Abstract
Background
Mycoplasma pneumoniae is a major cause of community-acquired pneumonia (CAP) that is particularly prevalent in school-aged children. This study explored the potential involvement of cytokines in children with Mycoplasma pneumoniae pneumonia (MPP) infection.
Material/Methods
Children aged 3–7 years who were hospitalized due to CAP infection were enrolled and divided into 2 groups: an MPP group (n=33) and a NMPP group (n=38), along with 21 age-matched healthy controls. Clinical characteristics and laboratory data were recorded. Serum levels of IL-18, IL-33, IFN-γ, IL-5, IL-6, IL-8, and IL-13 were assessed using Luminex xMAP technology. Correlation analysis and ROC curves analysis were also performed to further explore the role of these detected cytokines in CAP.
Results
Compared with the healthy controls, the serum expression of IL-18, IL-33, IFN-γ, IL-5, IL-6, IL-8, and IL-13 were significantly higher in the MPP and NMPP groups. Furthermore, serum IL-18 expression was found to be significantly correlated with lgE, FeNO, IL-5, IL-8, and IL-13 concentrations. Significant differences were also observed between the MPP group and NMPP group patients in levels of IL-18, IL-5, and IL-6, and further ROC analysis showed that the area under the curve (AUC) of IL-18 and IL-5 were 0.813 (95% CI: 0.710–0.917; P<0.01) and 0.844 (95% CI: 0.756–0.933; P<0.01), respectively.
Conclusions
IL-18, IL-33, IFN-γ, IL-5, IL-6, IL-8, and IL-13 serum levels showed significant differences in children with CAP. IL-18 and IL-5 were much higher in the MPP group compared to the NMPP group patients, whereas IL-6 levels were significantly lower in these 2 groups.
MeSH Keywords: Cytokines; Luminescent Measurements; Nitric Oxide; Pneumonia; Pneumonia, Mycoplasma; Receptors, Interleukin-18
Background
Mycoplasma pneumoniae (M. pneumoniae) is a major cause of community-acquired pneumonia (CAP) that is particularly prevalent in school-aged children, and is a common cause of hospital admissions. Mycoplasma pneumoniae pneumonia (MPP) accounts for about 30% of all pediatric CAP cases in a general population, with fever and persistent dry cough being the typical clinical symptoms [1]. Evidence suggests that M. pneumoniae plays a more important role in upper and lower respiratory tract infections in pediatric patients than previously recognized, and it is also associated with a variety of pulmonary infections and extra-pulmonary manifestations, including neurologic complications, hematologic system complications, and skin manifestations [2,3]. Antibiotic therapy is the usual treatment for MPP infection in children, but antibiotic-resistant MPP is emerging, posing an additional challenge in treatment of MPP [4]. Despite improved prevention strategies, pneumonia infection remains the major cause of childhood morbidity and mortality worldwide [5]. Annually, more than 25% of children in the developing world have an episode of CAP during the first 5 years of life, and there were about 1 million deaths globally in 2015 [6,7].
Cytokines, including Th1-type (IL-2, IFN-γ, TNF-α, and IL-18) and Th2-type (IL-4, IL-5, IL-6, IL-10, and IL-13), can recruit or activate B cells, T cells, and NK cells to initiate and amplify the inflammatory/immune response, thus providing crucial functions in the host defense against bacterial or viral infections. M. pneumoniae can activate many cytokines during infection, which may be partially responsible for the pathogenesis of MPP infection [8,9]. Recent studies have indicated that IL-18 and IL-33 are important cytokines involved in airway hyperresponsiveness and airway remodeling, and can induce production of Th1/2-type cytokines such as IFN-γ, IL-4, IL-5, IL-8, IL-13, and IgE. High expression of IL-18 also has been detected in patients with asthma [10,11]. Unlike asthma, in MPP the potential roles of IL-18, and IL-33, and their relationship with other Th1/2 cytokines have not been thoroughly investigated.
In the present study, Luminex technology was used to assess the serum Th1/2 cytokines levels in CAP patients treated in our hospital, including 33 children with MPP and 38 with NMPP, as well as 21 healthy controls. Further tests and analysis were performed to investigate the possible roles and correlations of these cytokines in children with CAP with or without M. pneumoniae infection. This study aimed to elucidate the underlying mechanisms of CAP in children, and to provide references for understanding the potential role of the detected cytokines in MPP.
