Abstract
Objective
Fertility may be defined as a capacity to conceive and produce offspring. Infertility is characterized by failure to establish a clinical pregnancy after 12 months of regular and unprotected sexual intercourse. Infertility concerns an estimated 8–12% of the global population, and is associated with factors including time of unwanted non-conception, age of female partner and number of diseases impacting fertility. Unexplained infertility is described as idiopathic. This study aimed to analyse and evaluate the influence of mental disorders, often considered as reasons for idiopathic infertility, on female and male fertility, including stress, depression, sleep and eating disorders, and addictions.
Methods
This systematic review comprised a search of MEDLINE, Cochrane and PubMed databases for relevant articles that were analysed by two independent reviewers.
Results
A total of 106 articles published between 1955–2019 were included. Mental disorders modify endocrine gland and immune system functioning at both the tissue and cellular level, and are negatively associated with female and male fertility.
Conclusion
Mental disorders may negatively impact female and male fertility. Further studies are required to explain the exact role and contribution of mental disorders to fertility.
Keywords: Mental disorders, stress, depression, sleep disorders, eating disorders, infertility, idiopathic infertility
Introduction
Fertility may be defined as a capacity to conceive and thus to produce offspring.1 In contrast, infertility is defined as a disease characterized by a failure to establish a clinical pregnancy after 12 months of regular and unprotected sexual intercourse. Infertility in females may be categorised as primary, concerning females who have never been pregnant, and secondary, concerning females who have previously been pregnant.2
The problem of infertility is estimated to concern approximately 8–12% of the global population,3 with secondary infertility occurring more often than primary infertility.4 Furthermore, infertility is found to occur more often in less developed countries.5 Males are estimated to be responsible for 20–30% of infertility individually, and are co-responsible for half of all infertility cases.6
Factors that may be associated with decreased fertility include time of unwanted non-conception, age of the female partner and number of diseases impacting fertility.2 In addition, more recent research indicates that fertility is also influenced by male partner’s age.7 Infertility may be caused by various diseases related only to males (e.g. testicular deficiency), only to females (e.g. polycystic ovary syndrome [PCOS], endometriosis, uterine fibroids, or premature ovarian insufficiency) or diseases that can concern either sex (e.g. systematic diseases, infections, hyperprolactinaemia, or hypogonadotropic hypogonadism).2
Very often, it is impossible to establish the precise cause of infertility, and such a disorder is defined as idiopathic infertility. Idiopathic infertility may be explained by the role of mental disorders, such as stress, depression, sleep disorders, eating disorders, and addictions. The relationship between mental disorders and human physiology was first described in detail and highlighted by Hans Hugo Selye in 1955,8 who stated that the stressor acts on the target (the body or some part of it) directly and indirectly through the pituitary and the adrenal glands. The first mediator travels from the injured target area to the anterior pituitary resulting in discharging of adrenocorticotropic hormone (ACTH). ACTH alone, or in cooperation with other hormones, stimulates the adrenal cortex to discharge corticoids. Mineralocorticoids stimulate the proliferative ability and reactivity of connective tissue, thus enhancing the inflammatory potential.8
The aim of the present study was to systematically review the literature to analyse and evaluate the influence of mental disorders, such as stress, depression, sleep disorders, eating disorders, and addictions, on female and male fertility.
Materials and methods
This systematic review, based on analysis of available literature indexed in MEDLINE, Cochrane and PubMed databases, was conducted independently by two reviewers (FS and JK) between June 2019 and October 2019, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. Keywords used during the search of titles and abstracts were the combinations of (infertility OR fertility) AND (depression OR stress OR mental disorders OR antidepressants OR sleep disorders OR sleep disturbances OR sleep deprivation OR insomnia OR eating disorders OR anorexia nervosa OR bulimia nervosa OR binge-eating disorder OR addictions OR substance addictions OR alcohol OR smoking OR marijuana OR behavioural addictions OR [cell- OR mobile- OR smart-phone addiction]). Abstracts written in English and relevant to the topic were selected during screening. Full-text articles were critically studied and analysed in detail.
Results
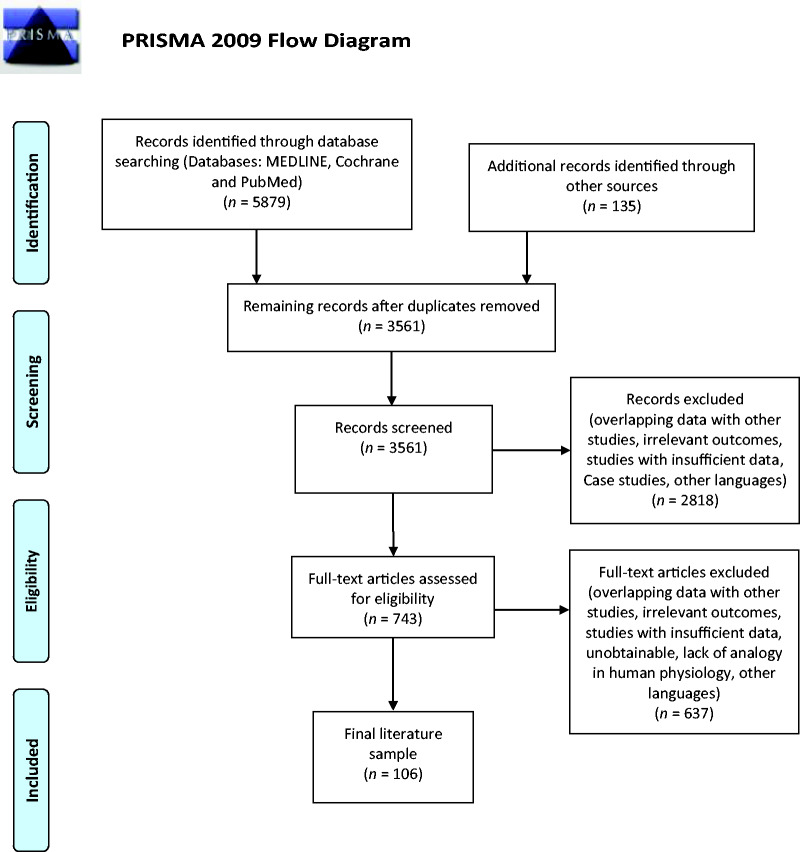
A total of 3 561 records were retrieved once duplicates had been removed, and following screening, 743 articles were assessed for eligibility. A final sample of 106 articles published between 1955 and 2019 were chosen for inclusion into the review (Figure 1).
