 | 5 (2) | 0 | 1.4 | 28 |
| Upadacitinib 15 mg* | 305 | 5 (1.6) | 0 | 2.9 | 34,62,63 |
| Upadacitinib 30 mg* | 488 | 9 (1.8) | 4 (0.8) | 5.4 | 34,62,63 |
| PBO | 219 | 1 (0.5) | 0 | 1.8 | 34,62,63 |
| Methotrexate | 216 | 1 (0.5) | 0 | 1.3 | 34 |
| Filgotinib 50 mg | 204 | 2 (1) | NS | 2.0 | 36,37 |
| Filgotinib 100 mg | 600 | 3 (0.5) | NS | 0.7 | 36–38,64 |
| Filgotinib 200 mg | 1012 | 8 (0.8) | NS | 1.2 | 36–38,64 |
| PBO | 276 | 1 (0.4) | NS | 0.7 | 36–38 |
| Methotrexate | 418 | 2 (0.5) | NS | 0.4 | 64 |
| Decernotinib 100 mg | 71 | 2 (2.8) | 0 | 5.6 | 43 |
| Decernotinib 150 mg | 72 | 1 (1.4) | 0 | 2.8 | 43 |
| Decernotinib 200 mg** | 144 | 3 (2.1) | 1 | 4.2 | 43 |
| PBO | 71 | 0 | 43 | ||
| Peficitinib 25 mg§ | 125 | 0 | NS | 0 | 46,47 |
| Peficitinib 50 mg§ | 135 | 0 | NS | 0 | 46,47 |
| Peficitinib 100 mg§ | 420 | 20 (0.5) | NS | 5.9 | 46,47,49,50 |
| Peficitinib 150 mg§ | 418 | 11 (2.6) | NS | 3.3 | 46,47,49,50 |
| PBO§ | 394 | 3 (0.8) | NS | 0.9 | 46,47,49,50 |
| Etanercept§ | 200 | 5 (2.5) | NS | 2.3 | 49 |
In most of the studies patients did not received vaccination. Upadacitinib data was calculated by adding also dosage of 6 mg and 12 mg twice daily equivalent of 15 mg and 30 mg, respectively.
PBO, placebo; NS, number not specified.
$the patient was receiving baricitinib 4 mg in association with MTX.
less than 5% add previous vaccination.
cumulative dose 200 mg, includes 200 mg once daily or 100 mg twice daily.
vaccination history not recorded.
The long-term extension study of baricitinib in RA (RA-BEYOND) [ClinicalTrials.gov identifier: NCT01885078] is on-going. Recently, this extension study reported an incidence rate of 2.5/100 PY when receiving a baricitinib dose of at least 4 mg for 128 weeks.69 A further extension study up to 52 weeks in a Japanese cohort indicated an overall higher risk of VZV reactivation during baricitinib treatment: 11 (7.8%) patients in the extension period developed herpes zoster infection with an incidence rate of 6.5.70
Among the selective JAKinib, the only data available are for a long-term extension study to 2 years for peficitinib. This extension of two global phase IIb trials in RA patients receiving peficitinib at various doses indicated an incidence rate of 1.5/100 PY for herpes zoster infections, including one herpes zoster ophthalmic infection.71
Integrated data from the various JAKinib clinical trials might give a broader insight regarding the risk of herpes zoster infection events in patients receiving JAKinibs with different selectivities. A recent meta-analysis of tofacitinib monotherapy and combination therapy in RA, indicated that the incidence rate of serious adverse events and discontinuation of treatment was lower in those receiving monotherapy compared with combination therapy (incidence rate for severe infective events for combination was 3.23 (95% CI: 2.55–4.03) compared with 1.65 (95% CI: 1.07–2.44) for combination therapy. Similarly, the incidence rate for VZV reactivation with tofacitinib monotherapy (2.48, 95% CI: 1.74–3.42) was lower than that reported for the combination group (4.51, 95% CI: 3.69–5.46). The zoster infections were limited in distribution and were responsive to antiviral treatment. Interestingly, the risk of developing herpes zoster infection was reduced in patients not previously exposed to glucocorticoids, independently of whether they received tofacitinib alone or in combination with csDMARDs.72 A pooled analysis confirmed that the risk for herpes zoster reactivation is increased when tofacitinib (5 mg or 10 mg) is used in combination with both csDMARDs and corticosteroids compared with monotherapy, with significantly high crude incidence rates of 4.82 versus 0.56 and 5.44 versus 2.19 for tofacitinib 5 mg and 10 mg, respectively.73 Corticosteroids appear to play a major role, and are estimated to approximately double the risk of VZV infection in patients treated with tofacitinib.74 Moreover, two recent meta-analysis reported that the overall risk of serious infectious adverse events in RA patients treated with tofacitinib is similar to the risk associated with the use of biologic DMARDs, including TNF inhibitors, abatacept, rituximab and tocilizumab.75,76
The incidence of VZV reactivation has also been evaluated in RA patients treated with baricitinib. In a meta-analysis, the incidence rate of VZV was 3.2/100 PY (95% CI 2.8–3.7); similar to that of tofacitinib, the zoster infection was usually mild, with no cases of disseminated disease.77 Pooled data from RA clinical trials and one ongoing long-term extension study recently reported higher exposure-adjusted incidence rates for VZ infections up to 5.5 years for baricitinib than placebo, similar to what was reported for tofacitinib.78 In particular, the herpes zoster incidence rate for baricitinib 4 mg (4.3) was significantly higher than the incidence rate in the placebo group (1.0), and higher for 4 mg (3.8) than for the 2 mg dose (2.7).78 As for tofacitinib, integrated analysis indicated that Japanese patients with RA treated with baricitinib had higher herpes zoster infection incidence rates (6.5, 95% CI: 4.9 ‒8.4) compared with RA patients overall (3.2, 95% CI: 2.8–3.7); of note, the majority of cases were mild, with few moderate infections involving more than one dermatome.79 In another meta-analysis, the odds ratio (OR) for serious adverse events, serious infections, and herpes zoster infection was estimated from five clinical trials; interestingly, although baricitinib was not associated with an increased risk of overall infections, the OR associated with herpes zoster was significantly higher than that of placebo (2.34, 95% CI: 0.27–20.47).80