Material and Methods
Subjects and study design
This study, we recruited patients age 3–7 years with signs or symptoms of CAP on admission. We enrolled 71 pneumoniae-infected children (35 girls and 36 boys) from January 2018 to March 2019 in our hospital. The diagnosis of M. pneumoniae was based on clinical and radiological findings, including fever, cough, abnormal lung auscultation, and a new infiltrate on chest radiograph [12]. MPP infection was confirmed based on serologic tests showing MP IgM positivity and antibody titer ≥1: 160, along with positive results for MPP polymerase chain reaction (PCR) tests of nasopharyngeal secretions (Daan Gene, Guangzhou) [13]. The CAP patients without M. pneumoniae infection were defined as having NMPP infection. We also enrolled 21 age-matched, healthy children without pneumoniae infection as healthy controls.
Exclusion criteria were: 1) did not meet the inclusion criteria, or incomplete clinical characteristics data; 2) congenital heart disease, tuberculosis infection, bronchial foreign body, or bronchiectasis; 3) history of personal or family allergies, including asthma, allergic dermatitis, and rhinitis; 4) history of glucocorticoid, bronchodilator, or leukotriene receptor antagonist administration within 2 weeks of admission; and 5) history of smoking or passive smoking [14].
The study was approved by the Ethics Committee of Zhejiang Integrated Traditional and Western Medicine Hospital, China. We obtained signed informed consent from the family members of all participators.
The clinical characteristics data of the enrolled participants were recorded at the time of admission. Peripheral blood was collected from each participant after an 8-h fast on the day of admission. Blood samples were centrifuged at 3000 g for 10 min at 4°C and the obtained serum samples were stored at −80°C for further tests.
Clinical laboratory tests
The serological tests with an enzyme-linked immunosorbent assay (ELISA) kit (Savyon, Israel) showed increased levels of the specific antibody IgM. Total serum IgE was measured using the Coat-A Count Total IgE IRMA kit with ELISA assay (Diagnostic Products Co., USA).
FeNO levels were measured by an online technique using a NIOX Flex kit (Aerocrine, Solna, Sweden) in accordance with international guidelines. The child inhaled and immediately exhaled at an exhalation rate of 50±5 ml/s maintaining the expiratory pressure at least 4 s, as previously described [15].
White blood cells (WBC) and neutrophils (NEU) were counted using an automated hematology analyzer (Coulter Counter STKS, Beckman Coulter, USA) with blood samples stored in tubes containing EDTA.
Serum cytokines analysis
The concentrations of the serum cytokines IL-18, IL-33, IFN-γ, IL-4, IL-5, IL-6, IL-8, IL-13, and IgE were determined using Luminex-based Milliplex xMAP technology with the Human Cytokine/Chemokine Magnetic Bead Panel Kit (EMD Millipore Corp., Billerica, MA, USA), following to the manufacturers’ instructions.
Statistical analysis
Normally distributed data are expressed as mean±standard deviation (SD). One-way ANOVA or Mann-Whitney test was used for comparison of continuous variables, if appropriate, and the chi-square test was used for comparison of categorical variables. Pearson’s correlation analysis was used to explore the relationships between cytokine concentrations and laboratory data. Receiver operating characteristic (ROC) curve analysis was performed to evaluate candidate indicators with regards to the assessment of patients with MPP. Statistical significance was considered as P<0.05.
Results
Clinical characteristics
A total of 92 participants were enrolled into 3 groups in this study, and the clinical characteristics of these groups are summarized in Table 1. There were 33 patients in the MPP group, 38 patients in the NMPP group, and 21 healthy children in the healthy control group. There were no significant differences in age, sex, or BMI among the 3 groups. In the healthy control group, the male: female ratio was 12: 9, and mean age was 4.88±1.40 years, and mean BMI was 15.62±2.12 kg/m2. In the MPP group, the male: female ratio was 15: 18, mean age was 5.04±1.58 years, and mean BMI was 15.46±2.20 kg/m2. In the NMPP group, the male: female ratio was 21: 17, mean age was 4.79±1.44 years, and mean BMI was 15.83±1.97 kg/m2. We also assessed presence of common clinical respiratory and extra-pulmonary symptoms. Compared with the healthy control group, children in the MPP group and NMPP group were more likely to have fever and cough, with no significantly differences between the 2 groups, and the incidences of sputum, wheezing, and chest pain were also higher. Extra-pulmonary manifestations, including skin manifestations and anemia, were also observed. Concerning the clinical course, we found that the duration was 7.88±3.50 days in the MP group and 8.13±3.62 days in the NMP group.
