Abstract
In December 2019, a novel coronavirus pneumonia (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) suddenly broke out in China and rapidly spread all over the world. Recently, a cell surface protein, known as angiotensin-converting enzyme II (ACE2), has been identified to be involved in receptor-mediated endocytosis for SARS-CoV-2 entry to the cells. Many studies have reported the clinical characteristics of COVID-19: sudden deterioration of disease around 1–2 weeks after onset; much lower level of lymphocytes, especially natural killer (NK) cells in peripheral blood; extremely high pro-inflammatory cytokines and C reactive protein (CRP). About 15.7% of patients develop severe pneumonia, and cytokine storm is an important factor leading to rapid disease progression. Currently, there are no specific drugs for COVID-19 and the cytokine storm it causes. Baricitinib intracellularly inhibits the proinflammatory signal of several cytokines by suppressing Janus kinase (JAK) JAK1/JAK2. It has been demonstrated clinical benefits for the patients with rheumatoid arthritis (RA), active systemic lupus erythematosus and atopic dermatitis with good efficacy and safety records. Baricitinib is expected to interrupt the passage and intracellular assembly of SARS-CoV-2 into the target cells mediated by ACE2 receptor, and treat cytokine storm caused by COVID-19. Several clinical trials are currently investigating the drug, and one of which has been completed with encouraging results. In this paper, we will elaborate the role of cytokine storm mediated by JAK-STAT pathway in severe COVID-19, the possible mechanisms of baricitinib on reducing the viral entry into the target cells and cytokine storm, the key points of pharmaceutical care based on the latest research reports, clinical trials progress and drug instruction from the US FDA, so as to provide reference for the treatment of severe COVID-19.
Keywords: COVID-19, Cytokine storm, Baricitinib, JAK-STAT, Pharmaceutical care
1. Background
Since December 2019, coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has broken out in Wuhan, China, and has spread rapidly in most countries of the world. Recently, a cell surface protein, known as angiotensin-converting enzyme II (ACE2), has been identified to be involved in receptor-mediated endocytosis for SARS-CoV-2 entry to the cells [1]. The team of Nanshan Zhong found that 87.9% patients had fever after hospitalization and 15.7% patients developed to severe pneumonia [2]. In this outbreak of COVID-19, some infected patients would suddenly worsen and developed acute respiratory distress syndrome (ARDS), and then shock, tissue perfusion disorders in the later stage, eventually die from multiple organ failure, and the aggravation was mainly caused by cytokine storm [3], [4]. That was described in detail in our last review [5]. Therefore, how to block the cytokine storm and when to initiate anti-inflammation therapy is critical for reducing death rate of COVID-19. The clinically severe phase of COVID-19 is accompanied by high levels of cytokine signalling, all of which signal through the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway. Baricitinib, is not only expected to interrupt the passage and intracellular assembly of SARS-CoV-2 into the target cells mediated by ACE2 receptor, but also promises to be a specific drug that blocks cytokine storms by suppressing JAK1/JAK2 [6]. However, the clinical experience and data of baricitinib in the treatment of COVID-19 are limited, and the mechanism of drug action, applicable patients and risks of the drug need to be recognized. In this paper, we will elaborate the role of cytokine storm mediated by JAK-STAT pathway in severe COVID-19, the possible mechanisms of baricitinib blocking SARS-CoV-2 entry into the target cells and controlling the cytokine storm, the key points of pharmaceutical care based on the latest research reports, clinical trial progress and drug instruction from the US FDA, so as to provide reference for the treatment of COVID-19.