Recently, a study compared the incidence rates of infections and VZV reactivation in RA patients treated with tofacitinib, baricitinib or upadacitinib using pooled data from clinical trials.81 Interestingly, the estimated incidence rate ratio of serious infections was not significantly different for these JAKinibs compared with placebo. However, baricitinib, but not tofacitinib or upadacitinib, was associated with a higher risk of developing VZV compared with placebo (incidence rate ratio 2.86).81
The differential geographical distribution of VZV reactivation in RA patients treated with tofacitinib has been highlighted; Japan and Korea have the highest incidence rates of zoster reactivation equal to 8.1/100 PY (95% CI: 7.0–9.4), followed by Australia, New Zealand and other Asian countries.66,73 Recently, a post hoc analysis of pooled data confirmed the higher susceptibility to zoster in the Asia-Pacific population compared with the global population [incidence rate (IR) (95% CI) of 0.7 (0.5, 1.0) versus 0.3 (0.2, 0.3), respectively].82 In contrast, population studies in Latin America treated with tofacitinib reported lower herpes zoster infection rates in this ethnic group compared with the global population [3.39 (2.68–4.29) versus 4.39 (4.00–4.83), respectively].83
Real-world data based on the global safety database for tofacitinib collected over 3 years following marketing authorisation did not highlight new safety risks on estimated exposure of 34,223 PYs.84 Specifically, the reported percentage of herpes zoster cases (2.2%) is consistent with the previously reported data. Currently, a post marketing national register for tofacitinib is in progress in Japan; a short-term report described an incidence rate of 6.81/100 PY, consistent with the data from clinical trials.85 Administrative health plan data from the US revealed a higher incidence rate for herpes zoster infection in patients treated with tofacitinib compared with biologics (3.87 versus 1.95–2.71 per 100 PY).86 This study specifically compared the risk of herpes zoster occurrence in RA patients treated with tofacitinib or with biologic agents, including anti-TNFs, abatacept, rituximab and tocilizumab, in a real-life setting; interestingly, the risk of zoster during tofacitinib treatment was roughly double the risk observed with the biologics drugs.86 Several risks factors, including age, sex, use of corticosteroids, history of infections and hospitalisations were reported to be associated with a higher risk of zoster occurrence; the concomitant use of corticosteroids and tofacitinib further doubled the risk of VZV occurrence, independent of MTX use.86
The higher susceptibility to develop herpes zoster infections in RA Japanese patients was also confirmed for baricitinib in a meta-analysis of clinical trials and long-term extension studies. Specifically, the occurrence of herpes zoster was higher in patients receiving baricitinib 4 mg compared with the placebo group or patients receiving MTX or adalimumab.87 In a 52 week extension of a study investigating the efficacy of baricitinib exclusively in Japanese patients with active RA, herpes infections were reported in 11 patients out of 141 (7.8%), representing the most common cause for discontinuation of baricitinib in this cohort. The reasons of the observed differences of herpes infections with JAKinibs between ethnic groups are currently unknown; different genetic polymorphisms might be relevant. Vaccination for herpes zoster was demonstrated to be protective and to prevent reactivation of the disease.27 In Japan, both varicella and zoster vaccination approvals came later compared with European countries. It can potentially contribute to the increased incidence in Japan, explained with reduced herd immunity and reduced access to specific vaccination programs. However, this is a pure speculative consideration as the population sample is very small, and registry data on the vaccination coverage are needed to validate it.
This evidence highlights the need for tailored guidelines in specific countries related to local herpes zoster occurrence rates, as well as the need for more comprehensive real-life data from different population studies. It should also be noted that data from clinical trials and extension studies in patients receiving various doses of tofacitinib indicate that the overall risk of serious infections and the specific risk of VZV infection is higher in subjects older than 65 years.88
Currently, there are no data available about the safety profile of the new selective JAKinibs in real world settings; these data will provide further insights regarding the possible mechanism behind the increase risk of VZV in patients treated with JAKinibs.
In summary, while JAKinibs are generally safe and well tolerated, they can be associated with adverse reactions such as infections, including opportunistic agents. VZV reactivations are usually mild and responsive to antiviral therapy. The data are still evolving, especially in real-world settings and for the new selective JAKinibs. Therefore, further studies, including long-term observational studies, are needed to define the risk of adverse events, particularly in high-risk populations.
Herpes virus infections and JAKinibs in other immune-mediated diseases
JAKinibs have been approved or are currently under investigation for the treatment of other immune-mediated diseases in various fields including dermatology, gastroenterology and rheumatology. The enlarged population of patients treated with JAKinibs will expand the experience concerning herpes zoster reactivation and help in understanding the underlying mechanisms.