Figure 1.
Flowchart of the studies identified in the systematic review.
Depression and stress
Approximately 350 million people suffer from depression worldwide, and many of those affected receive no proper treatment, mainly due to a global shortage of psychiatrists, ineffective treatment, ineffective mental health care system and stigmatization.9
Often weeks or even months are needed to obtain a response to antidepressant treatment.10 In addition, adverse effects such as withdrawal syndrome, sexual problems, weight gain, and addiction are common.11 Therefore, despite being more effective than placebo (odds ratios [ORs] of 1.15 to 1.55), antidepressants are associated with lower acceptability (ORs of 0.64 to 0.83).12
The problems of depression, stress, and anxiety significantly affect infertile people. A meta-analysis by Fallahzadeh et al.13 showed that depression scores in women who are infertile were significantly higher than scores for fertile couples. Furthermore, a study by Crawford et al.14 showed that women suffering from depression were less likely to undergo infertility treatment and more likely to be overweight or obese. Since biblical times, hypotheses have been raised about the relationship between depression, anxiety, and infertility. It is known that infertility may cause stress and even depression, but what remains unclear is whether depression, stress and anxiety affect fertility.15
Secretion of gonadotropin-releasing hormone (GnRH) pulses from the hypothalamus stimulates the pituitary gland to secrete luteinizing hormone (LH) and follicle-stimulating hormone (FSH).16 Both the amplitude and frequency of GnRH pulses are crucial for proper secretion of gonadotropins. LH secretion is stimulated by high frequency GnRH pulses, while low-frequency pulses stimulate FSH. During the follicular phase of the menstrual cycle, increased oestrogen levels lead to increased frequency of GnRH pulses. This results in increased LH secretion and ovulation. Many reproductive disorders in women are associated with abnormalities in GnRH secretion, and among others, this group includes hypogonadotropic hypogonadism, hyperprolactinaemia and PCOS.17
A study using a rat model of menopause and depression found that levels of cortisone, LH, FSH, and ACTH were significantly higher in groups subjected to mild stress factors compared with analogous groups not subjected to mild stress factors.18 On the other hand, dopamine levels were lower in the stress groups.18 While this is an interesting observation, similar effects have not been observed in humans. In addition, the effect of stress on the rat’s fertility in this study is unknown, thus, the topic requires further research.
According to a study among women with recurrent miscarriages, as many as 45% showed anxiety and 37% had depressive symptoms, and these results were significantly higher than in women with low risk of miscarriage.19 Furthermore, although major depression alone was not found to be associated with poorer results in female non-in vitro fertilization (IVF) fertility treatments, it lowered the chances of male partners to achieve conception.20 Meanwhile, the use of antidepressants, particularly non-selective serotonin reuptake inhibitor (SSRI), has been found to increase the risk of first-trimester pregnancy loss.20
There are a number of published investigations regarding the influence of antidepressant drugs on fertility and miscarriages. In a Finnish cohort study of 6 920 women living in Vantaa, a correlation was found between the risk of miscarriage, antidepressant use, and body mass index (BMI).21 Among women using antidepressants, the risk of miscarriage decreased as BMI increased. Women with low BMI who used antidepressants both before and during pregnancy were at the highest risk of miscarriage, and should be closely monitored to reduce the risk of preterm delivery.21 In their work, Norr et al.22 emphasized the negative impact of SSRIs on sperm parameters, particularly regarding dosage and duration of therapy. Spermatogenesis lasts at least 74 days, so at the beginning of therapy, sperm parameters may remain at their initial levels.22 A study using a mouse model showed that sperm parameters are weakened by amitriptyline, but increased by venlafaxine.23 At the same time, the study showed that amitriptyline combined with vitamin C reduces the negative effect of amitriptyline on sperm motility.23 Depression may be associated with an increased risk of premature ejaculation.24 A meta-analysis by Yi et al.25 showed that sertraline increases intravaginal ejaculation latency time and may be used for the treatment of premature ejaculation.
Depression, stress, and anxiety associated with infertility treatment are important issues. A study involving 842 patients undergoing IVF treatment showed that 39.4% of patients felt anxious, and 28.5% had depressive symptoms.26 Another study found a relationship between the duration of infertility and the incidence of depressive symptoms and anxiety, whereby symptoms were most common in the group of patients suffering from infertility for 4–6 years.27 A prospective cohort study of 72 patients treated with IVF showed that their salivary cortisol levels were higher than among the general female population. In addition, the highest levels were reached in a group undergoing IVF treatment for the first time. For this reason, relaxation techniques should be considered, especially during initial treatment.28
Depression, anxiety, stress, and antidepressants used in a patient’s treatment can play an essential role in the treatment of infertility. The impact of these factors on hormonal balance, ovulation, miscarriages in women, as well as on the quality of sperm and ejaculation disorders in men, should be taken into consideration. In the present authors’ view, patients’ reproductive plans should play a role in determining the most effective therapy, while minimizing side effects. It is worth noting that infertility may be the cause of many cases of stress, anxiety or depression. Furthermore, in such situations, successful infertility treatment may have a positive impact on mental health.