Table 1.
Clinical characteristics of the study subjects.
| HC (n=21) | MPP (n=33) | NMPP (n=38) | P | |
|---|---|---|---|---|
| Gender | ||||
| Male/Female, n | 12/9 | 15/18 | 21/17 | 0.622 |
| Age (years, mean±SD) | 4.88 ± 1.40 | 5.04 ± 1.58 | 4.79 ± 1.44 | 0.581 |
| BMI (kg/m2, mean±SD) | 15.62 ± 2.12 | 15.46 ± 2.20 | 15.83 ± 1.97 | 0.632 |
| Symptoms, n (%) | ||||
| Fever | ND | 28 (84.85) | 34 (89.47) | 0.559 |
| Cough | ND | 33 (100.00) | 37 (97.37) | 0.348 |
| Sputum | ND | 19 (57.58) | 25 (65.79) | 0.477 |
| Wheeze | ND | 7 (21.21) | 12 (31.58) | 0.325 |
| Chest pain | ND | 6 (18.18) | 11 (28.95) | 0.050 |
| Length of Duration (days) | ND | 7.88±3.50 | 8.13±3.62 | 0.181 |
HC – healthy control; MPP – Mycoplasma pneumoniae pneumonia; NMPP – non-Mycoplasma pneumoniae pneumonia.
Clinical laboratory data of the study participants
Laboratory data of the 3 groups are shown in Table 2. The levels of IgE, FeNO, and WBC and the percentages of NEU in children with MPP and NMPP infection were significantly higher than in healthy children (P<0.05). There were no significant differences in levels of FeNO, and WBC and the percentage of NEU between MPP and NMPP groups, but the level of the lgE in the MP group was significantly higher than in the NMP group (P<0.05).
Table 2.
Laboratory data of the children with or without Mycoplasma pneumoniae infection. (Data are presented as the mean±SD).
| Laboratory results | HC (n=21) | MPP (n=33) | NMPP (n=38) |
|---|---|---|---|
| IgE (IU/mL) | 165.29±50.49 | 272.28±59.66** | 213.68±36.24**,# |
| FeNO (ppb) | 11.27±4.07 | 22.13±9.81** | 24.14±14.29** |
| WBC (×109/L) | 6.34±1.76 | 8.25±3.11* | 9.82±3.15** |
| NEU, % | 50.39±11.23 | 58.78±13.75* | 65.94±22.22* |
HC – healthy control; MPP – Mycoplasma pneumoniae pneumonia; NMPP – non-Mycoplasma pneumoniae pneumonia; WBC – white blood cell; NEU – neutrophil; Fe-NO – fractional exhaled nitric oxide.
Comparing with healthy control group; P<0.05;
comparing with healthy control group, P<0.01;
comparing with MPP, P<0.05.
Comparison of the serum cytokines among groups
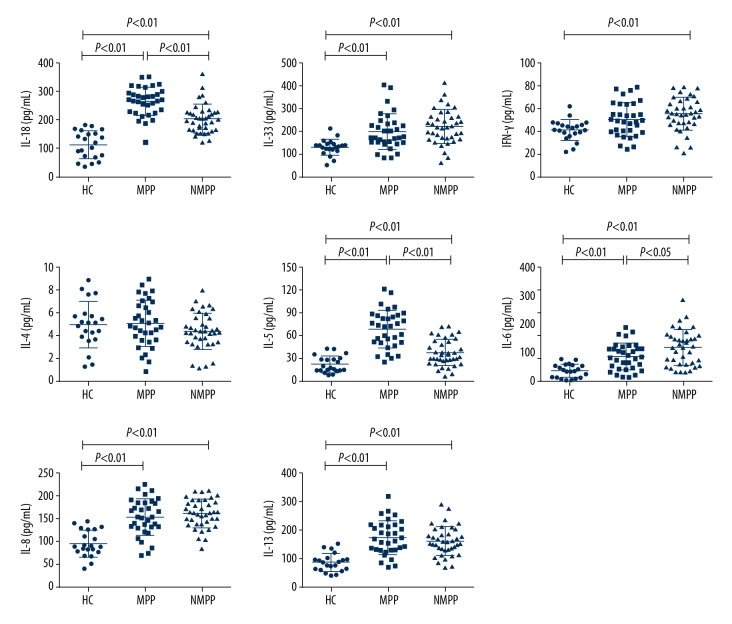
To investigate the roles of the inflammatory cytokines in pneumoniae, the levels of IL-18, IL-33, IFN-γ, IL-4, IL-5, IL-6, IL-8, and IL-13 in serum samples from 21 healthy controls and the 71 patients were measured with Luminex technology (Figure 1). The results showed that the expression of these cytokines was mostly higher in CAP patients compared with those in healthy controls. Serum levels of IL-18, IL-33, IL-5, IL-6, IL-8, and IL-13 in the MPP patient group were significantly higher than those in the healthy control group (P<0.01). Serum levels of IL-18, IL-33, IFN-γ, IL-5, IL-6, and IL-13 in the NMPP group were significantly higher than those in the healthy control group (P<0.01). Significantly differences were also observed between the MPP group and NMPP group in levels of IL-18, IL-5, and IL-6 (P<0.01, P<0.01, and P<0.05, respectively).
