Abstract
Background
The use of dental handpieces produces aerosols containing microbial agents, bacteria, and viruses representing a high-risk situation for airborne cross infections. The aim of this study was to map and quantify the biological contamination of a dental operatory environment using a biological tracer.
Methods
Streptococcus mutans suspension was infused into the mouth of a manikin, and an operator performed standardized dental procedures using an air turbine, a contra-angle handpiece, or an ultrasonic scaler. The presence of the tracer was measured at 90 sites on the dental unit and the surrounding surfaces of the operatory environment.
Results
All tested instruments spread the tracer over the entire dental unit and the surrounding environment, including the walls and ceiling. The pattern and degree of contamination were related to the distance from the infection source. The maximum distance of tracer detection was 360 centimeters for air turbine, 300 cm for contra-angle handpiece, and 240 cm for ultrasonic scaler. No surface of the operative environment was free from the tracer after the use of the air turbine.
Conclusions
Attention should be paid to minimize or avoid the use of rotary and ultrasonic instruments when concerns for the airborne spreading of pandemic disease agents are present.
Practical Implications
This study supports the recommendations of dental associations to avoid treatments generating aerosols, especially during pandemic periods. Guidelines for the management of dental procedures involving aerosols, as well as methods for the modification of aerosols aimed to inactivate the infective agent, are urgently needed.
Key Words: Aerosols, air microbiology, bacteria, communicable disease control, cross infection, decontamination, dental equipment, disease transmission, patient-to-professional, Streptococcus
Abbreviation Key: CFU, Colony-forming units; COVID-19, Coronavirus disease 2019; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
The risk of airborne contamination in dentistry is considered high1 owing to the unique characteristics of the dental equipment. The cooling spray of dental handpieces is 1 of the primary sources of spatter and aerosol in surgery.2, 3, 4 Spatters are air suspensions of liquid or solid particles having a particle size of approximately 100 micrometers or more, whereas aerosol particles have a smaller diameter (< 50 μm).5 Spatters are too large to be inhaled but can contaminate skin, eyes, hair, and clothing, in addition to the dental working area. Aerosol particles can remain suspended for a relatively long time (up to 30 minutes) after the end of an operative procedure and are easily spread throughout the operative environment via air currents.6, 7, 8 There is evidence that dental aerosol can reach a distance of 1 through 3 meters from its source.9 , 10 From this point of view, aerosol particles are vectors of infective agents that show potential for contamination not only of the dental care personnel and patients but also of all exposed surfaces of the dental unit and the operatory environment.5 In addition to microbial species from nonpathogenic oral flora, aerosols may contain pathogenic bacteria (such as Mycobacterium tuberculosis, Legionella pneumophila, and various species of Staphylococcus) and viruses (such as HIV, hepatitis B virus, hepatitis C virus, herpes simplex virus, influenza virus, and rhinovirus).11, 12, 13, 14 The problem of airborne contamination in the dental operatory environment returned to the spotlight owing to the outbreak of coronavirus disease 2019 (known as COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This infective agent owes its virulence to high contagiousness related to airborne transmission and to the fact that it can survive on surfaces for up to 72 hours.15 The spreading of SARS-CoV-2 places tremendous stress on health care systems worldwide.16 For this reason, additional preventive measures are being introduced and updated continuously in all health care settings, including dentistry, to reduce further dispersion of this disease.4 , 17, 18, 19, 20, 21, 22
In the past 50 years, attempts have been made to determine the topographic distribution of the airborne contamination caused by different rotary and vibrating-oscillating dental instruments.3 , 19 , 20 The relevance of these data is profound in terms of trying to direct the disinfection procedures rationally on areas of higher contamination and to control the spreading of diseases.
Different approaches have been used to map the contaminants, from dye tracers to microbiological evaluation of air and surface contamination.2 , 10 , 14 , 23 The literature shows that dental treatments significantly increase biological contamination of dental operatories to a higher level than public areas.14 , 24 Nevertheless, to our knowledge, no study has determined the topographic distribution of surface contamination in the dental operatory, although this information is essential in implementing protocols for disinfection procedures in areas with critical levels of contaminants.
In our study, we aimed to evaluate the contamination resulting from the use of rotary and vibrating oscillating instruments in a dental operatory using a biological tracer. The null hypotheses were that the presence of the tracer would be detected uniformly on the dental unit and the operatory environment surfaces and that the spread of the tracer would not be different when using different handpieces.