Sleep disturbances and infertility
Sleep disturbances are commonly known to be associated with a number of diseases concerning the cardiovascular, endocrine, and nervous systems.29 Furthermore, recent studies have shown that there is a relationship between sleep disturbances and reproductive health, which inevitably results in infertility.30
Polycystic ovary syndrome is characterized by hyperandrogenism, insulin resistance and oligoanovulation, and constitutes one of the most common endocrine system diseases among women of childbearing age.31 PCOS also reduces fertility and is a common cause of female infertility.30 Vgontzas et al.32 reported that women with PCOS were 30 times more likely to suffer from sleep disordered breathing than women without PCOS, and a separate study demonstrated a correlation between obstructive sleep apnoea (OSA) and PCOS, in that 44.4% of women with PCOS experience OSA compared with only 5.5% of women without PCOS.33 OSA is linked to increased reactive oxygen/nitrogen species and oxidative stress,34 and oxidative stress is a recognized factor in both female and male infertility.35,36 Further studies are needed to explain the exact role and contribution of OSA to the metabolic abnormalities in PCOS.37 Moreover, patients with OSA are often characterized by hyperprolactinaemia that may indicate reproductive dysfunction in both sexes, resulting in infertility.38 Effective continuous positive airway pressure therapy has been shown to decrease previously elevated serum prolactin levels in patients with OSA,39 however, this study was limited by a relatively small male only study population.
Sleep disturbances coexist with circadian dysrhythmia and hypothalamic-pituitary-adrenal axis activation, and these factors directly or indirectly affect the inflammatory response, melatonin level, the level of reproductive hormones, and uterine receptivity. All of these previously mentioned factors have a significant impact on infertility.30 Hypothalamic-pituitary-adrenal axis activation affects reproductive hormone levels, and results in disorders of follicle development and menstruation, finally leading to infertility.40–42 This phenomenon may be explained by a high level of ACTH and cortisol (stress hormone),43 and such a situation is particularly intensified in the case of chronic insomnia.30
Despite the fact that melatonin is primarily related to circadian function, it is also thought to affect the reproductive system.44 The exact role of melatonin in infertility is not entirely understood, but melatonin has significant antioxidant properties and protects the oocyte from oxidative stress, particularly at the time of ovulation.45,46 In a study involving night-shift workers, melatonin levels were shown to be lowered, whereas LH and FSH levels were increased.47 On the other hand, there is evidence that high melatonin level is associated with amenorrhea, hyperprolactinaemia, hypogonadotropic hypogonadism, especially among men with oligospermia and/or azoospermia.48–51 Melatonin administration has been shown to enhance LH and FSH, possibly through GnRH during the follicular phase, but not the luteal phase, indicating that the effect of melatonin on reproductive hormones may vary depending on the cycle phase.52 To summarize, both stable circadian rhythms and cyclic melatonin availability are critical for optimal ovarian physiology and placental function, however, further studies are needed to explain the exact role and contribution of melatonin to fertility.46
A high level of thyroid stimulating hormone (TSH) is responsible for anovulation, amenorrhea and recurrent miscarriages. Moreover, high TSH levels cause hyperprolactinaemia, which, as mentioned previously, is responsible for infertility in both partners.53,54 During partial sleep deprivation, TSH concentrations have been shown to increase significantly and remain elevated throughout the following day.55 Furthermore, Gary et al.56 showed that TSH concentrations increased significantly as a result of total sleep deprivation, however, TSH concentrations were reduced during the second sleep deprived night compared to the first night, probably as a result of negative feedback inhibition. While sleep disorders are not a primary cause of hypo- and hyperthyroidism, there is evidence that sleep disorders may affect TSH level.
Levels of LH and FSH that are too high or too low may cause infertility.57 Short periods of sleep time may result in a low FSH level and in consequence, in luteal phase dysfunction, and partial and total sleep deprivation could result in an increased LH amplitude.30
High androgen levels, particularly in women, are associated with PCOS and thus, with infertility.58 Women with higher testosterone levels have been shown to have less sleep discontinuity.59 For men, longer sleep duration results in higher testosterone levels.30 Sleep loss in the early part of the night does not affect testosterone, while early wakening and wakefulness during the second part of the night has been associated with reduced morning circulating testosterone.60
Irregular oestradiol secretion may result in infertility. Partial sleep deprivation may increase oestradiol level, whereas variable sleep schedules would result in diminished levels. Low progesterone level has been correlated with infertility, and sleep-disordered breathing and stress are associated with a low level of progesterone.30
Furthermore, low sleep quality, sleep loss and interrupted sleep cause an inflammatory response in the form of elevation of tumour necrosis factors (TNF), interleukin (IL)-6, and C-reactive protein,61,62 and all of these factors are correlated with unexplained infertility.63,64 TNF-α is a central regulator of inflammation that is associated with inflammatory mechanisms related to implantation, placentation, and pregnancy outcome. Obstetric complications related to the overproduction of TNF-α include recurrent pregnancy loss, early and severe pre-eclampsia, and recurrent implantation failure syndrome.65 Furthermore, TNF-α has been shown to be significantly higher in infertile men with reduced sperm motility compared with fertile men.66 IL-6 is a multifunctional cytokine with a crucial role in the inflammatory response, and is associated with embryo implantation and placental development, as well as the immune adaptations required to tolerate pregnancy.67 Increased IL-6 level is associated with idiopathic infertility, recurrent miscarriage, pre-eclampsia, and preterm delivery.64,67 An inflammatory state indicated by increased C-reactive protein level is positively correlated with adiposity and inversely correlated with pregnancy rates in women undergoing IVF. Furthermore, inflammation itself may suppress ovarian function, or indicate immune challenges that lead to ovarian suppression.68–70