Figure 1.
Serum concentrations of IL-18, IL-33, IFN-r, IL-4, IL-5, IL-6, IL-8, and IL-13 in subjects were determined by Luminex assay. HC – healthy control; MPP – Mycoplasma pneumoniae pneumonia; NMPP – non-Mycoplasma pneumoniae pneumonia. Differences among distributed variables were analyzed using the t test or Mann-Whitney U test, as appropriate. Data are presented as the mean±SD (n=21 for HC, n=33 for MPP, patients, n=38 for NMPP patients). P<0.05 was considered as indicating a statistically significant difference.
As some of the detected serum cytokines levels were significantly different between the MPP and NMPP groups, ROC curves analysis was performed. As shown in Table 3 and Figure 2, the ROC curves results showed that the area under the curve (AUC) of IL-18 and IL-5 were 0.813 (95%CI: 0.710–0.917; P<0.01) and 0.844 (95%CI: 0.756–0.933; P<0.01), respectively. When the cut-off value for IL-18 was set at 250.59 pg/mL, the sensitivity and specificity were 69.7% and 86.8%, respectively; and when the cut-off values for the IL-5 was set at 42.19 pg/mL, the sensitivity and specificity were 84.8% and 68.4%, respectively.
Table 3.
ROC curve analysis of differences in cytokines in MPP and NMPP.
| Independent factors | Cutoff value | Sensitivity | Specificity | AUC | P-value | 95% CI |
|---|---|---|---|---|---|---|
| IL-18 | 250.59 | 0.697 | 0.868 | 0.813 | <0.01 | 0.710–0.917 |
| IL-33 | 169.18 | 0.737 | 0.515 | 0.386 | 0.099 | 0.253–0.519 |
| IFN-γ | 26.78 | 0.970 | 0.079 | 0.386 | 0.099 | 0.252–0.519 |
| IL-4 | 5.06 | 0.485 | 0.737 | 0.604 | 0.134 | 0.469–0.739 |
| IL-5 | 42.19 | 0.848 | 0.684 | 0.844 | <0.01 | 0.756–0.933 |
| IL-6 | 128.90 | 0.711 | 0.879 | 0.350 | <0.05 | 0.222–0.478 |
| IL-8 | 210.55 | 0.091 | 0.974 | 0.446 | 0.433 | 0.308–0.583 |
| IL-13 | 185.46 | 0.455 | 0.763 | 0.555 | 0.426 | 0.418–0.692 |
Figure 2.