Tofacitinib has been recently approved for the treatment of PsA. In both biologic naïve patients and inadequate responders to anti-TNF treatment, tofacitinib at 5 mg and 10 mg twice daily was reported to be superior to placebo and non-inferior to adalimumab. In both clinical trials, the frequency of herpes zoster was up to 2% in the tofacitinib-treated group compared with no cases in the placebo and adalimumab control groups. One case in each study was evaluated as opportunistic infection with at least three dermatomes involved.89,90 Filgotinib and upadacitinib are currently under investigation for the treatment of active PsA. In a phase II clinical trial of filgotinib in PsA, one non-complicated cases of herpes zoster (1 out of 65 patients; 2%) was reported in this study.41
In dermatology, JAKinibs, have demonstrated efficacy in both oral and topical formulation, for treating different conditions such as psoriasis, atopic dermatitis and alopecia areata. Tofacitinib was shown to be effective in treating cutaneous psoriasis. A recent long-term extension study from phase II and III clinical trials reported that episodes of herpes zoster occurred in 176 (6.1%) patients out of 2867 patients treated with tofacitinib 10 mg and/or 5 mg for up to 66 months. Only seven cases were reported as severe and two of them presented as multidermatomal.91 Two phase II clinical trials investigating the efficacy of baricitinib and peficitinib in the treatment of moderate and severe psoriasis reported no cases of herpes zoster.31,53
In patients with atopic dermatitis, a phase II clinical trial of topical tofacitinib reported no herpes zoster or herpes simplex infections; however, the topical formulation and the short 4-week duration of the administration might explain the absence of this adverse event.92 In a 16-week phase II clinical trial of oral baricitinib in patients with moderate–severe atopic dermatitis, a single patient in the baricitinib 4 mg treatment arm developed herpes zoster.32 In a study of the selective JAK1 inhibitor, upadacitinib, in patients with atopic dermatitis, none of the 126 patients treated with upadacitinib for 16 weeks developed herpes zoster.93
In clinical trials investigating the efficacy of tofacitinib in alopecia areata, only one case of limited herpes zoster was reported in the 78 patients treated in these clinical trials.94,95 There are only a few case reports of baricitinib in the treatment of alopecia areata, with no complications of herpes zoster reported to date.96 The selective JAK1 and JAK2 inhibitor, ruxolitinib, was assessed in an open-label study in 12 patients for alopecia areata, with no cases of herpes zoster reported.97
In Crohn’s disease patients treated with tofacitinib 5 mg (85), 10 mg (86, or placebo (90), only two cases of localised herpes zoster were reported in the 10 mg group (3.3%).98 In the 48-week extension study, mild herpes zoster infections occurred in two patients in each tofacitinib group (5 mg = 62 patients; 10 mg + 88 patients). One case of primary varicella infection was reported in the tofacitinib 10 mg group. In addition, in the 5 mg group, there was also a case of disseminated zoster that was considered mild and treated with anti-viral treatment without suspending the tofacitinib treatment.99 In a phase II study in patients with active Crohn disease, filgotinib was well tolerated and only one case of herpes zoster was reported in a group a patients receiving filgotinib 200 mg for 10 weeks and 100 mg for the following 10 weeks (3%); in the 77 patients receiving filgotinib 200 mg consecutively for 20 weeks, there were no cases of zoster reported.43
A study recently investigated the risk of developing herpes zoster reactivation in patients with ulcerative colitis treated with tofacitinib by analysing data from different clinical trials and long-term extension studies; of a total of 1157 patients treated with tofacitinib, 65 cases of zoster were found (5.6%). Up to 74% of the cases were mild, with the remaining cases multi-dermatomal or disseminated; among those with disseminated disease, there was a case of invasive HZ encephalitis, recovered with intravenous and oral antiviral treatment.100
Implications for screening and vaccination
The first VZV vaccination was a live attenuated vaccine (Zostavax), with two scheduled doses, that become available in the early 1990s. This attenuated vaccine induces immunity effective at reducing primary varicella infections.101 The vaccine is generally safe and well tolerated – infections associated with the vaccine have been described only in severely immunosuppressed patients.102 This live attenuated vaccine, in higher concentration, is also available to prevent the latent virus reactivation, usually manifesting clinically as herpes zoster, and is widely available for people older than 60 years, with good safety and efficacy, and is generally well tolerated.103 Currently, zoster vaccination in patients with inflammatory arthritis is recommended over the age of 60 years old (as in the general population) unless relevant contraindications or precautions are present.104 The relevant European League against Rheumatism (EULAR) and American College of Rheumatology (ACR) guidelines suggest vaccinating patients with RA105,106; however, as this is a live vaccine, it is not recommended during biologic or JAKinib treatment. Rather it is suggested to start biologic or JAKinib treatment at least 2–4 weeks after live attenuated zoster vaccination.107 Low doses of immunosuppressant such as MTX (<0.4 mg/kg/week), do not represent a contraindication to the administration of the live zoster vaccine. However, in routine clinical practice, the vaccination is not widely used in patients with inflammatory arthritis as they may be receiving more than one immunosuppressive treatment, or as administration of the live vaccine would lead to delay in starting of new treatment required for arthritis, or occasionally itself cause the reactivation of herpes zoster symptoms in immune-suppressed patients.