Eating disorders
Anorexia nervosa is a severe psychiatric disorder characterized by extreme dissatisfaction with the size and/or shape of the patient’s body or body parts that finally leads to weight phobia and food aversion. Desired (very low) body weight is achieved thanks to a strict diet and/or excessive hyperactivity.71 Amenorrhea is estimated to occur in about 75% of women with anorexia nervosa, and oligomenorrhea is estimated to occur in about 8%.72–74 Anorexia nervosa is associated with low BMI levels, low caloric intake, and excessive hyperactivity.73 Menstrual cycle disturbances may be explained by a decrease in the leptin level and thus disruption of the pulsatile release of GnRH by the hypothalamus. Furthermore, in addition to regulation of GnRH secretion, leptin also affects LH level.75,76 All of these factors would result in ovulation disruption and hypogonadotropic hypogonadism, and in consequence, would affect fertility. Moreover, decreased sexual desire and increased sexual anxiety is associated with anorexia nervosa,77 and anorexia nervosa is also associated with an increased number of miscarriages and induced abortions.78
Like anorexia nervosa, bulimia nervosa is characterized by dissatisfaction with size and/or shape of body/body parts that finally leads to weight phobia and food aversion. However, in bulimia nervosa, a strict low-calorie diet is repeatedly interrupted by binge-eating episodes proceeding to feelings of losing control.71 These episodes are followed by a variety of procedures to counteract the food ingestion, including vomiting, starvation, and laxative misuse.79 Amenorrhea is reported in about 23% of patients with bulimia nervosa, whereas oligomenorrhea occurs in up to half of all bulimia nervosa cases.72,73 Moreover, there is evidence that among patients with bulimia nervosa and oligomenorrhea, the LH and FSH levels are pathologically low.80 PCOS was mentioned previously to diminish fertility, and it is also associated with bulimia nervosa. In fact, 76% of patients with bulimia nervosa are shown to have PCOS.81
Binge-Eating Disorder (BED) is characterized by binge-eating episodes proceeding to feelings of losing control, similar to bulimia nervosa. However, BED is not associated with a variety of procedures to counteract the food ingestion.71 As in the case of anorexia nervosa and bulimia nervosa, patients with BED also experience oligomenorrhea and amenorrhea.82 Moreover, there is evidence that binging leads to insulin increase. High insulin levels cause an increase in testosterone levels, which negatively affects fertility.83 What is more, similar to anorexia nervosa and bulimia nervosa, BED is associated with PCOS.73 BED is also associated with an elevated risk of miscarriages and worse sexual functioning compared with patients without BED who are obese.78 Furthermore, obesity, as one sign of BED, is a significant risk factor for endometrial cancer, and endometrial cancer treatment methods may lead to infertility.84
Addictions
Substance addiction is a neuropsychiatric disorder characterized by a recurring desire to continue taking the substance despite harmful consequences.85 It has a direct impact on fertility, primarily through the harmful effects of misused substances.
In the case of women, acute alcohol consumption may increase oestrogen levels leading to a decrease in FSH, finally resulting in ovulation disorders.86 What is more, alcohol intake has a negative effect on semen volume and semen morphology, and reduces testosterone level among males, and differences in these factors were more significant when comparing daily versus occasional alcohol consumers, rather than occasional versus non-consumers.87
Published literature emphasizes an association between cigarette smoking and infertility. The prevalence of infertility and time to conception is higher, whereas fecundity is lower, among smokers compared with non-smokers,88 and FSH levels are elevated among active and passive smokers compared with non-smokers.89 Moreover, urinary oestrogen excretion is lower among smokers, and smoking females may experience earlier menopause.90 What is more, among smoking females, there is an increased risk of spontaneous miscarriages.91 Smoking males have demonstrated a reduction in sperm density, motility and pathologies in sperm morphology.92
Women who smoke marijuana demonstrate increased serum testosterone levels, decreased prolactin secretion and suppressed or increased serum LH level in a menstrual stage-specific manner,93 all of which would result in menstrual cycle disorders with significant effects on fertility. Furthermore, female marijuana smokers undergoing in-vitro fertilization demonstrate poor oocyte retrieval rates.94 In the case of males, marijuana smoking may cause a reduction in the volume and number of spermatozoa and may cause morphology changes, which results in sperm hyperactivity and reduction of fertilization capacity.95
Mobile phone overuse (behavioural addiction) has been suggested to decrease sperm parameters (count, motility, viability, and morphology) and increase oxidative stress, however, the effects appear to be the result of emitted radiofrequency electromagnetic waves.96,97 Direct effects of other behavioural addictions on human fertility have not been proven. However, social media addiction, video game addiction, gambling problems, internet addiction, and exercise addiction are associated with depression, which is an infertility risk factor described previously in the current paper.98–101
It is worth noting that substance addiction is much more common in people suffering from eating disorders than in the general population. In addition, more than one-third of people with substance addiction report eating disorders.102,103
Furthermore, addictions are often associated with risky sexual behaviour, such as frequently changing sexual partners or unprotected sex with an unknown person.104 This leads to an increased risk of contracting sexually transmitted diseases which, if left untreated, are responsible for most cases of tubal factor infertility.105,106
Conclusion
An analysis of the literature carried out during the present systematic review highlights the negative impact of mental disorders, such as stress, depression, sleep disorders, eating disorders, and addictions on female and male fertility. These disorders modify the functioning of endocrine glands and the immune system at both the tissue and cellular level, all of which may result in reduced fertility.