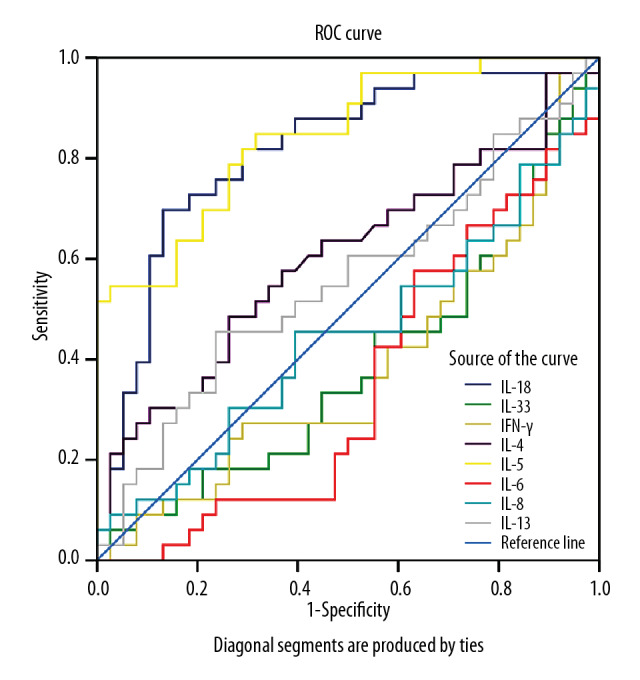
ROC curves of the cytokines differentiating MPP among CAP cases. ROC – receiver operating characteristic; MPP – Mycoplasma pneumoniae pneumonia; CAP – community-acquired pneumonia.
Correlation analysis of IL-18, IL-33, and other factors
The correlations between IL-18, IL-33, and IFN-γ, IL-5, IL-6, IL-8, IL-13, and FeNO are shown in Figure 3. The results indicated that the correlations between the levels of IL-18 and IL-5, IL-6, IL-8, IL-13, FeNO (r=0.552, P<0.001; r=0.234, P<0.05; r=0.461, P<0.001; r=0.457, P<0.001; r=0.206, P<0.05, respectively), as well as the correlations between the levels of IL-33 and IL-4, IL-6, IL-8, FeNO (r=−0.258, P<0.05; r=0.218, P<0.05; r=0.284, P<0.05; r=0.226, P<0.05, respectively) were statistically significant.
Figure 3.
Correlations between IL-18, IL-33, and other serum cytokines. Correlations were tested using the Person’s test, and P<0.05 was considered as indicating a statistically significant difference. Feno – fractional exhaled nitric oxide.
Discussion
MPP is a frequent cause of community-acquired respiratory infections in school-aged children. Regarding the pathogenesis of MPP, many studies have revealed that cytokines secreted from various types of cells during M. pneumoniae infection are critically important. In the present study, we enrolled 92 participators to evaluate the concentrations of serum cytokines to determine whether these cytokines are correlated with pneumonia infection, and whether could differentiate M. pneumoniae infection from CAP. We found that concentrations of most of the cytokines (IL-18, IL-33, IFN-γ, IL-5, IL-6, IL-8, and IL-13) were significantly higher in the 2 patient groups compared with the healthy control group. More importantly, significant differences were also observed between the MPP group and NMPP group in levels of IL-18, IL-5, and IL-6, and ROC curves analysis showed that IL-18 and IL-5 may be useful in differentiating between MPP and NMPP.
Researchers have found that the cell cytokines-mediated inflammation and immune response of the host play an important role in mycoplasmal pathogenicity, especially Th1-type and Th2-type cytokines [16]. IL-18 is considered to be a Th1 pro-inflammatory cytokine and acts as a co-factor for both Th1/Th2 cells activation and lgE production. Repeated administration of IL-18 can stimulate T cells and macrophages to secrete IFN-γ, IL-5, and IL-6, which in turn induces neutrophilia and eosinophilia in mice [17]. IL-18 also has an important role in allergic disease and pulmonary disease [18,19]. We found that serum levels of IL-18 were significantly higher in MPP and NMPP patients compared with the healthy controls (P<0.05), which is consisted with previous studies. A study of 34 patients and 32 control subjects found that serum levels of IL-18 were significantly higher in patients with M. pneumoniae infection than in control subjects, and after therapy, the increased levels of IL-18 were significantly reduced [20]. Significantly higher serum IL-18 levels in severe cases than in mild cases were also found in MPP-infected adults, suggested an important role of IL-18 in the immunopathologic responses in MPP [21]. Significant correlations between IL-18 and the Th1/Th2 cytokines IL-5, IL-8, IL-13, and lgE were also observed, suggested that IL-18 is involved in inducing Th1/Th2 cytokines production and pneumonia inflammation. Previous experimental studies also reported that splenic T cells in IL-18 transgenic mice produce higher levels of IFN-γ, IL-4, IL-5, and IL-13 than in control wild-type mice [22]. Furthermore, significant differences in IL-18 levels were also observed between MPP and NMPP patients in this study, indicated a potential role of IL-18 in pathogenesis of CAP caused by M. pneumoniae, but further investigation with more subjects and in-depth study are need to clarify the precise role of IL-18 in MPP infection.