Methods
Operatory
We used a 598 centimeter by 376 cm by 270 cm (length, width, and height, respectively) operative environment located in the dental clinic at San Paolo Hospital, University of Milan, Milan, Italy, for the study. Both the raised floor and false ceiling were made of 60 cm by 60 cm–sized polyvinyl chloride panels. The air-conditioning system for the operatory was isolated by means of sealing the inlet. The operatory was equipped with a dental unit (Skema 4, Castellini), 2 dental stools, and a 4-door cabinet located behind the dental chair.
We measured the presence of a biological tracer in 22 sites on the dental unit and 68 sites in the operatory. On the dental unit, we placed 14 sites on the dental chair, 1 on the assistant pad, 1 on the instrument tray, 1 on the cuspidor cup, 1 on the water glass tray, 3 on the overhead dental unit light, and 1 on the foot pedal. In the operatory, we placed 48 sites on the floor, 4 on the wall in front of the dental unit, 1 on the lateral column, 5 on the back wall, 6 on the ceiling, and 4 on the cabinet (Figures 1 and 2 ). We used the same sites throughout all the experiments.
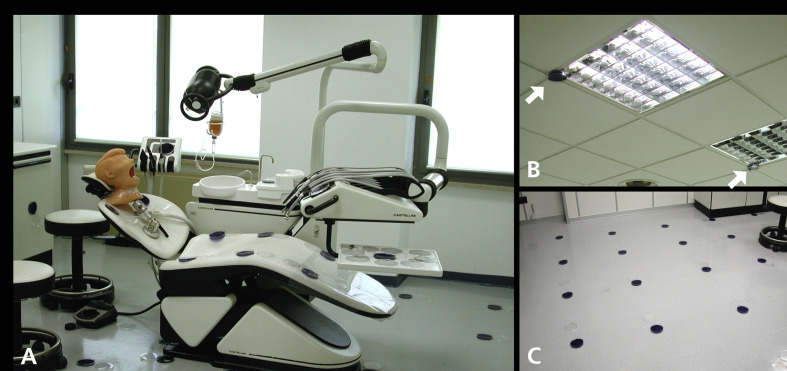
Figure 1.
The dental unit inside the operative environment. A. Opened agar plates that were used to evaluate the blank can be seen. The manikin head was mounted in a working position, and the bacterial suspension was attached with a drip. B. Arrows show the locations of 2 of the 6 plates that mapped tracer presence on the ceiling. C. The location of the detection sites on the floor, 60 centimeters apart from each other.
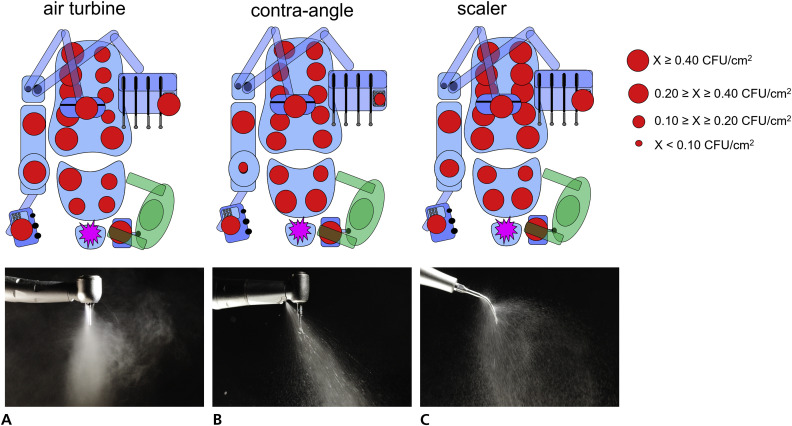
Figure 2.
Schemes representing the topographic distribution and the tracer levels after the dental procedures using the following: air turbine (A), contra-angle (B), and ultrasonic scaler (C) handpieces. Each red dot represents a measurement site on the dental unit. The 3 sites on the dental light source were averaged. The dimension of the dot represents the tracer level. The spreading pattern of spatter and aerosols close to the tip of each handpiece is displayed under each corresponding scheme. Air turbine produced the finest and farthest-spreading microparticles. CFU: Colony-forming units. cm2: Square centimeter. X: Contamination value of each site.