The recent update of the EULAR recommendations for vaccination recommend considering the use of herpes zoster vaccination in high-risk patients with RMDs. Despite the EULAR task force on vaccination general recommendation to avoid live attenuated vaccines in immunosuppressed RMD patients, varicella and herpes zoster vaccine are exceptions and should be considered on a case-by-case basis.106 The live zoster vaccine was demonstrated to be safe and effective in a cohort of 54 RA patients older than 50 years, when administered up to 3 weeks before starting tofacitinib.108 The risk of zoster reactivation with tofacitinib started 2–3 weeks after attenuated vaccination was very low and judged to be acceptable – the study also indicated that the vaccine was effective in inducing effective immunity. During this study, only one patient developed primary disseminated varicella consequent upon lack of pre-existing immunity; this infection was responsive to antiviral therapy and resolved after discontinuation of tofacitinib.108
Further studies are needed to confirm these data with regards to other JAKinibs while large prospective trials sufficiently powered to assess the safety of this live-attenuated vaccine are still lacking. A recent study indirectly estimated the effect of live zoster vaccination administered in a clinical trial cohort, before receiving tofacitinib monotherapy, tofacitinib plus MTX or adalimumab plus MTX, and calculated incidence rates of zoster infection in the non-vaccinated patients of 1.0, 2.2 and 2.1, respectively, compared with 1.5, 3.0 and 0, respectively, in the vaccinated RA patients.109
Recently, a recombinant VZV vaccine (Shingrix), containing surface protein and adjuvants, has been developed.110 This vaccine was shown to induce an effective immune response and appears cost-effective compared with the live attenuated vaccine.111,112 The recombinant vaccine represents a safe alternative for immunosuppressed patients in whom the previous attenuated vaccine is contraindicated.113 The recent EULAR guidelines on vaccination include the possibility to use the recently approved non-live recombinant zoster vaccine, when commercially available, as it demonstrated to be equally effective although safer than the live vaccine in immunosuppressed adults.108,113
Currently, routine screening for previous exposure to HVZ of asymptomatic subjects is not explicitly recommended in the ACR treatment guidelines for RA.105 In contrast, documenting the history of chronic viral infections, including varicella-zoster, and the vaccination status is recommended in all patients with chronic inflammatory rheumatic diseases in Europe.114 As a note of prudence, the recent EULAR vaccination task force advised documenting varicella zoster serostatus before administration of the live attenuated zoster vaccine, which is more potent than the varicella vaccine, in order to avoid primary infection after vaccination.106 In clinical practice, medical history is often used to decide whether screening patients for chronic viral infections is indicated; commonly, screening for antibodies against varicella is performed when prior infection status is not certain. Moreover, pre-screening for varicella may potentially not be cost-effective or time effective, potentially incurring increased healthcare costs and delayed treatment start. A systematic literature review investigating the risk of zoster occurrence in rheumatologic patients receiving only MTX estimated that there is no association of MTX and VZV infection in RA patients, such that the authors declare themselves against pre-MTX screening for varicella.115
Accordingly, serological screening and vaccination against herpes zoster are commonly underused in patients with inflammatory arthritis.116 As highlighted before, treatment with MTX appears to be associated with a low risk of VZV reactivation, while different biologic treatments seem to carry a similar risk for serious infections compared with tofacitinib in clinical trial settings.76 In contrast, tofacitinib appears to roughly double the risk of zoster infections when compared with biologic DMARDs, including anti-TNFs, abatacept, rituximab and tocilizumab in real-life analysis,86 particularly when administrated in combination with corticosteroids.74 It remains controversial whether routine pre-treatment screening of patients with rheumatic disease who are about to start a new treatment should be routinely performed. However, the high zoster reactivation rate associated with JAKinibs highlights the need for increased vigilance in these patients. It remains to be established whether the use of routine screening and vaccination reduces the risk of VZV infection, and is cost-effective, in patients receiving JAKinibs in real world settings.109 There are no current treatment recommendations regarding the clinical approach in cases of reactivation during JAKinib treatment.
Conclusion
Patients with inflammatory RMDs are often immunocompromised, by both the disease itself and by immunomodulatory therapies, putting them at higher risk of opportunistic infections, including VZV. Several risks factors for zoster infections, such as medications, age, ethnicity, diabetes and sex, can be readily captured on clinical history, allowing identification of high-risk patients and helping to guide treatment choices. The JAKinibs are promising new treatments for a range of immune-mediated inflammatory diseases. However, the incidence of herpes zoster appears higher than with the current biologic agents and csDMARDs. JAKinibs have a wider pleiotropic biological effect than current biologic agents; compared with the inhibition of one single cytokine or cytokine receptor, JAKinibs are able to simultaneously suppress the action of different cytokines, even if transitorily. This intrinsic characteristic of the JAKinibs can explain the higher risk of zoster compared with other biologic drugs. Although the risk of herpes zoster in phase II/III clinical trials appears similar for the various JAKinibs, further studies are needed to appropriately determine the safety profile of JAKinibs with different selectivities and at various doses. Emerging and pooled data may suggest that inhibition of JAK 2 and JAK 3 may be associated with a higher risk of VZV infection, while selectivity for JAK 1 may be associated with a lower risk of zoster infection. In particular, JAK 3 inhibition is theoretically able to inhibit CD8 and B cells maturation and to unleash the herpes zoster reactivation. However, the ability of different JAKinibs to inhibit JAK 2 appears to be associated with a slightly higher risk of VZV reactivation, as suggested in the pooled data comparing tofacitinib, upadacitinib and baricitinib. It is still early to point which is the JAK associated with the highest risk of zoster reactivation, so greater attention will be required to investigate this further in the next years. In fact, data are still evolving and derived mainly from meta-analysis of phase II/III clinical trials. Real-world longitudinal data with larger numbers of patients treated with different JAK selectivity are required to better define the safety of JAKinibs, particularly relating to the prevalence of VZV infections and to determine the role of screening and vaccination to prevent its occurrence.
Footnotes
Conflict of interest statement: FS: nothing to disclose. IBM has received honoraria or research funding from Abbvie, Lilly, Pfizer, BMS, UCB and Celgene. SS has received honoraria or research funding from Abbvie, Pfizer, Janssen, BMS, UCB, Celgene, Boehringer Ingelheim and Novartis
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Iain McInnes  https://orcid.org/0000-0003-4449-8501
https://orcid.org/0000-0003-4449-8501
Contributor Information
Flavia Sunzini, University of Glasgow College of Medical Veterinary and Life Sciences, Glasgow, UK.
Iain McInnes, University of Glasgow College of Medical Veterinary and Life Sciences, 120 University Place, Glasgow, G12 8TA, UK.
Stefan Siebert, University of Glasgow College of Medical Veterinary and Life Sciences, Glasgow, UK.