Despite these associations, it is not entirely clear to what extent mental disorders affect fertility and to what extent infertility affects mental health. Further studies are certainly required in order to explain the exact role of mental disorders in fertility and the contribution they have to infertility. Furthermore, a comprehensive approach to the diagnosis and treatment of infertility is critically needed, that includes analysis of the mental state of the couple desiring a child, in order to significantly reduce the number of idiopathic infertility diagnoses.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Piotr Szkodziak https://orcid.org/0000-0002-3582-067X
References
- 1.Zegers-Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril 2017; 108: 393–406. [DOI] [PubMed] [Google Scholar]
- 2.Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem 2018; 62: 2–10. [DOI] [PubMed] [Google Scholar]
- 3.Ombelet W, Cooke I, Dyer S, et al. Infertility and the provision of infertility medical services in developing countries. Hum Reprod Update 2008; 14: 605–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nachtigall RD. International disparities in access to infertility services. Fertil Steril 2006; 85: 871–875. [DOI] [PubMed] [Google Scholar]
- 5.Mascarenhas MN, Flaxman SR, Boerma T, et al. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 2012; 9: e1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal A, Mulgund A, Hamada A, et al. A unique view on male infertility around the globe. Reprod Biol Endocrinol 2015; 13: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lotti F, Maggi M. Sexual dysfunction and male infertility. Nat Rev Urol 2018; 15: 287–307. [DOI] [PubMed] [Google Scholar]
- 8.Selye H. Stress and disease. Science 1955; 122: 625–631. [DOI] [PubMed] [Google Scholar]
- 9.Smith K. Mental health: a world of depression. Nature 2014; 515: 181. [DOI] [PubMed] [Google Scholar]
- 10.Machado-Vieira R, Salvadore G, Luckenbaugh DA, et al. Rapid onset of antidepressant action: a new paradigm in the research and treatment of major depressive disorder. J Clin Psychiatry 2008; 69: 946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cartwright C, Gibson K, Read J, et al. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence 2016; 10: 1401–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 2018; 391: 1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fallahzadeh H, Zareei Mahmood Abadi H, Momayyezi M, et al. The comparison of depression and anxiety between fertile and infertile couples: a meta-analysis study. Int J Reprod Biomed (Yazd) 2019; 17: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crawford NM, Hoff HS, Mersereau JE. Infertile women who screen positive for depression are less likely to initiate fertility treatments. Hum Reprod 2017; 32: 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rooney KL, Domar AD. The relationship between stress and infertility. Dialogues Clin Neurosci 2018; 20: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaiser UB, Conn PM, Chin WW. Studies of gonadotropin-releasing hormone (GnRH) action using GnRH receptor-expressing pituitary cell lines. Endocr Rev 1997; 18: 46–70. [DOI] [PubMed] [Google Scholar]
- 17.Tsutsumi R, Webster NJG. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr J 2009; 56: 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu S, Jing L, Li Y, et al. Stress induced hormone and neuromodulator changes in menopausal depressive rats. Front Psychiatry 2018; 9: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao L, Qu J, Wang AY, et al. Anxiety, depression and social support in pregnant women with a history of recurrent miscarriage: a cross-sectional study. J Reprod Infant Psychol Epub ahead of print 14 Aug 2019. DOI: 10.1080/02646838.2019.1652730. [DOI] [PubMed]
- 20.Evans-Hoeker EA, Eisenberg E, Diamond MP, et al. Major depression, antidepressant use, and male and female fertility. Fertil Steril 2018; 109: 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laine MK, Masalin S, Rönö K, et al. Risk of preterm birth in primiparous women with exposure to antidepressant medication before pregnancy and/or during pregnancy – impact of body mass index. Ann Med 2019; 51: 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nørr L, Bennedsen B, Fedder J, et al. Use of selective serotonin reuptake inhibitors reduces fertility in men. Andrology 2016; 4: 389–394. [DOI] [PubMed] [Google Scholar]
- 23.Bandegi L, Anvari M, Vakili M, et al. Effects of antidepressants on parameters, melondiadehyde, and diphenyl-2-picryl-hydrazyl levels in mice spermatozoa. Int J Reprod Biomed (Yazd) 2018; 16: 365–372. [PMC free article] [PubMed] [Google Scholar]
- 24.Xia Y, Li J, Shan G, et al. Relationship between premature ejaculation and depression: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2016; 95: e4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi ZM, Chen SD, Tang QY, et al. Efficacy and safety of sertraline for the treatment of premature ejaculation: systematic review and meta-analysis. Medicine (Baltimore) 2019; 98: e15989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H, Ouyang N, Li R, et al. The effects of anxiety and depression on in vitro fertilization outcomes of infertile Chinese women. Psychol Health Med 2017; 22: 37–43. [DOI] [PubMed] [Google Scholar]