Bacteria
We obtained all reagents and culture media from Becton-Dickinson (BD Diagnostics-Difco). We isolated a wild strain of Streptococcus mutans on mitis salivarius-bacitracin agar from the dental operator who performed all dental procedures. We performed biochemical identification of the isolated strain using an automatic device (Vitek 2, BioMerieux). Then we obtained a pure suspension of S. mutans in trypticase soy broth from a single colony grown on the selective medium after a 12-hour incubation at 37°C in a 5%-supplemented carbon dioxide environment. We harvested cells via centrifugation (1.500 × gravity, 19°C, 5 minutes), washed them twice with sterile phosphate-buffered saline, and resuspended them in the same buffer. We subjected the cell suspension to sonication (Sonifier model B-150, Branson; 7 watt energy output, 30 seconds) to disperse bacterial chains and adjusted it to 1.0 McFarland standard. We obtained a fresh cell suspension in the logarithmic phase before the beginning of each experiment.
Experimental setup
We adapted a manikin head to the chair headrest in a standard working position (Figure 1A). The jaws inside the manikin head were equipped with resin teeth (Columbia Dentoform). We connected the drainpipe of the manikin head to a high-speed suction. After that, the operator performed 3 standardized dental procedures on the mandibular right first molar. For the first procedure, the operator prepared a class I cavity using an air turbine handpiece (Bora Led, Bien-Air Dental) equipped with a cylindrical diamond bur (835KR.314.016, Komet Italia Srl). The air pressure was 3.3 atmospheres, and the speed was 320 revolutions per minute. For the second procedure, the operator used a contra-angle handpiece (CA 1:1, Bien-Air) with a round tungsten carbide bur (H1SM.204.020, Komet) inside the already prepared cavity at a speed of 50 revolutions per minute to mimic removal of deep carious tissue and cavity refining. In the third procedure, the operator used an ultrasonic scaler (Suprasson, Satelec–Acteon) operating at 28 kilohertz oscillation frequency and equipped with an A2 insert to reach below the gingival line of the labial, lingual, and interproximal surfaces of the same tooth. Each procedure lasted 240 seconds, and we replaced the resin tooth after performing the 3 procedures. We infused a continuous flow of S. mutans suspension (30 milliliters per minute) in the mouth of the manikin head on the lingual surface of the mandibular right second molar throughout all procedures using a drip device. The operator wore biohazard-protective full suit, including shoe covers, gloves, filtering face piece respirator-2 or N95 mask without valve, and face shield to protect against possible infections by the tracer agent.
Operatory and microbiological procedures
Before the beginning of each procedure, we coated 90 mitis salivarius-bacitracin agar plates and placed 1 in each of the corresponding sites, keeping the lid closed. The operator took his position, and then a coworker, equipped with the same biohazard protections as the operator, opened the lids of every plate. After that, the operator opened the suspension drip and performed a 4-minute procedure. The coworker closed the plates 26 minutes after the end of the procedure to allow aerosols to settle. Then he immediately transferred the plates to the microbiological laboratory. We incubated the plates at 37◦C for 48 hours in a 5% supplemented carbon dioxide environment. At the end of the incubation, we counted the colonies and expressed the results as colony-forming units (CFU) per square centimeter.
We repeated the procedure 15 times for each handpiece. Between procedures, we disinfected the environment overnight using an ambient decontamination device (Phileas 75, Devea). The operator repeated the same procedures once without using the bacterial suspension, and we considered the results for the tested dental unit and the operative environment as the tracer's blank.
Statistical analysis
We used a statistical software (JMP Version 10.0, SAS Institute) to analyze microbiological data belonging to the tracer presence on the dental unit and the operatory. We applied the Shapiro-Wilk test to check the normality of the data distribution and used the Bartlett test to check the homogeneity of variances preliminarily. Because the data distribution was not normal, we log-transformed the data to approach a normal distribution. We used a 2-way analysis of variance, considering the handpiece and the topography as fixed factors, and Tukey honest significant difference post-hoc test to highlight significant differences between groups, at a level of significance of .05.
Results
Figures 2 and 3 show the topographic distribution of the tracer. Figure 4 shows the mean (standard deviation [SD]) tracer levels. An apparent decrease in tracer presence with an increasing distance from the infection source can be seen in the operating environment, independently from the tested handpiece. However, air turbine spread the tracer at a significantly greater distance than contra-angle handpiece, which in turn spread the tracer farther than the ultrasonic scaler. All sites of the dental unit generally obtained high tracer presence; the highest was found on the cuspidor cup when the air turbine was used, whereas the lowest was found on the same site when the contra-angle handpiece was operated.