References
- 1. Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994; 264: 1415–1421. [DOI] [PubMed] [Google Scholar]
- 2. Levy DE, Darnell JE., Jr. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 2002; 3: 651–662. [DOI] [PubMed] [Google Scholar]
- 3. Jatiani SS, Baker SJ, Silverman LR, et al. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer 2010; 1: 979–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med 2013; 368: 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Minegishi Y, Saito M, Tsuchiya S, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 2007; 448: 1058–1062. [DOI] [PubMed] [Google Scholar]
- 6. Liongue C, O'Sullivan LA, Trengove MC, et al. Evolution of JAK-STAT pathway components: mechanisms and role in immune system development. PLoS One 2012; 7: e32777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal 2017; 15: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schroder K, Hertzog PJ, Ravasi T, et al. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 2004; 75: 163–189. [DOI] [PubMed] [Google Scholar]
- 9. Bacon CM, McVicar DW, Ortaldo JR, et al. Interleukin 12 (IL-12) induces tyrosine phosphorylation of JAK2 and TYK2: differential use of Janus family tyrosine kinases by IL-2 and IL-12. J Exp Med 1955; 181: 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O'Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity 2008; 28: 477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Z, Laurence A, O'Shea JJ. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Seminars Immunol 2007; 19: 400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Panhuys N, Tang S-C, Prout M, et al. In vivo studies fail to reveal a role for IL-4 or STAT6 signaling in Th2 lymphocyte differentiation. Proc Natl Acad Sci. 2008; 105: 12423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tripathi P, Kurtulus S, Wojciechowski S, et al. STAT5 is critical to maintain effector CD8+ T cell responses. J Immunol 2010; 185: 2116–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cui W, Liu Y, Weinstein JS, et al. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity 2011; 35: 792–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 2012; 36: 542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Onda M, Ghoreschi K, Steward-Tharp S, et al. Tofacitinib suppresses antibody responses to protein therapeutics in murine hosts. J Immunol 2014; 193: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wolff MH, Schünemann S, Schmidt A. (eds). Varicella-zoster virus. Molecular biology, pathogenesis, and clinical aspects (Contributions to Microbiology). Vol. 3 Basel: Karger, 1999, pp. 21–42. [Google Scholar]
- 18. Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers 2015; 1: 15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Torigo S, Ihara T, Kamiya H. IL-12, IFN-gamma, and TNF-alpha released from mononuclear cells inhibit the spread of varicella-zoster virus at an early stage of varicella. Microbiol Immunol 2000; 44: 1027–1031. [DOI] [PubMed] [Google Scholar]
- 20. Zerboni L, Sen N, Oliver SL, et al. Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev Microbiol 2014; 12: 197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jarosinski KW, Carpenter JE, Buckingham EM, et al. Cellular stress response to varicella-zoster virus infection of human skin includes highly elevated interleukin-6 expression. Open Forum Infect Dis 2018; 5: ofy118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abendroth A, Slobedman B, Lee E, et al. Modulation of major histocompatibility class II protein expression by varicella-zoster virus. J Virol 2000; 74: 1900–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vandevenne P, Lebrun M, El Mjiyad N, et al. The varicella-zoster virus ORF47 kinase interferes with host innate immune response by inhibiting the activation of IRF3. PLoS One 2011; 6: e16870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fleischmann R, Kremer J, Cush J, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012; 367: 495–507. [DOI] [PubMed] [Google Scholar]
- 25. van der Heijde D, Tanaka Y, Fleischmann R, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month Phase III randomized radiographic study. Arthritis Rheum 2013; 65: 559–570. [DOI] [PubMed] [Google Scholar]
- 26. Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014; 370: 2377–2386. [DOI] [PubMed] [Google Scholar]
- 27. Fleischmann R, Mysler E, Hall S, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a Phase IIIb/IV, double-blind, head-to-head, randomised controlled trial. Lancet 2017; 390: 457–468. [DOI] [PubMed] [Google Scholar]
- 28. Dougados M, van der Heijde D, Chen YC, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis 2017; 76: 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smolen JS, Kremer JM, Gaich CL, et al. Patient-reported outcomes from a randomised phase III study of baricitinib in patients with rheumatoid arthritis and an inadequate response to biological agents (RA-BEACON). Ann Rheum Dis 2017; 76: 694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med 2017; 376: 652–662. [DOI] [PubMed] [Google Scholar]
- 31. Papp KA, Menter MA, Raman M, et al. A randomized phase 2b trial of baricitinib, an oral Janus kinase (JAK) 1/JAK2 inhibitor, in patients with moderate-to-severe psoriasis. Br J Dermatol 2016; 174: 1266–1276. [DOI] [PubMed] [Google Scholar]
- 32. Guttman-Yassky E, Silverberg JI, Nemoto O, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol 2019; 80: 913–921.e9. [DOI] [PubMed] [Google Scholar]
- 33. Wallace DJ, Furie RA, Tanaka Y, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2018; 392: 222–231. [DOI] [PubMed] [Google Scholar]
- 34. Burmester GR, Kremer JM, Van den Bosch F, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018; 391: 2503–2512. [DOI] [PubMed] [Google Scholar]
- 35. Genovese MC, Fleischmann R, Combe B, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet 2018; 391: 2513–2524. [DOI] [PubMed] [Google Scholar]