IL-5 is secreted by Th2 cells and is critically linked to atopic diseases such as allergies and asthma. A study showed that IL-5-deficient mice failed to develop pulmonary eosinophilia and airway hyperresponsiveness after allergen challenge, indicated an important role of IL-5 in the respiratory tract [23]. In children with wheezing, serum levels of IL-5 were significantly higher in subjects with acute M. pneumoniae infection than in those without infection (P<0.001) [24]. In another study, serum levels of IL-5 and IL-18 in a pneumonia patient group were significantly higher than in controls, and IL-5 levels in the pneumonia patients with M. pneumoniae tended to be higher than in patients without M. pneumoniae infection [25]. In the present study, IL-5 concentrations in serum were also measured, showing that IL-5 levels were significantly higher in MPP patients compared with the healthy controls and NMPP patients (P<0.01), and ROC curves analysis suggested a significant role of IL-5 in MPP and NMPP (AUC: 0.844, 95%CI: 0.756–0.933; P<0.01). Combined with previous reports, the present findings suggest that IL-5 is critically involved in M. pneumoniae infection in CAP, and this might provide a new perspective on CAP pathogenesis.
IL-6 is an important pro-inflammatory cytokine and is a useful biomarker for prediction of mortality in hospitalized patients with CAP [26]. In our study, IL-6 levels were significantly higher in MPP patients compared with the healthy controls, and significant differences were also found between MMP and NMMP patients (P<0.05). In Michelow et al. investigated serum concentrations of 15 cytokines in 55 hospitalized children with well-characterized CAP, showing that IL-6 levels were significantly different among CAP patients with M. pneumoniae, Chlamydophila pneumoniae, Streptococcus pneumoniae, viruses, mixed infections, or unidentified pathogens, and IL-6 was also found to be correlated with disease severity [27]. A prospective observational study of CAP children showed that serum IL-6 levels in the MPP group were significantly higher than in the control group, and multivariate logistic regression model analysis showed that moderately elevated IL-6 level predicted development of MPP among CAP patients [28].
The presence of allergic diseases or respiratory infections specifically increase IgE levels. Our study also found that IgE levels were significantly higher in CAP patients compared with the controls. Wang et al. also reported that the serum levels of IgE in M. pneumoniae infection patients and asthma patients with M. pneumoniae infection were significantly higher than in healthy subjects, and IgE levels were positively correlated with IL-5 and TNF-α [29]. A study of 150 MPP patients showed that lg E levels were significantly higher in patients with extra-pulmonary disease compared with pulmonary disease patients [30]. Another study reported that patients developing MPP-related extra-pulmonary diseases had significantly higher levels of IgE than in children with non-mycoplasma respiratory disease [31]. These results indicate that respiratory inflammation can increase serum levels of IgE, and IgE appears to be correlated with MP infection, especially extra-pulmonary manifestations.
FeNO is a marker of respiratory disease and is used in clinical practice to evaluate the inflammatory status of patients with various respiratory disorders, especially asthma. The increased FeNO levels are caused by pro-inflammatory cytokines inducing NOS2. Higher FeNO levels were reported to be associated with ever physician-diagnosed pneumonia in a cross-sectional study among 507 children aged 5 years [32]. Lee et al. showed that FeNO levels were significantly higher in patients with acute eosinophilic pneumonia than in those without acute eosinophilic pneumonia, and the elevated FeNO levels were reduced after treatment [33]. In the present study, higher levels of FeNO were also observed in CAP patients, and correlation analysis showed that the concentrations of FeNO were significantly correlated with IL-18 and IL-33 levels, suggesting that FeNO levels in CAP are closely associated with production of pro-inflammatory cytokines and respiratory tract inflammation.