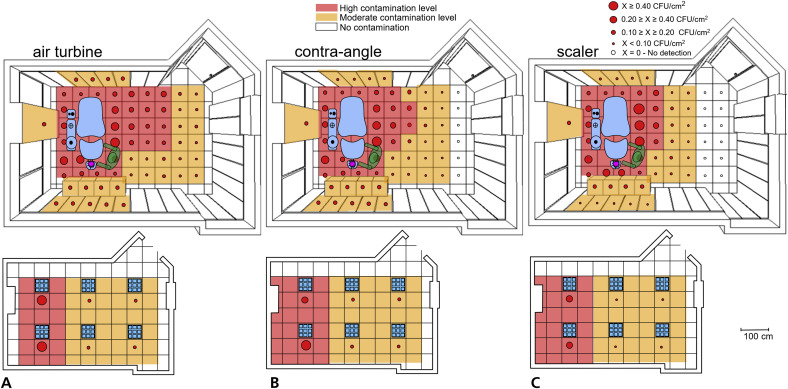
Figure 3.
Schemes representing the topographic distribution and the tracer levels after the dental procedures using the following: air turbine (A), contra-angle (B), and ultrasonic scaler (C) handpieces. Each red dot represents a measurement site of the operatory, including the ceilings, depicted below the main schemes. Values greater than 0.20 colony-forming units per square centimeter (CFU/cm2) are considered as high contamination levels, and the corresponding surfaces are displayed in red. Lower values indicate moderate contamination, and the corresponding surfaces are displayed in orange. White dots and surfaces represent no detection of the tracer.
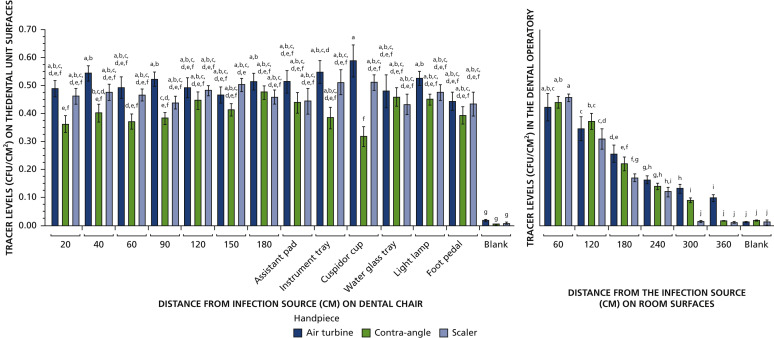
Figure 4.
Tracer presence on the different locations of the dental unit and in the operating environment expressed as colony-forming units per square centimeter (CFU/cm2). Mean (standard deviation) values are indicated, and different superscript letters indicate significant differences between groups (Tukey test, P < .05).
Tracer presence on dental chair unit
The analysis of variance results showed a highly significant difference in tracer levels between the tested handpieces (P < .01). The mean (SD) levels of tracer were air turbine, 0.51 (0.17); higher than ultrasonic scaler, 0.47 (0.14); higher than contra-angle handpiece, 0.41 (0.14). We found no significant difference between different sites on the dental unit when considering the distance from the infection source (P > .05). Also, we did not find an interaction between the considered factors (P = .08). When considering the 2 sides of the dental chair, the left side showed higher tracer levels than the right side when the air turbine was used (P = .01). We did not notice any significant differences in tracer presence when using contra-angle handpiece or ultrasonic scaler (P = .98 and P = .48, respectively).
Tracer presence in the operatory
The use of all handpieces spread the tracer all over the surrounding environment of the dental operatory. The mean (SD) levels of tracer were air turbine, 0.26 (0.38); higher than contra-angle handpiece, 0.20 (0.26); higher than ultrasonic scaler, 0.17 (0.27). The pattern and the tracer levels were related to the distance from the infection source. We found a highly significant interaction between factors (P < .0001). This result shows that the topographic distribution of the tracer varied depending on the tested handpiece. When the ultrasonic scaler was used, the tracer reached a maximum distance of 240 cm, whereas during the use of the contra-angle handpiece, the tracer reached 300 cm. When using the air turbine, however, no site remained free from tracer presence, reaching the maximum distance recorded, that is, 360 cm. Sites located on the floor behind the operator showed low tracer presence. The tracer presence on the walls and the ceiling was low, except for the ceiling area just over the dental unit, where we found a high tracer presence (Figure 3). Morphologic observation of plates after incubation showed high variability in the distribution of the colonies on the surfaces of the plates located within 150 cm from the tracer source.
