Abstract
Objective
To assess the efficacy of herbal medicine (cinnamon/fennel/ginger) for treating primary dysmenorrhea.
Methods
Relevant studies were searched in multiple databases. The weighted mean difference (WMD) was used as the effect indicator for measurement data, and each effect size was given estimates and 95% confidence intervals (CIs).
Results
Nine studies with 647 patients were selected. Compared with the results in the control group, pain intensity was significantly relieved in the trial group when assessed by the intervention (cinnamon vs. placebo: WMD = 1.815, 95% CI = 1.330–2.301; fennel vs. placebo: WMD = 0.528, 95% CI = 0.119–6.829; ginger vs. placebo: WMD = 2.902, 95% CI = 2.039–3.765), observation period (one cycle: WMD = 2.061, 95% CI = 0.815–3.307; one cycles: WMD = 1.831, 95% CI = 0.973–2.690), and study quality (high quality: WMD = 2.224, 95% CI = 1.488–2.960). Pain duration was significantly shorter in the trial group (cinnamon vs. placebo: WMD = 16.200, 95% CI = 15.271–17.129). No publication bias was observed for either outcome.
Conclusions
For primary dysmenorrhea, cinnamon/fennel/ginger effectively reduced pain intensity, and cinnamon shortened the duration of pain. Further studies are needed to confirm our results.
Keywords: Primary dysmenorrhea, herbal medicine, cinnamon, fennel, ginger, meta-analysis
Introduction
Primary dysmenorrhea, or painful menstruation in the absence of pelvic pathology, is a common menstrual disorder among menstruating women. It is characterized by cramps and colicky spasms of pain in the lower abdomen that interfere with daily activity.1,2 The prevalence of primary dysmenorrhea varies widely in women of reproductive age, ranging from 16% to 91%, and 2% to 29% of patients experience severe dysmenorrhea.3 In adolescence, the prevalence of dysmenorrhea is relatively higher, reaching approximately 75%.4 It is usually believed that imbalanced or excessive levels of prostanoids and possibly eicosanoids released from the endometrium during menstruation are associated with the occurrence of dysmenorrhea.5 The uterus is induced to contract irregularly and frequently, leading to increased basal tone and active pressure, and pain is induced by uterine hypercontractility, increased peripheral nerve hypersensitivity, and decreased uterine blood flow. At present, non-steroidal anti-inflammatory drugs are mainly used to treat primary dysmenorrhea, but the failure rate can reach 20% to 25%, excluding adverse reactions including headache, indigestion, and drowsiness.6,7 Thereby, it is indispensable to seek an alternative therapy to relieve menstrual discomfort in women.
In recent years, herbal medicine has been commonly used to treat primary dysmenorrhea.8 In traditional Chinese medicine, dysmenorrhea results from the stagnation of cold dampness in the uterine collaterals caused by the intake of cold drinks or exposure to rain and wading. The blood coagulates at the time of cold invasion, leading to unsmooth uterine collaterals, namely stagnation, leading to the pain. Hence, the therapeutic principles of dysmenorrhea should focus on warming meridians, dispersing cold, and removing dampness.
As commonly used natural products, cinnamon (Cinnamomum zeylanicum), fennel (Foeniculum vulgare), and ginger (Zingiber officinale) all warm meridians, disperse cold, and remove dampness. Cinnamon, an aromatic spice, has been used to treat various inflammatory disorders and chronic diseases, such as menstrual pain,9 arthritis,10 diabetes mellitus,11 and Alzheimer’s disease.12 With its contrastimulant and analgesic effects, fennel is considered an acceptable herbal treatment for dysmenorrhea despite its unpleasant taste.13 Ginger is helpful for relieving pain associated with dysmenorrhea, rheumatoid arthritis, osteoarthritis, and gastrointestinal symptoms such as diarrhea, nausea, and vomiting. Some studies also revealed that ginger can be used to relieve pain among women with dysmenorrhea.5,14 Nevertheless, there has been no attempt to date to integrate the available evidence supporting the efficacy of these three herbs for treating primary dysmenorrhea. In this review, the current evidence supporting the efficacy of herbal medicines (cinnamon/fennel/ginger) in the treatment of primary dysmenorrhea was systematically evaluated.
