Abstract
Juvenile myelomonocytic leukemia (JMML) has a poor prognosis in general, with hematopoietic stem cell transplant (HSCT) remaining the standard of care for cure. The hypomethylating agent, azacitidine, has been used as a bridging therapy to transplant. However, no patients have been treated with azacitidine without an HSCT post azacitidine. We report on an infant with JMML with somatic KRAS G12A mutation and monosomy 7 who achieved sustained remission following azacitidine monotherapy. He also developed an aberrant B-lymphoblast population which declined with similar kinetics as his JMML-associated abnormalities, suggesting that a B-lymphoblast population in JMML does not always progress to acute leukemia.
Keywords: azacitidine, B-lymphoblasts, JMML
1 ∣. INTRODUCTION
Juvenile myelomonocytic leukemia (JMML) is a unique myelodysplastic/myeloproliferative clonal stem cell neoplasm of early childhood. For most patients with JMML, early allogeneic hematopoietic stem cell transplant (HSCT) is the therapy of choice.1,2 None of the current approaches to therapy prior to HSCT3,4 have been shown to reduce the relapse rate, occurring with a cumulative incidence of 35%.5 Specifically, there is no evidence that conventional chemotherapy prior to stem cell transplantation significantly reduces tumor burden or improves outcome following HSCT.1,2,6 Epigenetic profiling has revealed aberrant DNA methylation patterns,2,7-9 lending support to the use of hypomethylating agents such as 5-azacitidine. However, azacitidine has primarily been used as a bridging therapy prior to HSCT.10 Here we describe the case of a child with KRAS-mutated JMML with monosomy 7 who achieved sustained remission with azacitidine monotherapy without subsequent HSCT. His course was additionally complicated by the novel finding of an aberrant precursor B-lymphoblast population.
2 ∣. CASE
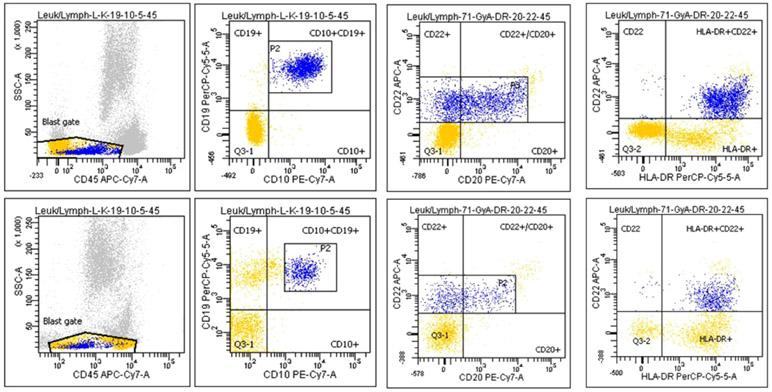
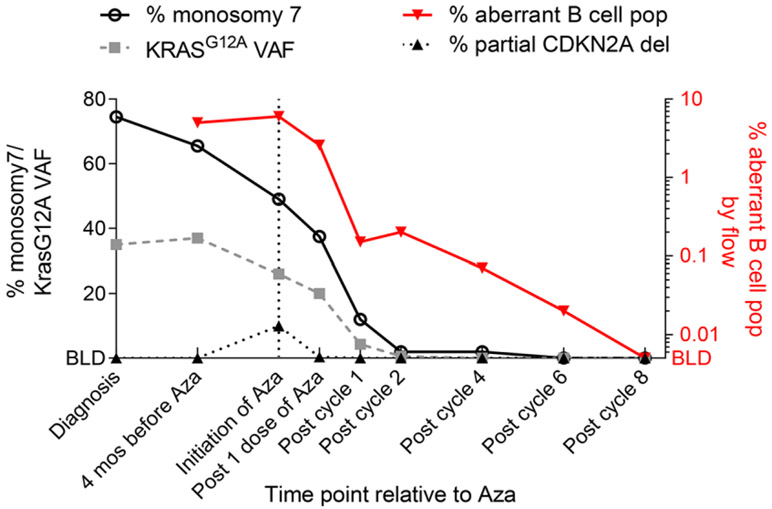
An 11-month-old male was referred for mosaic monosomy 7, identified on peripheral blood single-nucleotide-polymorphism array, sent for evaluation of speech delay and structural brain anomalies (Dandy-Walker malformation, thin corpus callosum, and cerebellar vermis hypoplasia). He had splenomegaly on exam (spleen was palpable 3 cm below the costal margin). Complete blood count showed a normal white blood cell count (11.4 × 109/L; reference: 5-14.5 × 109/L) and lymphocyte count (6.05 × 109/L; reference: 4-10.5 × 109/L), with neutropenia (absolute neutrophil count: 0.61 × 109/L; reference: 1.5-8 × 109/L), thrombocytopenia (69 × 109/L; reference:150-450 × 109/L), and monocytosis (absolute monocyte count: 4.15 × 109/L; normal: <1 × 109/L). Hemoglobin and hemoglobin F (HbF) were normal for age. Peripheral blood smear showed monocytosis, but no circulating myeloid precursors. (Figure S1). Monosomy 7 was seen in 74.5% of cells on bone marrow aspirate by fluorescent in situ hybridization (FISH). A next-generation