Discussion
The danger of cross infection through spatters and aerosols has long been considered 1 of the main concerns in the dental practice.2 , 3 , 14 , 20 , 24 Air-spray cooled handpieces, such as air turbines, contra-angle handpieces, and scalers, produce spatters and aerosols that can reach a considerable distance, carrying potentially infective agents.6 , 14 , 19 , 22 Despite being necessary, the use of air-spray cooling is recognized as 1 of the primary sources of contamination in the dental setting.23 In fact, even before the outbreak of SARS-CoV-2, the potential airborne spreading of life-threatening infections was well recognized.13 , 14 , 25 Nevertheless, there are few data available on the topographic distribution of contamination induced by aerosol-generating devices. The SARS-CoV-2 outbreak highly increased the need for such experimental data,22 , 26 , 27 to address rationally the need for operative and disinfection procedures yielding the lowest possible contamination levels.
The contamination usually produced directly by the patients themselves (talking, breathing, sneezing, coughing) or during high-risk medical procedures (tracheal intubation, manipulation of the oxygen mask, bronchoscopy, noninvasive ventilation, insertion of a nasogastric tube) shows a high variability owing to interindividual differences.14 , 28 Furthermore, aerosols produced by patients show different behaviors depending on the particle size. Indeed, it has been observed that the size of a pathogen dictates the size of the particle that is carrying that pathogen. For instance, aerosol particles that carry viral particles are much smaller than particles carrying larger pathogens such as bacteria.12 This may not be the case in the dental setting because aerosols and spatters are mechanically produced and thus have a particle size that depends on the functioning parameters of each handpiece. Our study shows that dental handpieces generate a contamination pattern with relatively low variability. This phenomenon is due to the direct production of the aerosol via handpieces in a standardized way following defined operating parameters. Studies have shown that aerosols and spatters produced via dental handpieces are able to carry and diffuse any pathogen that is present in the oral environment and in saliva. These pathogens include bacteria and viruses from the nose, throat, and respiratory tract.6 SARS-CoV-2 is an infective pathogen that is harbored mainly in these locations, and therefore it is prone to be carried via aerosol generated during dental procedures.
Our findings allowed us to reject both null hypotheses, implying that the presence of the tracer was not detected uniformly on the dental unit and the operatory environment surfaces and that the spread of the tracer was significantly different when the tested handpieces were used. Our results reveal the existence of heavy contamination involving the whole dental unit as well as the surrounding surfaces of the operatory. Values higher than 0.10 CFU/cm2 exceeded the guideline value for good hygiene, indicating moderate contamination.29 Values higher than 0.20 CFU/cm2 were considered arbitrarily as a high contamination level. Furthermore, the area contaminated by the biological tracer via spatters and aerosols was wide, reaching a maximum distance of 360 cm from the infection source when we operated the air turbine. Subsequently, no surface of the operative environment was left free from the biological tracer after the tested dental procedures involving air turbine.
When looking at the contamination in the dental operatory, the contra-angle handpiece yielded lower tracer levels than the air turbine, and the ultrasonic scaler showed the lowest tracer levels overall. The same sequence was evidenced when considering the maximum distance at which the tracer was detected. The high variability in the distribution of bacterial colonies on the sites within 150 cm from the infection source after using the handpieces likely suggests that spatters were the primary vector of the tracer. This result may suggest that the primary source of contamination for both dental operators and dental unit surfaces may be spatters rather than aerosols. However, relatively regular distribution of the bacterial colonies at a higher distance suggests aerosols as the primary vector of the tracer, and this finding was not dependent on the type of handpiece.
Walls and ceiling showed a relatively regular distribution of the colonies, being seemingly reached by aerosols. This result is relevant because, as far as we are aware, no other study in the literature has shown the possibility for aerosols to reach such surfaces. These findings suggest the need for disinfection protocols to include such surfaces. Regarding the topographic distribution of the tracer on the dental chair, the distance from the infection source did not influence tracer levels except for air turbine, which caused a higher degree of tracer presence on the left side of the chair, likely owing to the fact that the operator was right-handed. Also, lower tracer presence on the floor behind the operator probably was due to the barrier effect caused by the operator's position.