- 27.Gdańska P, Drozdowicz-Jastrzębska E, Grzechocińska B, et al. Anxiety and depression in women undergoing infertility treatment. Ginekol Pol 2017; 88: 109–112. [DOI] [PubMed] [Google Scholar]
- 28.Miller N, Herzberger EH, Pasternak Y, et al. Does stress affect IVF outcomes? A prospective study of physiological and psychological stress in women undergoing IVF. Reprod Biomed Online 2019; 39: 93–101. [DOI] [PubMed] [Google Scholar]
- 29.Panossian LA, Avidan AY. Review of sleep disorders. Med Clin North Am 2009; 93: 407–425. [DOI] [PubMed] [Google Scholar]
- 30.Kloss JD, Perlis ML, Zamzow JA, et al. Sleep, sleep disturbance, and fertility in women. Sleep Med Rev 2015; 22: 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrison CL, Teede HJ, Joham AE, et al. Breastfeeding and obesity in PCOS. Expert Rev Endocrinol Metab 2016; 11: 449–454. [DOI] [PubMed] [Google Scholar]
- 32.Vgontzas AN, Legro RS, Bixler EO, et al. Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance. J Clin Endocrinol Metab 2001; 86: 517–520. [DOI] [PubMed] [Google Scholar]
- 33.Fogel RB, Malhotra A, Pillar G, et al. Increased prevalence of obstructive sleep apnea syndrome in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 2001; 86: 1175–1180. [DOI] [PubMed] [Google Scholar]
- 34.Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia–revisited–the bad ugly and good: implications to the heart and brain. Sleep Med Rev 2015; 20: 27–45. [DOI] [PubMed] [Google Scholar]
- 35.Bisht S, Faiq M, Tolahunase M, et al. Oxidative stress and male infertility. Nat Rev Urol 2017; 14: 470–485. [DOI] [PubMed] [Google Scholar]
- 36.Szkodziak P, Wozniak S, Czuczwar P, et al. Infertility in the light of new scientific reports - focus on male factor. Ann Agric Environ Med 2016; 23: 227–230. [DOI] [PubMed] [Google Scholar]
- 37.Tasali E, Van Cauter E, Ehrmann DA. Polycystic ovary syndrome and obstructive sleep apnea. Sleep Med Clin 2008; 3: 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Capozzi A, Scambia G, Pontecorvi A, et al. Hyperprolactinemia: pathophysiology and therapeutic approach. Gynecol Endocrinol 2015; 31: 506–510. [DOI] [PubMed] [Google Scholar]
- 39.Macrea MM, Martin TJ, Zagrean L. Infertility and obstructive sleep apnea: the effect of continuous positive airway pressure therapy on serum prolactin levels. Sleep Breath 2010; 14: 253–257. [DOI] [PubMed] [Google Scholar]
- 40.Louis GMB, Lum KJ, Sundaram R, et al. Stress reduces conception probabilities across the fertile window: evidence in support of relaxation. Fertil Steril 2011; 95: 2184–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gordon CM. Clinical practice. Functional hypothalamic amenorrhea. N Engl J Med 2010; 363: 365–371. [DOI] [PubMed] [Google Scholar]
- 42.Ferin M. Clinical review 105: stress and the reproductive cycle. J Clin Endocrinol Metab 1999; 84: 1768–1774. [DOI] [PubMed] [Google Scholar]
- 43.Vgontzas AN, Bixler EO, Lin HM, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab 2001; 86: 3787–3794. [DOI] [PubMed] [Google Scholar]
- 44.Tamura H, Takasaki A, Miwa I, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res 2008; 44: 280–287. [DOI] [PubMed] [Google Scholar]
- 45.Srinivasan V, Spence WD, Pandi-Perumal SR, et al. Melatonin and human reproduction: shedding light on the darkness hormone. Gynecol Endocrinol 2009; 25: 779–785. [DOI] [PubMed] [Google Scholar]
- 46.Reiter RJ, Tamura H, Tan DX, et al. Melatonin and the circadian system: contributions to successful female reproduction. Fertil Steril 2014; 102: 321–328. [DOI] [PubMed] [Google Scholar]
- 47.Davis S, Mirick DK, Chen C, et al. Night shift work and hormone levels in women. Cancer Epidemiol Biomarkers Prev 2012; 21: 609–618. [DOI] [PubMed] [Google Scholar]
- 48.Puig-Domingo M, Webb SM, Serrano J, et al. Brief report: melatonin-related hypogonadotropic hypogonadism. N Engl J Med 1992; 327: 1356–1359. [DOI] [PubMed] [Google Scholar]
- 49.Macchi MM, Bruce NJ. Human pineal physiology and functional significance of melatonin. Front Neuroendocrinol 2004; 25: 177–195. [DOI] [PubMed] [Google Scholar]
- 50.Karasek M, Pawlikowski M, Nowakowska-Jankiewicz B, et al. Circadian variations in plasma melatonin, FSH, LH, and prolactin and testosterone levels in infertile men. J Pineal Res 1990; 9: 149–157. [DOI] [PubMed] [Google Scholar]
- 51.Berga SL, Mortola JF, Yen SS. Amplification of nocturnal melatonin secretion in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab 1988; 66: 242–244. [DOI] [PubMed] [Google Scholar]
- 52.Cagnacci A, Paoletti AM, Soldani R, et al. Melatonin enhances the luteinizing hormone and follicle-stimulating hormone responses to gonadotropin-releasing hormone in the follicular, but not in the luteal, menstrual phase. J Clin Endocrinol Metab 1995; 80: 1095–1099. [DOI] [PubMed] [Google Scholar]
- 53.Arredondo F, Noble LS. Endocrinology of recurrent pregnancy loss. Semin Reprod Med 2006; 24: 33–39. [DOI] [PubMed] [Google Scholar]