sequencing panel (University of California, San Francisco) detected a KRAS c.35G > C, p.G12A mutation (variant allele fraction [VAF]: 35%). Germline testing of skin fibroblasts was negative for monosomy 7 and KRAS mutation. No suitable related or unrelated HSCT donors were identified. As the patient was clinically stable and transfusion independent, we followed him clinically. Other than multiple reactive airway disease exacerbations and hospitalization for diarrhea and dehydration, the child was well. Splenomegaly resolved by 14 months of age; peripheral blood counts improved, though with continued mild thrombocytopenia and neutropenia, which were worse at times of acute illness (Figure S2). However, his cytogenetic and molecular abnormalities persisted. Based on expert opinion and case-report evidence,10,11 we thus elected to trial 5-azacitidine. A pre-azacitidine bone marrow aspirate was done. Flow cytometry from this aspirate was reported after administration of the first dose. This showed an abnormal B-lymphoid blasts population (6%) (Figure 1), which had low side scatter with characteristic flow findings including positive CD45 (dim), CD19, CD10 (dim), CD20, CD22, CD34, HLA-DR, CD52, CD99, CD58, and CD38. Additionally, FISH detected a new partial CDKN2A deletion in 10% of interphase cells. We held subsequent doses of azacitidine given concerns for emerging B-lymphoblastic leukemia, possibly driven by the new CDKN2A deletion. On flow sorting the B lymphoblasts, partial CDKN2A deletion was seen in only 1.25% of cells, whereas 100% were positive for monosomy 7, suggesting this population had emerged from his original JMML clone. Re-analysis of flow from a marrow done 4 months prior revealed this same aberrant B-lymphoblast population with a similar frequency (5%). The stability of this population over the course of months was not consistent with an evolving lymphoid blast crisis. On a repeat marrow done 3 weeks later, this population had decreased to 2.5%. Reassured, we proceeded with azacitidine 100 mg/m2 intravenous daily for 5 days every 28 days,10 which was well tolerated. CDKN2A deletion, KRAS mutation, and monosomy 7 resolved after 1, 4, and 6 cycles of azacitidine, respectively (Table S1). The B-lymphoblast population declined to <0.01% after the eighth cycle of azacitidine (Figure 2). The patient is now over 2 years post completion of azacitidine and remains in hematologic, cytogenetic, and molecular remission.
FIGURE 1.
Reduction of the aberrant B-lymphoblast population in bone marrow aspirates on flow cytometry; flow plots on the top panel prior to azacitidine therapy (left to right) show the expression pattern of CD45, CD10/19, CD20/22, and HLA-DR/CD22 on the aberrant B-lymphoblast population constituting 6% of bone marrow mononuclear cells. Flow plots on the bottom panel show a decrease in this flow-defined B-lymphoblast population to 2.6% of bone marrow mononuclear cells after one dose of azacitidine
FIGURE 2.