- 36. Smolen JS, Pangan AL, Emery P, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet 2019; 393: 2303–2311. [DOI] [PubMed] [Google Scholar]
- 37. Fleischmann R, Pangan AL, Song IH, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase 3, double-blind, randomized controlled trial. Arthritis Rheumatol 2019; 71: 1788–1800. [DOI] [PubMed] [Google Scholar]
- 38. Kavanaugh A, Kremer J, Ponce L, et al. Filgotinib (GLPG0634/GS-6034), an oral selective JAK1 inhibitor, is effective as monotherapy in patients with active rheumatoid arthritis: results from a randomised, dose-finding study (DARWIN 2). Ann Rheum Dis 2017; 76: 1009–1019. [DOI] [PubMed] [Google Scholar]
- 39. Westhovens R, Taylor PC, Alten R, et al. Filgotinib (GLPG0634/GS-6034), an oral JAK1 selective inhibitor, is effective in combination with methotrexate (MTX) in patients with active rheumatoid arthritis and insufficient response to MTX: results from a randomised, dose-finding study (DARWIN 1). Ann Rheum Dis 2017; 76: 998–1008. [DOI] [PubMed] [Google Scholar]
- 40. Genovese MC, Kalunian K, Gottenberg JE, et al. Effect of Filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: the FINCH 2 randomized clinical trial. JAMA 2019; 322: 315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mease P, Coates LC, Helliwell PS, et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): results from a randomised, placebo-controlled, phase 2 trial. Lancet 2018; 392: 2367–2377. [DOI] [PubMed] [Google Scholar]
- 42. van der Heijde D, Baraliakos X, Gensler LS, et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active ankylosing spondylitis (TORTUGA): results from a randomised, placebo-controlled, phase 2 trial. Lancet 2018; 392: 2378–2387. [DOI] [PubMed] [Google Scholar]
- 43. Vermeire S, Schreiber S, Petryka R, et al. Clinical remission in patients with moderate-to-severe Crohn's disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 2017; 389: 266–275. [DOI] [PubMed] [Google Scholar]
- 44. Fleischmann RM, Damjanov NS, Kivitz AJ, et al. A randomized, double-blind, placebo-controlled, twelve-week, dose-ranging study of decernotinib, an oral selective JAK-3 inhibitor, as monotherapy in patients with active rheumatoid arthritis. Arthritis Rheumatol 2015; 67: 334–343. [DOI] [PubMed] [Google Scholar]
- 45. Genovese MC, van Vollenhoven RF, Pacheco-Tena C, et al. VX-509 (Decernotinib), an oral selective JAK-3 inhibitor, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheumatol 2016; 68: 46–55. [DOI] [PubMed] [Google Scholar]
- 46. Genovese MC, Yang F, Østergaard M, et al. Efficacy of VX-509 (decernotinib) in combination with a disease-modifying antirheumatic drug in patients with rheumatoid arthritis: clinical and MRI findings. Ann Rheum Dis 2016; 75: 1979–1983. [DOI] [PubMed] [Google Scholar]
- 47. Sands BE, Sandborn WJ, Feagan BG, et al. Peficitinib, an oral janus kinase inhibitor, in moderate-to-severe ulcerative colitis: results from a randomised, phase 2 study. J Crohns Colitis 2018; 12: 1158–1169. [DOI] [PubMed] [Google Scholar]
- 48. Genovese MC, Greenwald M, Codding C, et al. Peficitinib, a JAK inhibitor, in combination with limited conventional synthetic disease modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol 2017; 69: 932–942. [DOI] [PubMed] [Google Scholar]
- 49. Kivitz AJ, Gutierrez-Ureña SR, Poiley J, et al. Peficitinib, a JAK inhibitor, in the treatment of moderate-to-severe rheumatoid arthritis in patients with an inadequate response to methotrexate. Arthritis Rheumatol 2017; 69: 709–719. [DOI] [PubMed] [Google Scholar]
- 50. Takeuchi T, Tanaka Y, Iwasaki M, et al. Efficacy and safety of the oral Janus kinase inhibitor peficitinib (ASP015K) monotherapy in patients with moderate to severe rheumatoid arthritis in Japan: a 12-week, randomised, double-blind, placebo-controlled phase IIb study. Ann Rheum Dis 2016; 75: 1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tanaka Y, Takeuchi T, Tanaka S, et al. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to conventional DMARDs: a randomised, double-blind, placebo-controlled phase III trial (RAJ3). Ann Rheum Dis 2019; 78: 1320–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takeuchi T, Tanaka Y, Tanaka S, et al. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III randomised, double-blind, placebo-controlled trial (RAJ4) in Japan. Ann Rheum Dis 2019; 78: 1305–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Papp K, Pariser D, Catlin M, et al. A phase 2a randomized, double-blind, placebo-controlled, sequential dose-escalation study to evaluate the efficacy and safety of ASP015K, a novel Janus kinase inhibitor, in patients with moderate-to-severe psoriasis. Br J Dermatol 2015; 173: 767–776. [DOI] [PubMed] [Google Scholar]
- 54. Westhovens R. Clinical efficacy of new JAK inhibitors under development. Just more of the same? Rheumatology (Oxford) 2019; 58(Suppl. 1): i27–i33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kremer JM, Bloom BJ, Breedveld FC, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum 2009; 60: 1895–1905. [DOI] [PubMed] [Google Scholar]
- 56. Kremer J, Li ZG, Hall S, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 2013; 159: 253–261. [DOI] [PubMed] [Google Scholar]
- 57. Chakravarty EF, Michaud K, Katz R, et al. Increased incidence of herpes zoster among patients with systemic lupus erythematosus. Lupus 2013; 22: 238–244. [DOI] [PubMed] [Google Scholar]
- 58. Kim H, Cho SK, Lee J, et al. Increased risk of opportunistic infection in early rheumatoid arthritis. Int J Rheum Dis 2019; 22: 1239–1246. [DOI] [PubMed] [Google Scholar]
- 59. Smitten AL, Choi HK, Hochberg MC, et al. The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum 2007; 57: 1431–1438. [DOI] [PubMed] [Google Scholar]