Materials and Methods
Literature search
We systematically searched randomized controlled trials (RCTs) related to the efficacy of cinnamon/fennel/ginger in the treatment of primary dysmenorrhea in databases updated on December 19, 2019, including PubMed, Embase, Cochrane Library (Cochrane Center Register of Controlled Trials), and Web of Science. The search strategy used a combination of subject headings (“cinnamon/fennel/ginger” AND “dysmenorrhea” AND trial) and free words. The free words were successively as follows: (1) cinnamon, “Cinnamomum zeylanicum” OR “Cinnamomum verum” OR “Cinnamon” OR “Cinnamons;” (2) fennel, “Foeniculum” OR “Foeniculums” OR “Foeniculum vulgare” OR “Foeniculum vulgares” OR “vulgares, Foeniculum” OR “Fennel” OR “Fennels;” (3) ginger, “Ginger” OR “Gingers” OR “Zingiber officinale” OR “Zingiber officinales” OR “officinales, Zingiber;” (4) dysmenorrhea, “Dysmenorrheas” OR “Pain, Menstrual” OR “Menstrual Pain” OR “Menstrual Pains” OR “Pains, Menstrual” OR “Menstruation, Painful” OR “Menstruations, Painful” OR “Painful Menstruation” OR “Painful Menstruations” OR “Primary dysmenorrhea;” and (5) trial, “Randomized controlled trial” OR “RCT.” This study was conducted according to PRISMA guidelines. Owing to the nature of this review, ethics approval was not required.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) RCTs related to the efficacy of cinnamon/fennel/ginger in the treatment of primary dysmenorrhea; (2) research subjects were women diagnosed with primary dysmenorrhea; (3) patients in the trial group received treatment with herbal medicine (cinnamon/fennel/ginger), and those in the control group were treated with placebo; (4) pain intensity and/or duration was the outcome indicator; and (5) published in English.
The exclusion criteria were as follows: (1) observational studies; (2) reviews, meta-analyses, letters, or editorial articles; (3) animal experiments; and (4) duplicated studies or studies from which effective data could not be extracted.
Data extraction and quality assessment
Two authors (Yincong Xu and Qinglin Yang) were involved in data extraction according to the inclusion and exclusion criteria. A third author (Xiaoping Wang) resolved conflicts if disagreements occurred during the process of data extraction. The collected information was as follows: first author, year of publication, participants, number of cases and intervention in the trial and control groups, observation period, quality assessment, and outcome indicators.
The modified Jadad scale (Table 1) was used to assess the quality of the included studies.15 This scoring system mainly depends on the study design (generation of randomization, allocation concealment, blinding method, and withdrawal) to evaluate study quality. In the scale, scores of 1 to 3 indicate low quality, whereas those of 4 to 7 indicate high quality. The quality of each included study was evaluated independently by two authors (Yincong Xu and Qinglin Yang).
Table 1.
The modified Jadad scale.
| Classification | Score | Description |
|---|---|---|
| Randomization | ||
| Inappropriate | 0 | Semi-randomized or quasi-randomized trials |
| Unclear | 1 | Randomized trials without describing methods for generating random sequences |
| Appropriate | 2 | Random sequences produced by a computer or random number table |
| Allocation concealment | ||
| Inappropriate | 0 | Regular grouping |
| Unclear | 1 | Only use of a random number table or other random assignment scheme |
| Appropriate | 2 | A method for assigning sequences without prediction |
| Blinding | ||
| Inappropriate | 0 | Use of double blinding without an appropriate method |
| Unclear | 1 | Only a mention double blinding |
| Appropriate | 2 | A description of the specific and appropriate method of double blinding |
| Withdrawals or dropouts | ||
| No | 0 | No description of withdrawal or dropouts |
| Yes | 1 | A description of withdrawal or dropouts |
Statistical analysis
In this meta-analysis, STATA 15.1 software (Stata Corporation, College Station, TX, USA) was used to analyze the data. The weighted mean difference (WMD) was used as the effect indicator for measurement data, and each effect size was given estimates and 95% confidence intervals (CIs). Heterogeneity among studies was evaluated using the I2 statistic and tested with a significance level of P < 0.1. The I2 statistic represents the proportion of variability among the included studies. The fixed-effect model was applied when I2 < 50%, whereas the random-effect model was used when I2 ≥ 50%. Publication bias was evaluated using Begg’s test. Subgroup analyses and meta-regression analysis were performed if significant heterogeneity was present. P < 0.05 denoted statistical significance.