Trend of molecular and cytogenetic abnormalities; the left Y-axis represents the percentage of monosomy 7 and CDKN2A deletion by fluorescent in situ hybridization, and KRAS G12A variant allele fraction (VAF) by next-generation sequencing in bone marrow aspirates; the right Y-axis represents the percentage of aberrant B-cell lymphoblasts by flow cytometry; the X-axis denotes the time points during azacitidine (Aza) therapy at which that the molecular and cytogenetic data were obtained; BLD, below limit of detection
3 ∣. DISCUSSION
JMML is a biologically heterogeneous disorder.7 In genome methylation studies, patients with KRAS mutation appear to have an intermediate methylation signature, which translates into a lower recurrence rate following HSCT than that noted in PTPN11-mutated JMML.2,8 JMML with KRAS mutation can be difficult to distinguish from RAS-associated lymphoproliferative disorder (RALD); the latter can have identical somatic KRAS mutations and monocytosis but is characterized by an indolent, nonmalignant course.1,12 Presence of monosomy 7 is suggestive of a malignant process and considered an exclusion criterion for RALD. In our patient, thus, the diagnosis of JMML was favored. The clinical phenotype with structural brain abnormalities is reminiscent of RAS-opathies, raising the possibility of mosaicism. Germline testing on fibroblasts and remission bone marrows have been negative for the KRAS mutation and monosomy 7, suggesting these lesions were somatic.
Interestingly, there was a downward trend in the KRAS VAF and monosomy 7 prior to initiation of azacitidine, suggesting that our patient may have been heading towards spontaneous resolution. However, the rate of decline was discordant with the improvement of cytopenias and clinical exam. Based on the concern for poor outcome in the presence of ≥2 somatic alterations,7 we pursued treatment with azacitidine, which was anticipated to be tolerated with few side effects and had the potential to decrease disease burden. Additionally, the patient subsequently developed a third somatic alteration, CDKN2A deletion. The rapidity with which the genetic alterations declined thereafter was indicative of a striking therapeutic effect of azacitidine.
Spontaneous remissions have been reported in JMML.13,14 Matsuda et al found that patients with an NRAS or KRAS G12S substitution had a milder course.13 However, these patients also had good clinical prognostic predictors (age <2 years, HbF <15%, and platelet count >33 × 109/L), similar to those in our patient. In the genotype-phenotype correlation by the European Working Group of Myelodysplastic Syndromes in Childhood, patients with RAS mutations who did well without transplant also had better platelet counts and a normal HbF.14
Use of azacitidine in JMML was first described by Furlan et al in a similar patient with somatic KRAS mutation and monosomy 7, who achieved cytogenetic and molecular remission after eight cycles of azacitidine before HSCT.11 Another series reported three of nine patients who received pre-HSCT azacitidine, achieved complete remission, and proceeded to transplant after 7-11 azacitidine cycles. However, no patients with JMML have been followed without HSCT after receiving azacitidine. Prospective trials are ongoing to characterize which patients can be followed post azacitidine without HSCT.10
The finding of an aberrant B-lymphoblast population was another unique feature for our patient. The blast phase of JMML is typically myeloid, similar to chronic myeloid leukemia (CML), though B- and T-cell transformations have been reported.15-18 In CML, a minority of patients will have a small abnormal B-lymphoblast population that does not necessarily signify impending progression to a B-lymphoblastic phase.19 However, this has not been reported in JMML. The B-lymphoblast population in our patient was noted about 6 months after diagnosis. We used it as a surrogate minimal residual disease marker, as levels declined after each cycle of azacitidine with similar kinetics as the decline of his KRAS and monosomy 7 (but with a lower limit of detection). We opted to stop therapy when the levels were undetected by flow after eight cycles of azacitidine (Figure 2).
This is the longest follow-up of a patient with JMML treated exclusively with azacitidine, suggesting that for a specific subset of patients with JMML, azacitidine may represent a curative option. Further studies in cohorts of patients with JMML are needed to define factors predictive of response to azacitidine. Additionally, detection of a small, stable abnormal B-lymphoblast population in patients with JMML does not imply eventual transition to a B-lymphoblastic phase.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to our patient and his family, and would like to thank our colleagues for helpful discussion regarding this case. This work was supported by grant K08CA201611 from the National Institutes of Health.