- 60. He Y, Wong AY, Chan EW, et al. Efficacy and safety of tofacitinib in the treatment of rheumatoid arthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord 2013; 14: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med 2016; 374: 1243–1252. [DOI] [PubMed] [Google Scholar]
- 62. Fleischmann R, Schiff M, van der Heijde D, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol 2017; 69: 506–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Genovese MC, Smolen JS, Weinblatt ME, et al. Efficacy and safety of ABT-494, a selective JAK-1 inhibitor, in a phase IIb study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol 2016; 68: 2857–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Westhovens R, Rigby WFC, van der Heijde D, et al. Efficacy and safety of filgotinib for patients with rheumatoid arthritis naïve to methotrexate therapy: FINCH 3 primary outcome results. EULAR 2019; Abstract LB0003 Presentation. [Google Scholar]
- 65. Wollenhaupt J, Lee EB, Curtis JR, et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther 2019; 21: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang Z, Deng W, Wu Q, et al. Tuberculosis, hepatitis B and herpes zoster in tofacitinib-treated patients with rheumatoid arthritis. Immunotherapy 2019; 11: 321–333. [DOI] [PubMed] [Google Scholar]
- 67. Yamanaka H, Tanaka Y, Takeuchi T, et al. Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with background methotrexate, in Japanese patients with rheumatoid arthritis: an open-label, long-term extension study. Arthritis Res Ther 2016; 18: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li ZG, Liu Y, Xu HJ, et al. Efficacy and safety of tofacitinib in Chinese patients with rheumatoid arthritis. Chin Med J (Engl) 2018; 131: 2683–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Keystone EC, Genovese MC, Schlichting DE, et al. Safety and efficacy of baricitinib through 128 weeks in an open-label, longterm extension study in patients with rheumatoid arthritis. J Rheumatol 2018; 45: 14–21. [DOI] [PubMed] [Google Scholar]
- 70. Tanaka Y, Ishii T, Cai Z, et al. Efficacy and safety of baricitinib in Japanese patients with active rheumatoid arthritis: a 52-week, randomized, single-blind, extension study. Mod Rheumatol 2018; 28: 20–29. [DOI] [PubMed] [Google Scholar]
- 71. Genovese MC, Greenwald MW, Gutierrez-Ureña SR, et al. Two-year safety and effectiveness of peficitinib in moderate-to-severe rheumatoid arthritis: a phase IIb, open-label extension study. Rheumatol Ther 2019; 6: 503–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kivitz AJ, Cohen S, Keystone E, et al. A pooled analysis of the safety of tofacitinib as monotherapy or in combination with background conventional synthetic disease-modifying antirheumatic drugs in a Phase 3 rheumatoid arthritis population. Semin Arthritis Rheum 2018; 48: 406–415. [DOI] [PubMed] [Google Scholar]
- 73. Winthrop KL, Curtis JR, Lindsey S, et al. Herpes zoster and tofacitinib: clinical outcomes and the risk of concomitant therapy. Arthritis Rheumatol 2017; 69: 1960–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Curtis JR, Xie F, Yang S, et al. Risk for Herpes zoster in tofacitinib-treated rheumatoid arthritis patients with and without concomitant methotrexate and glucocorticoids. Arthritis Care Res (Hoboken) 2019; 71: 1249–1254. [DOI] [PubMed] [Google Scholar]
- 75. Strand V, Ahadieh S, DeMasi R, et al. Meta-analysis of serious infections with baricitinib, tofacitinib and biologic DMARDs in rheumatoid arthritis. Ann Rheum Dis 2017; 76(Suppl. 2): 284. [Google Scholar]
- 76. Vieira MC, Zwillich SH, Jansen JP, et al. Tofacitinib versus biologic treatments in patients with active rheumatoid arthritis who have had an inadequate response to tumor necrosis factor inhibitors: results from a network meta-analysis. Clin Ther 2016; 38: 2628–2641.e5. [DOI] [PubMed] [Google Scholar]
- 77. Genovese MC, Smolen JS, Takeuchi T, et al. Safety profile of baricitinib for the treatment of rheumatoid arthritis up to 5.5 years: an updated integrated safety analysis. Arthritis Rheumatol 2017; 69: Abstract 511. Presented at ACR/ARHP Annual Meeting, 5 November, 2017. [Google Scholar]
- 78. Smolen JS, Genovese MC, Takeuchi T, et al. Safety profile of baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J Rheumatol 2019; 46: 7–18. [DOI] [PubMed] [Google Scholar]
- 79. Harigai M, Takeuchi T, Smolen JS, et al. Safety profile of baricitinib in Japanese patients with active rheumatoid arthritis with over 1.6 years median time in treatment: an integrated analysis of Phases 2 and 3 trials. Mod Rheumatol 2019; 20: 1–8. [DOI] [PubMed] [Google Scholar]
- 80. Kunwar S, Collins CE, Constantinescu F. Baricitinib, a Janus kinase inhibitor, in the treatment of rheumatoid arthritis: a systematic literature review and meta-analysis of randomized controlled trials. Clin Rheumatol 2018; 37: 2611–2620. [DOI] [PubMed] [Google Scholar]
- 81. Bechman K, Subesinghe S, Norton S, et al. A systematic review and meta-analysis of infection risk with small molecule JAK inhibitors in rheumatoid arthritis. Rheumatology (Oxford) 2019; 58: 1755–1766. [DOI] [PubMed] [Google Scholar]
- 82. Lee EB, Yamanaka H, Liu Y, et al. Efficacy and safety of tofacitinib for the treatment of rheumatoid arthritis in patients from the Asia-Pacific region: post-hoc analyses of pooled clinical study data. Int J Rheum Dis 2019; 22: 1094–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Castañeda OM, Romero FJ, Salinas A, et al. Safety of tofacitinib in the treatment of rheumatoid arthritis in Latin America compared with the rest of the world population. J Clin Rheumatol 2017; 23: 193–199. [DOI] [PubMed] [Google Scholar]
- 84. Cohen S, Curtis JR, DeMasi R, et al. Worldwide, 3-year, post-marketing surveillance experience with tofacitinib in rheumatoid arthritis. Rheumatol Ther 2018; 5: 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tamura N, Kuwana M, Atsumi T, et al. Infection events in Japanese patients with rheumatoid arthritis treated with tofacitinib: interim all-case post-marketing surveillance [abstract]. Arthritis Rheumatol 2018; 70(Suppl. 10). [Google Scholar]