Results
Description and characteristics of included studies
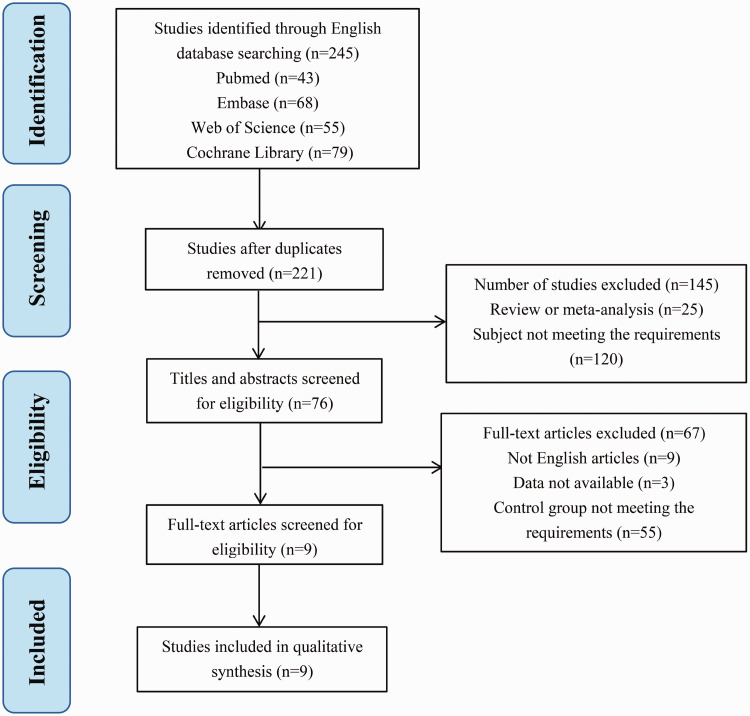
In total, 245 studies were identified by searching the databases, including PubMed, Embase, Cochrane Library, and Web of Science. From these studies, 221 remained after removing the duplicates. After screening titles and abstracts, 25 review articles and meta-analyses and 120 studies that did not match the inclusion criteria were eliminated and 76 potentially eligible studies were identified. After excluding nine studies written in languages other than English, three studies for which valid data could not be extracted, and 55 with control group not meeting the requirements, nine studies13,16–23 were finally included in this meta-analysis. The PRISMA flowchart describing the study selection process is presented in Figure 1.
Figure 1.
The PRISMA flowchart describing the study selection process.
In this meta-analysis, nine studies enrolling 333 patients in the trial group and 314 patients in the control group were finally selected, including two studies comparing cinnamon and placebo, three studies comparing fennel and placebo, and four studies comparing ginger and placebo. The quality of the included studies was relatively high, and only two studies were considered low quality. The characteristics of the included studies are shown in Table 2.
Table 2.
The characteristics of the included studies.
| First author | Year of publication | Participants |
Number of cases/age, years |
Intervention methods |
Observation period | Jadad scores | Outcome indicators | ||
|---|---|---|---|---|---|---|---|---|---|
| Trial group | Control group | Trial group | Control group | ||||||
| Omidvar et al. [16] | 2012 | Virgin patients aged 15–24 years | 25/22 ± 0.5 | 25/22 ± 0.5 | Capsules containing 30 mg of fennel, 4 times/day for 3 days | Capsules containing wheat flour, 4 times/day for 3 days | Two cycles | 3 | Pain intensity via VAS |
| Rahnama et al. [17] | 2012 | College students | 59/21.4 ± 2.0 | 46/21.3 ± 2.2 | Capsule containing 500 mg of ginger powder, 3 times/day for 5 days (first cycle) + 3 days (second cycle) | Capsule containing 500 mg of toast powder, 3 times/day for 5 days (first cycle) + 3 days (second cycle) | Two cycles | 6 | Pain intensity via VAS, pain duration |