Considering airborne transmission, bacterial and viral infectious agents may be carried by aerosols, which can remain suspended for a considerable amount of time and travel relatively long distances.5 , 6 , 14 , 18 , 27 , 30 However, as far as we are aware, there is no evidence in the literature that bacteria behave differently from viruses when spread by an aerosol. In our study, we used a biological tracer to simulate clinical conditions as closely as possible and to allow both quantitative and topographic evaluation of aerosol diffusion. We selected the bacterial tracer (S. mutans) to simulate the diffusion of any infective agent by aerosol. The choice of a relatively low pathogenic microorganism as a tracer and of a passive method of sampling was motivated by health-risk concerns and ethical reasons. Owing to the peculiar characteristics of the analysis techniques we used in our study, we found relatively large variability of the data, as expected. We performed a high number of replications (15) of the experiments to control such effects.
We have to distinguish between studies using an active sampling method and those using a passive one. An active sampling method is based on suction systems coupled with filters or agar plates that collect the infective agents in specific sampling locations. This technique has been used to characterize the different types of infective agents of an aerosol.30 , 31 However, it does not allow for mapping the surface spreading of the contamination. Passive methods are based mainly on detection of surface contamination by aerosols, most often using agar plates or sampling filters that collect the droplets coming into contact with the surface after a specified amount of time. The latter method, therefore, allows for precise mapping and evaluation of the variability of the contamination at a specific site.
Few studies have mapped the operatory surfaces reached by aerosols produced by dental handpieces, and, to our knowledge, none were based on the use of a biological tracer under standardized conditions. Miller and colleagues3 used a setup similar to that of our study, and they showed the presence of a high degree of bacterial contamination at about 240 cm (the measured maximum distance) using an air turbine. Hackney and colleagues32 used viridans streptococci as biological indicators of oral contamination of the operatory because they are known to be abundant in human saliva. These bacteria were detected on operatory surfaces after dental treatments were finished and surfaces were disinfected, confirming the validity of using a biological tracer. The approach is similar to the 1 used in our study, but it was performed without a true standardization of the infection source. Contrary to the setup of that study, the experimental conditions we applied in our study allowed us to define the topographic distribution of the contamination and to measure this parameter reliably. A comparison between the contaminating effect of the different tested handpieces was, therefore, possible.
Rautemaa and colleagues20 collected fallout samples on blood agar plates (measured maximum distance, 200 cm) in the operatory after using air turbine. The results showed significant contamination at all sampled distances. These findings are in agreement with those of our investigation on air turbine contamination. Using a similar experimental setup as in our study, Purohit and colleagues33 evaluated the effect of rinses with an antibacterial mouthrinse on the reduction of airborne contamination measured at a maximum distance of 60 cm. Contrary to our results, the ultrasonic scaler produced significantly higher contamination than the air turbine. Higher variability in contamination data at the recorded distance may explain the differences between the findings. The results of the investigation by Chuang and colleagues34 showed that bacterial aerosols could reach a 100 cm horizontal and a 50 cm vertical distance (measured maximum distances) from a patient's oral cavity, remaining suspended for 20 minutes. The findings of our study show that the distances reached by dental aerosols are underestimated severely.
Possible limitations of our investigation are related to the operatory, which provided space constraints to the source of infection, and the absence of data regarding the operator contamination. When looking at the topographic distribution of the tracer, it is reasonable to conclude that the operator was exposed to highest tracer levels. Also, the air-conditioning intake was blocked in our setup; therefore, the effects of air currents on aerosols are not known.
Further research is needed to find alternative approaches to the threat represented by aerosol generation in dentistry. A possible solution could be modifying the composition of the aerosols produced by handpieces. This could be achieved via the addition of water spray with a disinfectant agent with low toxicity that is able to inactivate the pathogen but avoid deterioration of dental unit waterlines, such as 0.5% hydrogen peroxide for coronaviruses. In this way, the disinfectant agent could be active both in the aerosol spreading phase and once deposited on surrounding surfaces within 1 minute.35 Another topic for future research should be to study the influence of additional protection procedures, such as the use of high-volume suction systems and rubber dams, on the spread of contamination.
Conclusions
Dental procedures involving rotary and oscillating handpieces spread the biological tracer throughout the dental operatory. Therefore, attention should be paid to minimize their use, especially during a pandemic by an airborne spreading agent. The results of our study highlight the need to disinfect all surfaces of the dental operatory within 360 cm of the infection source (patient's oral cavity). Furthermore, because the maximum contamination was found in the dental unit area, the highest attention must be paid to the use of personal protection equipment and decontamination procedures of the operators.