- 86. Curtis JR, Xie F, Yun H, et al. Real-world comparative risks of herpes virus infections in tofacitinib and biologic-treated patients with rheumatoid arthritis. Ann Rheum Dis 2016; 75: 1843–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tanaka Y, Atsumi T, Amano K, et al. Efficacy and safety of baricitinib in Japanese patients with rheumatoid arthritis: subgroup analyses of four multinational phase 3 randomized trials. Mod Rheumatol 2018; 28: 583–591. [DOI] [PubMed] [Google Scholar]
- 88. Curtis JR, Schulze-Koops H, Takiya L, et al. Efficacy and safety of tofacitinib in older and younger patients with rheumatoid arthritis. Clin Exp Rheumatol 2017; 35: 390–400. [PubMed] [Google Scholar]
- 89. Mease P, Hall A, FitzGerald O, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. New Engl J Med 2017; 377: 1537–1550. [DOI] [PubMed] [Google Scholar]
- 90. Gladman D, Rigby W, Azevedo VF, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med 2017; 377: 1525–1536. [DOI] [PubMed] [Google Scholar]
- 91. Valenzuela F, Korman NJ, Bissonnette R, et al. Tofacitinib in patients with moderate-to-severe chronic plaque psoriasis: long-term safety and efficacy in an open-label extension study. Br J Dermatol 2018; 179: 853–862. [DOI] [PubMed] [Google Scholar]
- 92. Deleanu D, Nedelea I. Biological therapies for atopic dermatitis: an update. Exp Ther Med 2019; 17: 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol 2020; 145: 877–884. [DOI] [PubMed] [Google Scholar]
- 94. Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight 2016; 1: e89776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Jabbari A, Sansaricq F, Cerise J, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol 2018; 138: 1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Jabbari A, Dai Z, Xing L, et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EBioMedicine 2015; 2:351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mackay-Wiggan J, Jabbari A, Nguyen N, et al. Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata. JCI Insight 2016; 1: e89790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Panés J, Sandborn WJ, Schreiber S, et al. Tofacitinib for induction and maintenance therapy of Crohn's disease: results of two phase IIb randomised placebo-controlled trials. Gut 2017; 66: 1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Panés J, D'Haens GR, Higgins PDR, et al. Long-term safety and tolerability of oral tofacitinib in patients with Crohn's disease: results from a phase 2, open-label, 48-week extension study. Aliment Pharmacol Ther 2019; 49: 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Winthrop KL, Melmed GY, Vermeire S, et al. Herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis 2018; 24: 2258–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Baxter R, Ray P, Tran TN, et al. Long-term effectiveness of varicella vaccine: a 14-Year, prospective cohort study. Pediatrics 2013; 131: e1389–e1396. [DOI] [PubMed] [Google Scholar]
- 102. Bhalla P, Forrest GN, Gershon M, et al. Disseminated, persistent, and fatal infection due to the vaccine strain of varicella-zoster virus in an adult following stem cell transplantation. Clin Infect Dis 2015; 60: 1068–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352: 2271–2284. [DOI] [PubMed] [Google Scholar]
- 104. Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57: 1–30. [PubMed] [Google Scholar]
- 105. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016; 68: 1–26. [DOI] [PubMed] [Google Scholar]
- 106. Furer V, Rondaan C, Heijstek MW, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2020; 79: 39–52. [DOI] [PubMed] [Google Scholar]
- 107. Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58: 309–318. [DOI] [PubMed] [Google Scholar]
- 108. Winthrop KL, Wouters AG, Choy EH, et al. The safety and immunogenicity of live zoster vaccination in patients with rheumatoid arthritis before starting tofacitinib: a randomized phase II trial. Arthritis Rheumatol 2017; 69: 1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Calabrese LH, Abud-Mendoza C, Lindsey SM, et al. Live zoster vaccine in patients with rheumatoid arthritis treated with tofacitinib with or without methotrexate, or adalimumab with methotrexate. Arthritis Care Res (Hoboken) 2020; 72: 353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372: 2087–2096. [DOI] [PubMed] [Google Scholar]
- 111. Heineman TC, Cunningham A, Levin M. Understanding the immunology of Shingrix, a recombinant glycoprotein E adjuvanted herpes zoster vaccine. Curr Opin Immunol 2019; 59: 42–48. [DOI] [PubMed] [Google Scholar]
- 112. Carpenter CF, Aljassem A, Stassinopoulos J, et al. A cost-effectiveness analysis of an adjuvanted subunit vaccine for the prevention of herpes zoster and post-herpetic neuralgia. Open Forum Infect Dis 2019; 6: ofz219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Berkowitz EM, Moyle G, Stellbrink HJ, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis 2015; 211: 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Baillet A, Gossec L, Carmona L, et al. Points to consider for reporting, screening for and preventing selected comorbidities in chronic inflammatory rheumatic diseases in daily practice: a EULAR initiative. Ann Rheum Dis 2016; 75: 965–973. [DOI] [PubMed] [Google Scholar]
- 115. Zhang N, Wilkinson S, Riaz M, et al. Does methotrexate increase the risk of varicella or herpes zoster infection in patients with rheumatoid arthritis? A systematic literature review. Clin Exp Rheumatol 2012; 30: 962–971. [PubMed] [Google Scholar]
- 116. Reimold A. Viruses and arthritis: new challenges in diagnosis, therapy, and immunization. Am J Med Sci 2010; 339: 549–556. [DOI] [PubMed] [Google Scholar]