| Moslemi [18] | 2012 | Female university medical students | 22/25.05 ± 2.16 | 21/25.9 ± 2.12 | Capsule of fennel extract containing 46 mg of hydroalcoholic fennel fruit extract mixed with starch | Placebo (the description is unknown) | Two cycles | 4 | Pain intensity via VAS |
| Jenabi [19] | 2013 | Female university students | 35/21.33 ± 1.16 | 34/21.54 ± 1.78 | Capsule containing 500 mg of ginger powder, 3 times/day for 3 days | Capsule containing placebo, 3 times/day for 3 days | One cycle | 5 | Pain intensity via VAS |
| Bokaie [13] | 2013 | Female university students | 29/21.07 ± 1.8 | 30/21.17 ± 1.6 | Fennel drop 2% and mefenamic acid cap 250 mg | Mefenamic acid cap 250 mg | One cycle | 3 | Pain intensity via VAS |
| Kashefi et al. [20] | 2014 | Female high school students | 45/15–18 | 42/15–18 | Capsules containing 250 mg of ginger powder, 3 times/day for 4 days | Capsules containing lactose, 3 times/day for 4 days | Two cycles | 5 | Pain intensity via VAS |
| Jaafarpour et al. [21] | 2015 | Female college students | 38/20.7 ± 1.1 | 38/21.3 ± 1.5 | Capsule containing 420 mg of cinnamon (two capsules each time), 3 times/day for 3 days | Empty capsules containing starch, 3 times/day for 3 days | One cycle | 5 | Pain intensity via VAS, pain duration |
| Jahangirifar et al. [22] | 2018 | Single female college students | 30/22.2 ± 2.2 | 28/22.3 ± 2.7 | 1000 mg of cinnamon, 3 times/day for 3 days | 1000 mg of starch, 3 times/day for 3 days | Two cycles | 4 | Pain intensity via VAS |
| Pakniat et al. [23] | 2019 | Medical students aged 18–25 years | 50/22.8 ± 1.8 | 50/22.3 ± 1.6 | Ginger capsules (Zingiber officinale) 500 mg per day | Placebo capsule (Dineh Co.) containing 250 g of mefenamic acid twice per day | Two cycles | 4 | Pain intensity via VAS, pain duration |
Note: VAS = visual analog scale.
Pain intensity
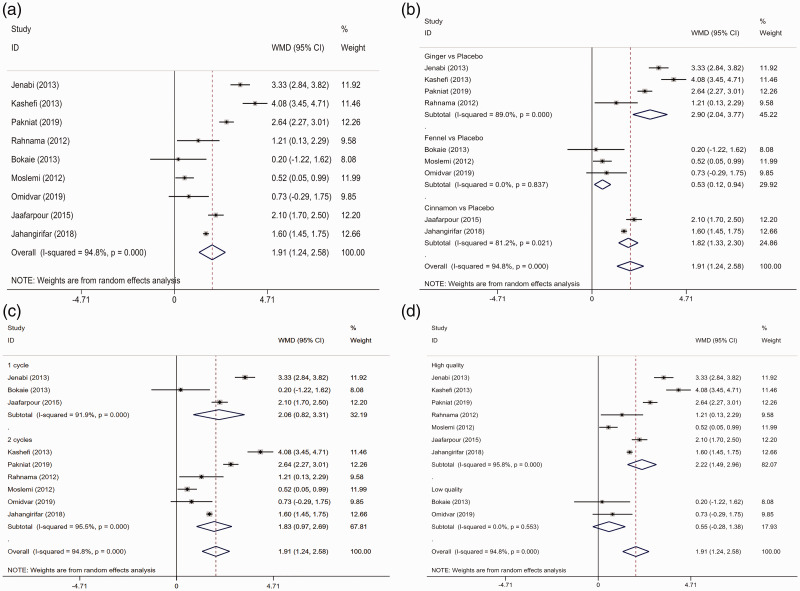
Pain intensity was reported as the outcome indicator in nine studies,13,16–23 but significant heterogeneity was detected (I2 =94.8%). Using the random-effect model, pain intensity was significantly weaker in the trial group than in the control group (WMD = 1.913, 95% CI = 1.244–2582, P < 0.001; Figure 2a and Table 3).
Figure 2.
Forest plot comparing pain intensity between the trial and control groups. a. Overall analysis. b. Subgroup analysis based on the intervention. c. Subgroup analysis based on the observation period. d. Subgroup analysis based on the quality assessment.
Table 3.