Biographies
Dr. Ionescu is an adjunct professor and postdoctorate researcher, Oral Microbiology and Biomaterials Laboratory, Department of Biomedical, Surgical and Dental Sciences, University of Milan, Milan, Italy.
Dr. Cagetti is an associate professor, Department of Biomedical, Surgical and Dental Sciences, University of Milan, Milan, Italy.
Dr. Ferracane is a professor and the chair of restorative dentistry and division director of biomaterials and biomechanics, Oregon Health and Science University, Portland, OR.
Dr. Garcia-Godoy is a professor and the director, Bioscience Research Center, and the director of clinical research, College of Dentistry, University of Tennessee Health Science Center, Memphis, TN; and a senior clinical investigator, Forsyth Center, Cambridge, MA.
Dr. Brambilla is an associate professor and the head of the Oral Microbiology and Biomaterials Laboratory, Department of Biomedical, Surgical and Dental Sciences, University of Milan, Milan, Italy.
This article has an accompanying online continuing education activity available at: http://jada.ada.org/ce/home.
Footnotes
Disclosure. None of the authors reported any disclosures.
References
- 1.Volgenant C.M.C., de Soet J.J. Cross-transmission in the dental office: does this make you ill? Curr Oral Health Rep. 2018;5(4):221–228. doi: 10.1007/s40496-018-0201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrel S.K., Barnes J.B., Rivera-Hidalgo F. Aerosol and splatter contamination from the operative site during ultrasonic scaling. JADA. 1998;129(9):1241–1249. doi: 10.14219/jada.archive.1998.0421. [DOI] [PubMed] [Google Scholar]
- 3.Miller R.L., Micik R.E., Abel C., Ryge G. Studies on dental aerobiology, II: microbial splatter discharged from the oral cavity of dental patients. J Dent Res. 1971;50(3):621–625. doi: 10.1177/00220345710500031701. [DOI] [PubMed] [Google Scholar]
- 4.Szymanska J., Medicine E. Dental bioaerosol as an occupational hazard in a dentist's workplace. Ann Agric Environ Med. 2007;14(2):203–207. [PubMed] [Google Scholar]
- 5.Leggat P.A., Kedjarune U. Bacterial aerosols in the dental clinic: a review. Int Dent J. 2001;51(1):39–44. doi: 10.1002/j.1875-595x.2001.tb00816.x. [DOI] [PubMed] [Google Scholar]
- 6.Harrel S.K., Molinari J.J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. JADA. 2004;135(4):429–437. doi: 10.14219/jada.archive.2004.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larato D.C., Ruskin P.F., Martin A. Effect of an ultrasonic scaler on bacterial counts in air. J Periodontol. 1967;38(6):550–554. doi: 10.1902/jop.1967.38.6_part1.550. [DOI] [PubMed] [Google Scholar]
- 8.Shpuntoff H., Shpuntoff R. High-speed dental handpieces and spread of airborne infections. N Y State Dent J. 1993;59(1):21–23. [PubMed] [Google Scholar]
- 9.Logothetis D.D., Martinez-Welles J.M. Reducing bacterial aerosol contamination with a chlorhexidine gluconate pre-rinse. JADA. 1995;126(12):1634–1639. doi: 10.14219/jada.archive.1995.0111. [DOI] [PubMed] [Google Scholar]
- 10.Veena H.R., Mahantesha S., Joseph P.A., Patil S.R., Patil S.H. Dissemination of aerosol and splatter during ultrasonic scaling: a pilot study. J Infect Public Health. 2015;8(3):260–265. doi: 10.1016/j.jiph.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Cowling B.J., Ip D.K., Fang V.J. Aerosol transmission is an important mode of influenza A virus spread. Nat Commun. 2013;4:1935. doi: 10.1038/ncomms2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gralton J., Tovey E., McLaws M.L., Rawlinson W.D. The role of particle size in aerosolised pathogen transmission: a review. J Infect. 2011;62(1):1–13. doi: 10.1016/j.jinf.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marui V.C., Souto M.L.S., Rovai E.S., Romito G.A., Chambrone L., Pannuti C.M. Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol: a systematic review. JADA. 2019;150(12):1015–1026 e1. doi: 10.1016/j.adaj.2019.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Zemouri C., de Soet H., Crielaard W., Laheij A. A scoping review on bio-aerosols in healthcare and the dental environment. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0178007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Doremalen N., Bushmaker T., Morris D.H. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velavan T.P., Meyer C.G. The COVID-19 epidemic. Trop Med Int Health. 2020;25(3):278–280. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abichandani S.J., Nadiger R. Cross-contamination in dentistry: a comprehensive overview. J Edu Ethic Dent. 2013;4(1):51. [Google Scholar]