- 54.Verma I, Sood R, Juneja S, et al. Prevalence of hypothyroidism in infertile women and evaluation of response of treatment for hypothyroidism on fertility. Int J Appl Basic Med Res 2012; 2: 17–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baumgartner A, Dietzel M, Saletu B, et al. Influence of partial sleep deprivation on the secretion of thyrotropin, thyroid hormones, growth hormone, prolactin, luteinizing hormone, follicle stimulating hormone, and estradiol in healthy young women. Psychiatry Res 1993; 48: 153–178. [DOI] [PubMed] [Google Scholar]
- 56.Gary KA, Winokur A, Douglas SD, et al. Total sleep deprivation and the thyroid axis: effects of sleep and waking activity. Aviat Space Environ Med 1996; 67: 513–519. [PubMed] [Google Scholar]
- 57.Iliodromiti S, Nelson SM. Biomarkers of ovarian reserve. Biomark Med 2013; 7: 147–158. [DOI] [PubMed] [Google Scholar]
- 58.Haning RV, Jr, Hackett RJ, Flood CA, et al. Testosterone, a follicular regulator: key to anovulation. J Clin Endocrinol Metab 1993; 77: 710–715. [DOI] [PubMed] [Google Scholar]
- 59.Sowers MF, Zheng H, Kravitz HM, et al. Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep 2008; 31: 1339–1349. [PMC free article] [PubMed] [Google Scholar]
- 60.Schmid SM, Hallschmid M, Jauch-Chara K, et al. Sleep timing may modulate the effect of sleep loss on testosterone. Clin Endocrinol (Oxf) 2012; 77: 749–754. [DOI] [PubMed] [Google Scholar]
- 61.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab 2004; 89: 2119–2126. [DOI] [PubMed] [Google Scholar]
- 62.Okun ML, Coussons-Read M, Hall M. Disturbed sleep is associated with increased C-reactive protein in young women. Brain Behav Immun 2009; 23: 351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Naz RK, Butler A, Witt BR, et al. Levels of interferon-gamma and tumor necrosis factor-alpha in sera and cervical mucus of fertile and infertile women: implication in infertility. J Reprod Immunol 1995; 29: 105–117. [DOI] [PubMed] [Google Scholar]
- 64.Demir B, Guven S, Guven ES, et al. Serum IL-6 level may have role in the pathophysiology of unexplained infertility. Am J Reprod Immunol 2009; 62: 261–267. [DOI] [PubMed] [Google Scholar]
- 65.Alijotas-Reig J, Esteve-Valverde E, Ferrer-Oliveras R, et al. Tumor necrosis factor-alpha and pregnancy: focus on biologics. An updated and comprehensive review. Clin Rev Allergy Immunol 2017; 53: 40–53. [DOI] [PubMed] [Google Scholar]
- 66.Ghandehari-Alavijeh R, Zohrabi D, Tavalaee M, et al. Association between expression of TNF-α, P53 and HIF1α with asthenozoospermia. Hum Fertil (Camb) 2019; 22: 145–151. [DOI] [PubMed] [Google Scholar]
- 67.Prins JR, Gomez-Lopez N, Robertson SA. Interleukin-6 in pregnancy and gestational disorders. J Reprod Immunol 2012; 95: 1–14. [DOI] [PubMed] [Google Scholar]
- 68.Buyuk E, Asemota OA, Merhi Z, et al. Serum and follicular fluid monocyte chemotactic protein-1 levels are elevated in obese women and are associated with poorer clinical pregnancy rate after in vitro fertilization: a pilot study. Fertil Steril 2017; 107: 632–640.e3. [DOI] [PubMed] [Google Scholar]
- 69.Levin I, Gamzu R, Mashiach R, et al. Higher C-reactive protein levels during IVF stimulation are associated with ART failure. J Reprod Immunol 2007; 75: 141–144. [DOI] [PubMed] [Google Scholar]
- 70.Clancy KB, Klein LD, Ziomkiewicz A, et al. Relationships between biomarkers of inflammation, ovarian steroids, and age at menarche in a rural Polish sample. Am J Hum Biol 2013; 25: 389–398. [DOI] [PubMed] [Google Scholar]
- 71.Herpertz-Dahlmann B. Adolescent eating disorders: update on definitions, symptomatology, epidemiology, and comorbidity. Child Adolesc Psychiatr Clin N Am 2015; 24: 177–196. [DOI] [PubMed] [Google Scholar]
- 72.Poyastro Pinheiro A, Thornton LM, Plotonicov KH, et al. Patterns of menstrual disturbance in eating disorders. Int J Eat Disord 2007; 40: 424–434. [DOI] [PubMed] [Google Scholar]
- 73.Kimmel MC, Ferguson EH, Zerwas S, et al. Obstetric and Gynecologic Problems Associated with Eating Disorders. Int J Eat Disord 2016; 49: 260–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watson TL, Andersen AE. A critical examination of the amenorrhea and weight criteria for diagnosing anorexia nervosa. Acta Psychiatr Scand 2003; 108: 175–182. [DOI] [PubMed] [Google Scholar]
- 75.Hebebrand J, Muller TD, Holtkamp K, et al. The role of leptin in anorexia nervosa: clinical implications. Mol Psychiatry 2007; 12: 23–35. [DOI] [PubMed] [Google Scholar]
- 76.Singhal V, Misra M, Klibanski A. Endocrinology of anorexia nervosa in young people: recent insights. Curr Opin Endocrinol Diabetes Obes 2014; 21: 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pinheiro AP, Raney TJ, Thornton LM, et al. Sexual functioning in women with eating disorders. Int J Eat Disord 2010; 43: 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Linna MS, Raevuori A, Haukka J, et al. Reproductive health outcomes in eating disorders. Int J Eat Disord 2013; 46: 826–833. [DOI] [PubMed] [Google Scholar]
- 79.Stewart DE, Robinson E, Goldbloom DS, et al. Infertility and eating disorders. Am J Obstet Gynecol 1990; 163: 1196–1199. [DOI] [PubMed] [Google Scholar]
- 80.Resch M, Szendei G, Haász P. Eating disorders from a gynecologic and endocrinologic view: hormonal changes. Fertil Steril 2004; 81: 1151–1153. [DOI] [PubMed] [Google Scholar]