The overall and subgroup analyses of clinical outcomes.
| Indicator | WMD (95% CI) | P | I2 |
|---|---|---|---|
| Pain intensity | |||
| Overall | 1.913 (1.244–2.582) | <0.001 | 94.8% |
| Intervention | |||
| Cinnamon vs. placebo | 1.815 (1.330–2.301) | <0.001 | 81.2% |
| Fennel vs. placebo | 0.528 (0.119–6.829) | 0.011 | 0.0 |
| Ginger vs. placebo | 2.902 (2.039–3.765) | <0.001 | 89.0% |
| Observation period | |||
| One cycle | 2.061 (0.815–3.307) | 0.001 | 91.9% |
| Two cycles | 1.831 (0.973–2.690) | <0.001 | 95.5% |
| Quality assessment | |||
| High quality | 2.224 (1.488–2.960) | <0.001 | 95.8% |
| Low quality | 0.551 (−0.276–1.378) | 0.191 | 0.0 |
| Pain duration | |||
| Overall | 7.151 (−5.977–20.279) | 0.286 | 99.8% |
| Intervention methods | |||
| Cinnamon vs. placebo | 16.200 (15.271–17.129) | <0.001 | NA |
| Ginger vs. placebo | 0.826 (−2.822–4.475) | 0.657 | 32.7% |
| Observation period | |||
| One cycle | 16.200 (15.271–17.129) | <0.001 | NA |
| Two cycles | 0.826 (−2.822–4.475) | 0.657 | 32.7% |
Note: WMD = weighted mean difference; 95% CI = 95% confidence interval; NA = missing value.
Both subgroup analysis and meta-regression analysis were performed according to the intervention, observation period, and quality assessment. Subgroup analysis illustrated that pain intensity was significantly relieved in the trial group compared with the control group (cinnamon vs. placebo: I2 = 81.2%, WMD = 1.815, 95% CI = 1.330–2.301, P < 0.001; fennel vs. placebo: I2 = 0.0, WMD = 0.528, 95% CI = 0.119–6.829, P = 0.011; ginger vs. placebo: I2 = 89.0%, WMD = 2.902, 95% CI = 2.039–3.765, P < 0.001; Figure 2b and Table 3). Significant differences were found between the two groups in terms of the observation period (one cycle I2 = 91.9%, WMD = 2.061, 95% CI = 0.815–3.307, P = 0.001; two cycles: I2 = 95.5%, WMD =1.831, 95% CI = 0.973–2.690, P < 0.001; Figure 2c and Table 3). Additionally, the difference was also pronounced in high-quality studies (I2 = 95.8%, WMD = 2.224, 95% CI = 1.488–2.960, P < 0.001; Figure 2d and Table 3).
Meta-regression analysis revealed that the intervention (ginger vs. cinnamon: P = 0.271; fennel vs. cinnamon: P = 0.406), observation period (P = 0.707), and study quality (P = 0.905) had no association with heterogeneity among the studies.
Pain duration
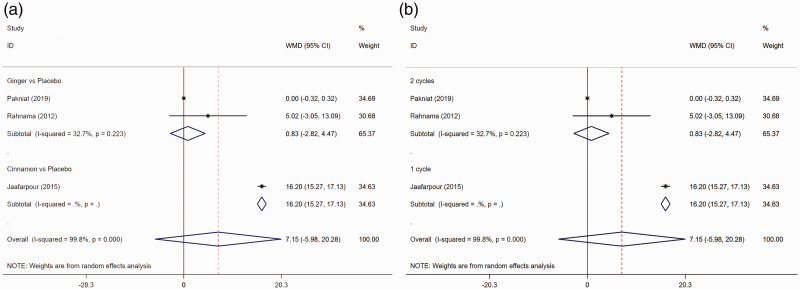
Only three studies reported outcomes for pain duration,17,21,23 and the results revealed no significant difference between the trial and control groups (I2 = 99.8%, WMD = 7.151, 95% CI = −5.977–20.279, P = 0.286; Table 3).
As shown in Table 3 and Figure 3a–b, subgroup analysis illustrated that the duration of pain was significantly shorter in patients treated with cinnamon than in those treated with placebo (WMD = 16.200, 95% CI = 15.271–17.129, P < 0.001), and the difference was distinct according to the observation period (one cycle: WMD = 16.200, 95% CI = 15.271–17.129, P < 0.001). Using meta-regression analysis, intervention methods (P = 0.114) and observation period (P = 0.114) were revealed to not be associated with heterogeneity among the studies.
Figure 3.
Forest plot comparing pain duration between the trial and control groups. a. Subgroup analysis based on the intervention. b. Subgroup analysis based on the observation period.
Publication bias
Publication bias was evaluated using Begg’s test. No publication bias was observed for pain intensity (Z = −0.10, P = 1.000) and pain duration (Z = 0.52, P = 0.602).