- 18.James R., Mani A. Dental aerosols: a silent hazard in dentistry! Int J Sci Res. 2016;5:1761–1763. [Google Scholar]
- 19.Prospero E., Savini S., Annino I. Microbial aerosol contamination of dental healthcare workers' faces and other surfaces in dental practice. Infect Control Hosp Epidemiol. 2003;24(2):139–141. doi: 10.1086/502172. [DOI] [PubMed] [Google Scholar]
- 20.Rautemaa R., Nordberg A., Wuolijoki-Saaristo K., Meurman J.H. Bacterial aerosols in dental practice–a potential hospital infection problem? J Hospital Infect. 2006;64(1):76–81. doi: 10.1016/j.jhin.2006.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng L, Hua F, Bian Z. Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J Dent Res. 2020:22034520914246. [DOI] [PMC free article] [PubMed]
- 22.Peng X., Xu X., Li Y., Cheng L., Zhou X., Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci. 2020;12(1):9. doi: 10.1038/s41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis D.L., Arens M., Appleton S.S. Cross-contamination potential with dental equipment. Lancet. 1992;340(8830):1252–1254. doi: 10.1016/0140-6736(92)92950-k. [DOI] [PubMed] [Google Scholar]
- 24.Kimmerle H., Wiedmann-Al-Ahmad M., Pelz K., Wittmer A., Hellwig E., Al-Ahmad A. Airborne microbes in different dental environments in comparison to a public area. Arch Oral Biol. 2012;57(6):689–696. doi: 10.1016/j.archoralbio.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Bruns S., Kniemeyer O., Hasenberg M. Production of extracellular traps against Aspergillusfumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog. 2010;6(4) doi: 10.1371/journal.ppat.1000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J., Li Y., Gan F., Du Y., Yao Y. Salivary glands: potential reservoirs for COVID-19 asymptomatic infection. J Dent Res. 2020;99(8) doi: 10.1177/0022034520918518. [DOI] [PubMed] [Google Scholar]
- 27.Ge Zi-Yu, Yang Lu-Ming, Xia Jia-Jia, Fu Xizo-Hui, Zhang Yan-Zhen Possible aerosol transmission of COVID-19 and special precautions in dentistry. J Zhejiang Uni Sci B. 2020;21(5):361–368. doi: 10.1631/jzus.B2010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson K.A., Pappachan J.V., Bennett A.M. EASE Study Consortium Influenza aerosols in UK hospitals during the H1N1 (2009) pandemic: the risk of aerosol generation during medical procedures. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houhala K., Rahkio M., Levo S., Sauna-Aho R., Valikyla T., editors. A Guide Book to Monitoring Surface Hygiene. Vammalan Kirjapaino Oy; Vammala, Finland: 1996. [Google Scholar]
- 30.Bennett A.M., Fulford M.R., Walker J.T., Bradshaw D.J., Martin M.V., Marsh P.D. Microbial aerosols in general dental practice. Br Dent J. 2000;189(12):664–667. doi: 10.1038/sj.bdj.4800859. [DOI] [PubMed] [Google Scholar]
- 31.Bârlean L., Iancu L.S., Minea M.L., Dãnilã I., Baciu D. Airborne microbial contamination in dental practices in Iasi, Romania. Oral Health Dental Management. 2010;9(1):16–20. [Google Scholar]
- 32.Hackney R.W., Jr., Crawford J.J., Tulis J.J. Using a biological indicator to detect potential sources of cross-contamination in the dental operatory. JADA. 1998;129(11):1567–1577. doi: 10.14219/jada.archive.1998.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purohit B., Priya H., Acharya S., Bhat M., Ballal M. Efficacy of pre-procedural rinsing in reducing aerosol contamination during dental procedures. J Infect Prev. 2009;10(6):190–192. [Google Scholar]
- 34.Chuang C.-Y., Cheng H.-C., Yang S., Fang W., Hung P.-C., Chuang S.-Y. Investigation of the spreading characteristics of bacterial aerosol contamination during dental scaling treatment. J Dent Sci. 2014;9(3):294–296. [Google Scholar]
- 35.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]