Conclusions
We found that the serum levels of IL-18, IL-5 (increased), and IL-6 (decreased) were significantly different between MPP and NMPP patients. These findings might help to better understand the immunological aspects of MPP pathogenesis and related complications. However, larger clinical and immunological investigations are needed to clarify the precise role of specific cytokines in MPP infection.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by the Medical Health Science and Technology Planning Project of Zhejiang Province, China (grant no. 2018KY607) and the Public Welfare Project of Zhejiang Traditional Chinese Medicine Administration, China (grant no. 2017C33241)
References
- 1.Atkinson TP, Balish MF, Waites KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev. 2008;32(6):956–73. doi: 10.1111/j.1574-6976.2008.00129.x. [DOI] [PubMed] [Google Scholar]
- 2.Principi N, Esposito S. Emerging role of Mycoplasma pneumoniae and Chlamydia pneumoniae in paediatric respiratory-tract infections. Lancet Infect Dis. 2001;1(5):334–44. doi: 10.1016/S1473-3099(01)00147-5. [DOI] [PubMed] [Google Scholar]
- 3.Poddighe D. Extra-pulmonary diseases related to Mycoplasma pneumoniae in children: Recent insights into the pathogenesis. Curr Opin Rheumatol. 2018;30(4):380–87. doi: 10.1097/BOR.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 4.Wagner K, Imkamp F, Pires VP, Keller PM. Evaluation of Lightmix Mycoplasma macrolide assay for detection of macrolide-resistant Mycoplasma pneumoniae in pneumonia patients. Clin Microbiol Infect. 2019;25(3):383.e5–e7. doi: 10.1016/j.cmi.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Zar HJ. Bacterial and viral pneumonia: New insights from the Drakenstein Child Health Study. Paediatr Respir Rev. 2017;24:8–10. doi: 10.1016/j.prrv.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: An updated systematic analysis. Lancet. 2015;385(9966):430–40. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 7.Principi N, Esposito S. Management of severe community-acquired pneumonia of children in developing and developed countries. Thorax. 2011;66(9):815–22. doi: 10.1136/thx.2010.142604. [DOI] [PubMed] [Google Scholar]
- 8.Chu HW, Honour JM, Rawlinson CA, et al. Effects of respiratory Mycoplasma pneumoniae infection on allergen-induced bronchial hyperresponsiveness and lung inflammation in mice. Infect Immun. 2003;71(3):1520–26. doi: 10.1128/IAI.71.3.1520-1526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimizu T, Kida Y, Kuwano K. Mycoplasma pneumoniae-derived lipopeptides induce acute inflammatory responses in the lungs of mice. Infect Immun. 2008;76(1):270–77. doi: 10.1128/IAI.00955-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding S, Wang X, Chen W, et al. Decreased Interleukin-10 responses in children with severe Mycoplasma pneumoniae pneumonia. PLoS One. 2016;11(1):e0146397. doi: 10.1371/journal.pone.0146397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gasiuniene E, Janulaityte I, Zemeckiene Z, et al. Elevated levels of interleukin-33 are associated with allergic and eosinophilic asthma. Scand J Immunol. 2019;89(5):e12724. doi: 10.1111/sji.12724. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Mei S, Zhou Y, et al. Cytokines as the good predictors of refractory Mycoplasma pneumoniae pneumonia in school-aged children. Sci Rep. 2016;6:37037. doi: 10.1038/srep37037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Zhou Y, Li S, et al. The clinical characteristics and predictors of refractory Mycoplasma pneumoniae pneumonia in children. PLoS One. 2016;11(5):e0156465. doi: 10.1371/journal.pone.0156465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan Q, Gu T, Li P, et al. Roles of T-cell immunoglobulin and mucin domain genes and toll-like receptors in wheezy children with Mycoplasma pneumoniae pneumonia. Heart Lung Circ. 2016;25(12):1226–31. doi: 10.1016/j.hlc.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Schoos AM, Christiansen CF, Stokholm J, et al. FeNO and exercise testing in children at risk of asthma. J Allergy Clin Immunol Pract. 2018;6(3):855–62.e2. doi: 10.1016/j.jaip.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, Hooper WC, Phillips DJ, Talkington DF. Cytokines in Mycoplasma pneumoniae infections. Cytokine Growth Factor Rev. 2004;15(2–3):157–68. doi: 10.1016/j.cytogfr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Ogura T, Ueda H, Hosohara K, et al. Interleukin-18 stimulates hematopoietic cytokine and growth factor formation and augments circulating granulocytes in mice. Blood. 2001;98(7):2101–7. doi: 10.1182/blood.v98.7.2101. [DOI] [PubMed] [Google Scholar]