Discussion
This meta-analysis was conducted to systematically evaluate the efficacy of herbal medicine (cinnamon/fennel/ginger) in the treatment of primary dysmenorrhea. Nine RCTs featuring 647 patients were analyzed, and the results revealed that cinnamon/fennel/ginger could effectively reduce pain intensity; and cinnamon shortened the duration of pain in patients with primary dysmenorrhea. However, these findings need to be further confirmed in a large number of studies with large sample sizes.
The occurrence of primary dysmenorrhea is closely associated with the increased release of prostaglandins (PGs). Nine PGs are present in the human body, of which PGE2 and PGF2α play important roles in the pathogenesis of dysmenorrhea.24 PGs are produced via the metabolism of arachidonic acid in vivo. Arachidonic acid is synthesized by phospholipids under the action of phospholipase A2, and the key enzyme for PG generation is cyclooxygenase (COX). Increased PG levels can cause myometrial spasmodic contractions, leading to local ischemia-hypoxia in the uterus.5 PGE2 has the dual functions of uterine contraction and expansion, whereas PGF2α can strongly promote the contraction of blood vessels and uterine smooth muscles and enhance the sensitivity of painful nerve terminals, thereby decreasing the threshold of pain.25
The essential oils in cinnamon mainly include cinnamaldehyde (55%–57%) and eugenol (5%–18%). It has been reported that cinnamaldehyde has an antispasmodic effect, and eugenol can inhibit the biosynthesis of PGs and alleviate inflammation.26 Therefore, cinnamon is considered to inhibit the prostanoid system, which is involved in PGE2 generation.27 Moreover, the effectiveness of cinnamon in the treatment of dysmenorrhea is ascribed to its potent tocolytic effect, which can reduce uterine activity regardless of how the force is produced.9 Our results illustrated that cinnamon effectively reduced the intensity and duration of pain, in line with the results of Jaafarpour et al.21,22 However, only two RCTs on cinnamon were included in this meta-analysis. Therefore, large-scale RCTs are required to further confirm these findings.
Fennel has been widely used as an herbal medicine worldwide.28 Its antispasmodic effect on spasms induced by oxytocin and PGE2 has been confirmed in uteri dissected from mice.29 A study on the association between fennel and colicky pain in infants suggested that fennel seed oil emulsion can reduce colic intensity in infants.30 In addition, fennel has been recommended for the treatment of primary dysmenorrhea in various studies.13,16,18,31 Nahid et al.31 found that pain intensity score was decreased from 5.3 to 3.0 after 2 months and to 0.5 after 3 months of treatment with multiple herbs including fennel, celery, and saffron. Through a systematic analysis, our results displayed that fennel could significantly alleviate the intensity of pain associate with primary dysmenorrhea.
Ginger contains a variety of useful substances, such as gingerols, free fatty acids, carbohydrates, and proteins. Its constituents are reported to have analgesic and anti-inflammatory effects.14 Ginger can inhibit leukotrienes and the synthesis of PGs by suppressing COX.32 With similar efficacy as ibuprofen, mefenamic acid, and Novafen, ginger can relieve pain in women with primary dysmenorrhea.33,34 This meta-analysis demonstrated that ginger could reduce pain intensity after one or two cycles. A meta-analysis on the efficacy of oral ginger for treating dysmenorrhea suggested that oral ginger is more effective in relieving pain severity than placebo.35 Another meta-analysis also demonstrated the effectiveness of ginger powder (750–2000 mg) for primary dysmenorrhea during the first 3 to 4 days of menstrual cycle.36 In combination with our results, it is speculated that ginger exerts a good effect on pain intensity for primary dysmenorrhea.
The superiority of this meta-analysis was that it made the first attempt at integrating the current available evidence supporting the efficacy of cinnamon/fennel/ginger in treating primary dysmenorrhea. The data extraction, quality assessment, and study selection were performed independently by two authors to minimize errors. Moreover, the quality of the included RCTs was relatively high, and no publication bias was identified. However, several limitations must also be cited. First, only RCTs published in English, but not observational studies, were included into the study, which may limit the sample size of our study. Second, the included RCTs were all conducted in Iran, which may affect the generalizability of the results. Third, this meta-analysis was not registered on PROSPERO, but future studies with larger sample sizes will use the registration system and compare the results.
Conclusions
For primary dysmenorrhea, cinnamon/fennel/ginger can effectively relieve the intensity of pain, and cinnamon can shorten the duration of pain. However, these findings must be further confirmed in a large number of studies with large sample sizes.