Abbreviations:
- FISH
fluorescent in situ hybridization
- HbF
hemoglobin F
- HSCT
hematopoietic stem cell transplant
- JMML
juvenile myelomonocytic leukemia
- RALD
RAS-associated lymphoproliferative disorder
- VAF
variant allele fraction
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
REFERENCES
- 1.Locatelli F, Niemeyer CM. How I treat juvenile myelomonocytic leukemia. Blood. 2015;125:1083–1090. [DOI] [PubMed] [Google Scholar]
- 2.Lipka DB, Witte T, Toth R, et al. RAS-pathway mutation patterns define epigenetic subclasses in juvenile myelomonocytic leukemia. Nat Commun. 2017;8:2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loh ML. Childhood myelodysplastic syndrome: focus on the approach to diagnosis and treatment of juvenile myelomonocytic leukemia. Hematol Am Soc Hematol Educ Program. 2010;2010:357–362. [DOI] [PubMed] [Google Scholar]
- 4.Loh ML, Mullighan CG. Advances in the genetics of high-risk childhood B-progenitor acute lymphoblastic leukemia and juvenile myelomonocytic leukemia: implications for therapy. Clin Cancer Res. 2012;18:2754–2767. [DOI] [PubMed] [Google Scholar]
- 5.Locatelli F, Nollke P, Zecca M, et al. Hematopoietic stem cell transplantation (HSCT) in children with juvenile myelomonocytic leukemia (JMML): results of the EWOG-MDS/EBMT trial. Blood. 2005;105:410–419. [DOI] [PubMed] [Google Scholar]
- 6.Bergstraesser E, Hasle H, Rogge T, et al. Non-hematopoietic stem cell transplantation treatment of juvenile myelomonocytic leukemia: a retrospective analysis and definition of response criteria. Pediatr Blood Cancer. 2007;49:629–633. [DOI] [PubMed] [Google Scholar]
- 7.Stieglitz E, Taylor-Weiner AN, Chang TY, et al. The genomic landscape of juvenile myelomonocytic leukemia. Nat Genet. 2015;47:1326–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stieglitz E, Mazor T, Olshen AB, et al. Genome-wide DNA methylation is predictive of outcome in juvenile myelomonocytic leukemia. Nat Commun. 2017;8:2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muramatsu H, Makishima H, Jankowska AM, et al. Mutations of an E3 ubiquitin ligase c-Cbl but not TET2 mutations are pathogenic in juvenile myelomonocytic leukemia. Blood. 2010;115:1969–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cseh A, Niemeyer CM, Yoshimi A, et al. Bridging to transplant with azacitidine in juvenile myelomonocytic leukemia: a retrospective analysis of the EWOG-MDS study group. Blood. 2015;125:2311–2313. [DOI] [PubMed] [Google Scholar]
- 11.Furlan I, Batz C, Flotho C, et al. Intriguing response to azacitidine in a patient with juvenile myelomonocytic leukemia and monosomy 7. Blood. 2009;113:2867–2868. [DOI] [PubMed] [Google Scholar]
- 12.Calvo KR, Price S, Braylan RC, et al. JMML and RALD (RAS-associated autoimmune leukoproliferative disorder): common genetic etiology yet clinically distinct entities. Blood. 2015;125:2753–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuda K, Shimada A, Yoshida N, et al. Spontaneous improvement of hematologic abnormalities in patients having juvenile myelomonocytic leukemia with specific RAS mutations. Blood. 2007;109:5477–5480. [DOI] [PubMed] [Google Scholar]
- 14.Flotho C, Kratz CP, Bergstrasser E, et al. Genotype-phenotype correlation in cases of juvenile myelomonocytic leukemia with clonal RAS mutations. Blood. 2008;111:966–967. [DOI] [PubMed] [Google Scholar]
- 15.Honda Y, Tsuchida M, Zaike Y, et al. Clinical characteristics of 15 children with juvenile myelomonocytic leukaemia who developed blast crisis: MDS Committee of Japanese Society of Paediatric Haematology/Oncology. Br J Haematol. 2014;165:682–687. [DOI] [PubMed] [Google Scholar]
- 16.Luna-Fineman S, Shannon KM, Atwater SK, et al. Myelodysplastic and myeloproliferative disorders of childhood: a study of 167 patients. Blood. 1999;93:459–466. [PubMed] [Google Scholar]
- 17.Lau RC, Squire J, Brisson L, et al. Lymphoid blast crisis of B-lineage phenotype with monosomy 7 in a patient with juvenile chronic myelogenous leukemia (JCML). Leukemia. 1994;8:903–908. [PubMed] [Google Scholar]
- 18.Osumi T, Kato M, Ouchi-Uchiyama M, et al. Blastic transformation of juvenile myelomonocytic leukemia caused by the copy number gain of oncogenic KRAS. Pediatr Blood Cancer. 2017;64:e2649. [DOI] [PubMed] [Google Scholar]
- 19.Vrotsos E, Gorgan M, DiGiuseppe J. Detection of small abnormal B-lymphoblast populations at diagnosis of chronic myelogenous leukemia, BCR-ABL11: incidence, phenotypic features, and clinical implications. Cytometry B Clin Cytom. 2017;92(4):275–278.. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.