- 18.Kandikattu HK, Upparahalli Venkateshaiah S, Mishra A. Synergy of Interleukin (IL)-5 and IL-18 in eosinophil mediated pathogenesis of allergic diseases. Cytokine Growth Factor Rev. 2019;47:83–98. doi: 10.1016/j.cytogfr.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dima E, Koltsida O, Katsaounou P, et al. Implication of Interleukin (IL)-18 in the pathogenesis of chronic obstructive pulmonary disease (COPD) Cytokine. 2015;74(2):313–17. doi: 10.1016/j.cyto.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Ding S, Wang X, Chen W, et al. Decreased Interleukin-10 responses in children with severe Mycoplasma pneumoniae pneumonia. PLoS One. 2016;11(1):e0146397. doi: 10.1371/journal.pone.0146397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka H, Narita M, Teramoto S, et al. Role of interleukin-18 and T-helper type 1 cytokines in the development of Mycoplasma pneumoniae pneumonia in adults. Chest. 2002;121(5):1493–97. doi: 10.1378/chest.121.5.1493. [DOI] [PubMed] [Google Scholar]
- 22.Hoshino T, Kawase Y, Okamoto M, et al. Cutting edge: IL-18-transgenic mice: In vivo evidence of a broad role for IL-18 in modulating immune function. J Immunol. 2001;166(12):7014–18. doi: 10.4049/jimmunol.166.12.7014. [DOI] [PubMed] [Google Scholar]
- 23.Hoek KL, Cassell GH, Duffy LB, Atkinson TP. Mycoplasma pneumoniae-induced activation and cytokine production in rodent mast cells. J Allergy Clin Immunol. 2002;109:470–76. doi: 10.1067/mai.2002.121951. [DOI] [PubMed] [Google Scholar]
- 24.Esposito S, Droghetti R, Bosis S, et al. Cytokine secretion in children with acute Mycoplasma pneumoniae infection and wheeze. Pediatr Pulmonol. 2002;34(2):122–27. doi: 10.1002/ppul.10139. [DOI] [PubMed] [Google Scholar]
- 25.Chung HL, Kim SG, Shin IH. The relationship between serum endothelin (ET)-1 and wheezing status in the children with Mycoplasma pneumoniae pneumonia. Pediatr Allergy Immunol. 2006;17(4):285–90. doi: 10.1111/j.1399-3038.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 26.Andrijevic I, Matijasevic J, Andrijevic L, et al. Interleukin-6 and procalcitonin as biomarkers in mortality prediction of hospitalized patients with community acquired pneumonia. Ann Thorac Med. 2014;9:162–67. doi: 10.4103/1817-1737.134072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michelow IC, Katz K, McCracken GH, Hardy RD. Systemic cytokine profile in children with community-acquired pneumonia. Pediatr Pulmonol. 2007;42(7):640–45. doi: 10.1002/ppul.20633. [DOI] [PubMed] [Google Scholar]
- 28.Xu XF, Li XJ, Liu JL, et al. Serum cytokine profile contributes to discriminating M. pneumoniae pneumonia in children. Cytokine. 2016;86:73–78. doi: 10.1016/j.cyto.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Chen Q, Shi C, et al. Changes of serum TNF-α, IL-5 and IgE levels in the patients of mycoplasma pneumonia infection with or without bronchial asthma. Int J Clin Exp Med. 2015;8(3):3901–6. [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Sun J, Liu Y, et al. Impact of atopy on the severity and extrapulmonary manifestations of childhood Mycoplasma pneumoniae pneumonia. J Clin Lab Anal. 2019;33(5):e22887. doi: 10.1002/jcla.22887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poddighe D, Comi EV, Brambilla I, et al. Increased total serum immunoglobulin E in children developing Mycoplasma pneumoniae-related extra-pulmonary diseases. Iran J Allergy Asthma Immunol. 2018;17(5):490–96. [PubMed] [Google Scholar]
- 32.Zhang X, Fan Q, Bai X, et al. Levels of fractional exhaled nitric oxide in children in relation to air pollution in Chinese day care centres. Int J Tuberc Lung Dis. 2018;22(7):813–19. doi: 10.5588/ijtld.17.0601. [DOI] [PubMed] [Google Scholar]
- 33.Lee JE, Rhee CK, Lim JH, et al. Fraction of exhaled nitric oxide in patients with acute eosinophilic pneumonia. Chest. 2012;141(5):1267–72. doi: 10.1378/chest.11-1303. [DOI] [PubMed] [Google Scholar]