Authors’ contributions
YCX designed the study and wrote and edited the manuscript. QLY and XPW contributed to data collection, analysis, and interpretation. All authors read and approved the final manuscript.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Yincong Xu https://orcid.org/0000-0002-6876-0714
References
- 1.Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update 2015; 21: 762–778. [DOI] [PubMed] [Google Scholar]
- 2.Koninckx PR, Ussia A, Adamyan L, et al. Primary Dysmenorrhea. J Obstet Gynaecol Can 2017; 39: 578–579. [DOI] [PubMed] [Google Scholar]
- 3.Ju H, Jones M, Mishra G. The prevalence and risk factors of dysmenorrhea. Epidemiol Rev 2014; 36: 104–113. [DOI] [PubMed] [Google Scholar]
- 4.Shetty GB, Shetty B, Mooventhan A. Efficacy of Acupuncture in the Management of Primary Dysmenorrhea: a Randomized Controlled Trial. J Acupunct Meridian Stud 2018; 11: 153–158. [DOI] [PubMed] [Google Scholar]
- 5.Oladosu FA, Tu FF, Hellman KM. Nonsteroidal antiinflammatory drug resistance in dysmenorrhea: epidemiology, causes, and treatment. Am J Obstet Gynecol 2018; 218: 390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng X, Wang X. Comparison of the efficacy and safety of non-steroidal anti-inflammatory drugs for patients with primary dysmenorrhea: a network meta-analysis. Molecular Pain 2018; 14: 174480691877032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marjoribanks J, Ayeleke RO, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev 2015; 30: 1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen HY, Lin YH, Su IH, et al. Investigation on Chinese herbal medicine for primary dysmenorrhea: implication from a nationwide prescription database in Taiwan. Complement Ther Med 2014; 22: 116–125. [DOI] [PubMed] [Google Scholar]
- 9.Alotaibi M. The effect of cinnamon extract on isolated rat uterine strips. Reprod Biol 2016; 16: 27–33. [DOI] [PubMed] [Google Scholar]
- 10.Rathi B, Bodhankar S, Mohan V, et al. Ameliorative Effects of a Polyphenolic Fraction of Cinnamomum zeylanicum L. Bark in Animal Models of Inflammation and Arthritis. Sci Pharm 2013; 81: 567–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranasinghe P, Galappaththy P, Constantine GR, et al. Cinnamomum zeylanicum (Ceylon cinnamon) as a potential pharmaceutical agent for type-2 diabetes mellitus: study protocol for a randomized controlled trial. Trials 2017; 18: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Momtaz S, Hassani S, Khan F, et al. Cinnamon, a promising prospect towards Alzheimer’s disease. Pharmacol Res 2018; 130: 241–258. [DOI] [PubMed] [Google Scholar]
- 13.Bokaie M, Farajkhoda T, Enjezab B, et al. Oral fennel (Foeniculum vulgare) drop effect on primary dysmenorrhea: effectiveness of herbal drug. Iran J Nurs Midwifery Res 2013; 18: 128–132. [PMC free article] [PubMed] [Google Scholar]
- 14.Ali BH, Blunden G, Tanira MO, et al. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol 2008; 46: 409–420. [DOI] [PubMed] [Google Scholar]
- 15.Gao Z, Liu Y, Zhang J, et al. Effect of Jianpi therapy in treatment of chronic obstructive pulmonary disease: a systematic review. J Tradit Chin Med 2013; 33: 1–8. [DOI] [PubMed] [Google Scholar]
- 16.Omidvar S, Esmailzadeh S, Baradaran M, et al. Effect of fennel on pain intensity in dysmenorrhoea: a placebo-controlled trial. Ayu 2012; 33: 311–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahnama P, Montazeri A, Huseini HF, et al. Effect of Zingiber officinale R. rhizomes (ginger) on pain relief in primary dysmenorrhea: a placebo randomized trial. BMC Complement Altern Med 2012; 12: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moslemi L, Bekhradi R, Galini Moghaddam T, et al. Comparative effect of fennel extract on the intensity of primary dysmenorrhea. Afr J Pharm Pharmacol 2012; 6: 1770–1773. [Google Scholar]