- 81.McCluskey S, Evans C, Lacey H, et al. Infertility and eating disorders. Am J Obstet Gynecol 1991; 165: 1576–1577. [DOI] [PubMed] [Google Scholar]
- 82.Mehler PS, Krantz MJ, Sachs KV. Treatments of medical complications of anorexia nervosa and bulimia nervosa. J Eat Disord 2015; 3: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ålgars M, Huang L, Von Holle AF, et al. Binge eating and menstrual dysfunction. J Psychosom Res 2014; 76: 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shaw E, Farris M, McNeil J, et al. Obesity and endometrial cancer. Recent Results Cancer Res 2016; 208: 107–136. [DOI] [PubMed] [Google Scholar]
- 85.Zou Z, Wang H, d’Oleire Uquillas F, et al. Definition of substance and non-substance addiction. Adv Exp Med Biol 2017; 1010: 21–41. [DOI] [PubMed] [Google Scholar]
- 86.Rossi BV, Abusief M, Missmer SA. Modifiable risk factors and infertility: what are the connections? Am J Lifestyle Med 2014; 10: 220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ricci E, Al Beitawi S, Cipriani S, et al. Semen quality and alcohol intake: a systematic review and meta-analysis. Reprod Biomed Online 2017; 34: 38–47. [DOI] [PubMed] [Google Scholar]
- 88.Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility: a committee opinion. Fertil Steril 2018; 110: 611–618. [DOI] [PubMed] [Google Scholar]
- 89.Cooper GS, Baird DD, Hulka BS, et al. Follicle-stimulating hormone concentrations in relation to active and passive smoking. Obstet Gynecol 1995; 85: 407–411. [DOI] [PubMed] [Google Scholar]
- 90.MacMahon B, Trichopoulos D, Cole P, et al. Cigarette smoking and urinary estrogens. N Engl J Med 1982; 307: 1062–1065. [DOI] [PubMed] [Google Scholar]
- 91.Ness RB, Grisso JA, Hirschinger N, et al. Cocaine and tobacco use and the risk of spontaneous abortion. N Engl J Med 1999; 340: 333–339. [DOI] [PubMed] [Google Scholar]
- 92.Pasqualotto FF, Umezu FM, Salvador M, et al. Effect of cigarette smoking on antioxidant levels and presence of leukocytospermia in infertile men: a prospective study. Fertil Steril 2008; 90: 278–283. [DOI] [PubMed] [Google Scholar]
- 93.Wang H, Dey SK, Maccarrone M. Jekyll and Hyde: two faces of cannabinoid signaling in male and female fertility. Endocr Rev 2006; 27: 427–448. [DOI] [PubMed] [Google Scholar]
- 94.Klonoff-Cohen HS, Natarajan L, Chen RV. A prospective study of the effects of female and male marijuana use on in vitro fertilization (IVF) and gamete intrafallopian transfer (GIFT) outcomes. Am J Obstet Gynecol 2006; 194: 369–376. [DOI] [PubMed] [Google Scholar]
- 95.Alvarez S. Do some addictions interfere with fertility? Fertil Steril 2015; 103: 22–26. [DOI] [PubMed] [Google Scholar]
- 96.Agarwal A, Deepinder F, Sharma RK, et al. Effect of cell phone usage on semen analysis in men attending infertility clinic: an observational study. Fertil Steril 2008; 89: 124–128. [DOI] [PubMed] [Google Scholar]
- 97.La Vignera S, Condorelli RA, Vicari E, et al. Effects of the exposure to mobile phones on male reproduction: a review of the literature. J Androl 2012; 33: 350–356. [DOI] [PubMed] [Google Scholar]
- 98.Younes F, Halawi G, Jabbour H, et al. Internet addiction and relationships with insomnia, anxiety, depression, stress and self-esteem in university students: a cross-sectional designed study. PLoS One 2016; 11: e0161126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Levit M, Weinstein A, Weinstein Y, et al. A study on the relationship between exercise addiction, abnormal eating attitudes, anxiety and depression among athletes in Israel. J Behav Addict 2018; 7: 800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grant JE, Odlaug BL, Chamberlain SR. Gambling disorder, DSM-5 criteria and symptom severity. Compr Psychiatry 2017; 75: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schou Andreassen C, Billieux J, Griffiths MD, et al. The relationship between addictive use of social media and video games and symptoms of psychiatric disorders: a large-scale cross-sectional study. Psychol Addict Behav 2016; 30: 252–262. [DOI] [PubMed] [Google Scholar]
- 102.The National Center on Addiction and Substance Abuse at Columbia University. Food for thought: substance abuse and eating disorders. Report, Columbia University, USA, December 2003. https://www.centeronaddiction.org/addiction-research/reports/food-thought-substance-abuse-and-eating-disorders
- 103.Krug I, Treasure J, Anderluh M, et al. Present and lifetime comorbidity of tobacco, alcohol and drug use in eating disorders: a European multicenter study. Drug Alcohol Depend 2008; 97: 169–179. [DOI] [PubMed] [Google Scholar]
- 104.Hallfors DD, Waller MW, Ford CA, et al. Adolescent depression and suicide risk: association with sex and drug behavior. Am J Prev Med 2004; 27: 224–231. [DOI] [PubMed] [Google Scholar]
- 105.Molitor F, Truax SR, Ruiz JD, et al. Association of methamphetamine use during sex with risky sexual behaviors and HIV infection among non-injection drug users. West J Med 1998; 168: 93–97. [PMC free article] [PubMed] [Google Scholar]
- 106.Tsevat DG, Wiesenfeld HC, Parks C, et al. Sexually transmitted diseases and infertility. Am J Obstet Gynecol 2017; 216: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]