- 19.Jenabi E. The effect of ginger for relieving of primary dysmenorrhoea. J Pak Med Assoc 2013; 63: 8–10. [PubMed] [Google Scholar]
- 20.Kashefi F, Khajehei M, Tabatabaeichehr M, et al. Comparison of the effect of ginger and zinc sulfate on primary dysmenorrhea: a placebo-controlled randomized trial. Pain Manag Nurs 2014; 15: 826–833. [DOI] [PubMed] [Google Scholar]
- 21.Jaafarpour M, Hatefi M, Najafi F, et al. The effect of cinnamon on menstrual bleeding and systemic symptoms with primary dysmenorrhea. Iran Red Crescent Med J 2015; 17: e27032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jahangirifar M, Taebi M, Dolatian M. The effect of Cinnamon on primary dysmenorrhea: a randomized, double-blind clinical trial. Complement Ther Clin Pract 2018; 33: 56–60. [DOI] [PubMed] [Google Scholar]
- 23.Pakniat H, Chegini V, Ranjkesh F, et al. Comparison of the effect of vitamin E, vitamin D and ginger on the severity of primary dysmenorrhea: a single-blind clinical trial. Obstet Gynecol Sci 2019; 62: 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruoff G, Lema M. Strategies in pain management: new and potential indications for COX-2 specific inhibitors. J Pain Symptom Manage 2003; 25: S21–S31. [DOI] [PubMed] [Google Scholar]
- 25.Harel Z. Cyclooxygenase-2 specific inhibitors in the treatment of dysmenorrhea. J Pediatr Adolesc Gynecol 2004; 17: 75–79. [DOI] [PubMed] [Google Scholar]
- 26.Mirabi P, Alamolhoda SH, Esmaeilzadeh S, et al. Effect of medicinal herbs on primary dysmenorrhoea- a systematic review. Iran J Pharm Res 2014; 13: 757–767. [PMC free article] [PubMed] [Google Scholar]
- 27.Marzouk TM, El-Nemer AM, Baraka HN. The effect of aromatherapy abdominal massage on alleviating menstrual pain in nursing students: a prospective randomized cross-over study. Evid Based Complement Alternat Med 2013; 2013: 742421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Proctor ML, Murphy PA, Pattison HM, et al. Behavioural interventions for primary and secondary dysmenorrhoea. Cochrane Database Syst Rev 2007; 3: CD002248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Modaress Nejad V, Asadipour M. . Comparison of the effectiveness of fennel and mefenamic acid on pain intensity in dysmenorrhoea. East Mediterr Health J 2006; 12: 423–427. [PubMed] [Google Scholar]
- 30.Alexandrovich I, Rakovitskaya O, Kolmo E, et al. The effect of fennel (Foeniculum Vulgare) seed oil emulsion in infantile colic: a randomized, placebo-controlled study. Altern Ther Health Med 2003; 9: 58–61. [PubMed] [Google Scholar]
- 31.Nahid K, Fariborz M, Ataolah G, et al. The effect of an Iranian herbal drug on primary dysmenorrhea: a clinical controlled trial. J Midwifery Womens Health 2009; 54: 401–404. [DOI] [PubMed] [Google Scholar]
- 32.Gonlachanvit S, Chen YH, Hasler WL, et al. Ginger reduces hyperglycemia-evoked gastric dysrhythmias in healthy humans: possible role of endogenous prostaglandins. J Pharmacol Exp Ther 2003; 307: 1098–1103. [DOI] [PubMed] [Google Scholar]
- 33.Ozgoli G, Goli M, Moattar F. Comparison of effects of ginger, mefenamic acid, and ibuprofen on pain in women with primary dysmenorrhea. J Altern Complement Med 2009; 15: 129–132. [DOI] [PubMed] [Google Scholar]
- 34.Adib Rad H, Basirat Z, Bakouei F, et al. Effect of Ginger and Novafen on menstrual pain: a cross-over trial. Taiwan J Obstet Gynecol 2018; 57: 806–809. [DOI] [PubMed] [Google Scholar]
- 35.Chen CX, Barrett B, Kwekkeboom KL. Efficacy of Oral Ginger (Zingiber officinale) for Dysmenorrhea: a Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med 2016; 2016: 6295737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daily JW, Zhang X, Kim DS, et al. Efficacy of Ginger for Alleviating the Symptoms of Primary Dysmenorrhea: a Systematic Review and Meta-analysis of Randomized Clinical Trials. Pain Med 2015; 16: 2243–2255. [DOI] [PubMed] [Google Scholar]