2. Cytokine storm mediated by JAK-STAT pathway in severe COVID-19
Cytokines, a group of small proteins secreted by stromal cells and immune system cells, used mainly for inter-cell signaling and communication and have a variety of biological functions such as regulating innate immunity, adaptive immunity, hematopoiesis, cell growth and differentiation, and repairing damaged tissues by binding to receptors. Cytokines mainly include interleukin (IL), interferon (IFN), tumor necrosis factor (TNF), colony stimulating factor (CSF), chemokine and growth factor. When the human body is invaded by bacteria and viruses, the immune system will release a large number of cytokines [7], [8]. Excessive and uncontrolled release of pro-inflammatory cytokines contributes to cytokine storm. In this outbreak of COVID-19, some infected patients would suddenly worsen in the later stage and eventually die from multiple organ failure, and the aggravation was mainly caused by cytokine storm [4]. These pathological mechanisms may be closely relevant to the death of COVID-19 patients [9]. A number of recently researches have analyzed the clinical characteristics of COVID-19 patients, and consistently found that the lymphocyte count was significantly reduced in patients with pneumonia, especially those with severe pneumonia, while numerous cytokines (such as IL-1β, IL-2, IL- 6, IL-8, IL-10, TNF-α and IFN-γ) were significantly increased, which was the main cause of the cytokine storm, especially IL-6 [9], [10], [11], [12], [13]. Previous therapy for controlling cytokine storm with large doses of glucocorticoid may delay the clearance of coronavirus and cause complications of glucocorticoid therapy. In addition to clinical trials, glucocorticoids should not be used to treat lung injury or shock caused by COVID-19 [14].
JAK are a group of cytoplasmic enzymes with tyrosine kinase activity that facilitate the transmission of signals from the cell surface to its interior, which are extremely important in pro-inflammatory cytokine-mediated signaling process. STAT, one of the most crucial cytokine-activated transcription factors in the process of immune response, is phosphorylated by JAK, dimerized, and then transports into the nucleus through the nuclear membrane to regulate the expression of related genes. This pathway is so called JAK-STAT signaling pathway that promotes the cytokine storm mediated by excessive amounts of cytokine in a simple and efficient way [15]. There are 4 JAK members: JAK1, JAK2, JAK3, and TYK2 and 7 STAT members: STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6 [16], [17]. JAK1, JAK2 and TYK2 are expressed indiscriminately throughout the body, whereas JAK3 is expressed in the haematopoietic cells [18]. Activation of the JAK-STAT pathway is induced by cytokine binding to surface receptors and culminates in regulation of transcription by STATs. Various cytokines and growth factors transmit signals through the JAK-STAT signaling pathway, including IL-2 ~ 7, GM-CSF, epidermal growth factor (EGF), platelet derived growth factor (PDGF), and IFN [16]. For example, IFN-γ signals via JAK1 and JAK2 which in turn activate STAT1, whereas IL-6, a JAK-STAT dependent cytokine, can signal via JAK1, JAK2, and/or TYK2 and activates STAT3 in T cells [19]. As cytokine storm is relatively common in severe case and often leads to the exacerbation of COVID-19, anti-inflammation therapy may help in preventing further injury. As we know, there are a variety of anti-inflammatory medications, including JAK inhibitor. Such drugs are predicted to be of particular importance in the treatment of severe COVID-19.
3. Possible mechanism of baricitinib in the treatment of COVID-19
Receptor-mediated endocytosis is the most common pathway for virus entry to the cells. Recently, a cell surface protein, known as angiotensin-converting enzyme II (ACE2), has been identified for SARS-CoV-2 in humans and animals. ACE2, expressed by cells in the kidney, blood vessels, heart, and most importantly in lung AT2 alveolar epithelial cells, is the receptor used by SARS-CoV-2 to infect lung cells [1]. The ACE2 receptor has several regulators among which AP2-associated protein kinase-1 (AAK1) and cyclin G-associated kinase (GAK), two pivotal regulators, mediate clathrin-dependent endocytosis [20]. Baricitinib was expected to have a high binding affinity to AAK1 and GAK and interrupt the passage and intracellular assembly of SARS-CoV-2 into the target cells [21]. Accordingly, baricitinib is suggested to be trialed on patients with SARS-CoV-2 acute respiratory disease in order to reduce the viral entry and the associated inflammation (NCT04321993).
Baricitinib not only interrupts the passage and intracellular assembly of SARS-CoV-2 into the target cells but it also reduces the inflammation in patients with ARDS. In severe COVID-19 cases, the JAK-STAT signaling pathway participates the cytokine storm. Excessive amounts of cytokines bind to their receptors and activates JAKs, which occurs upon ligand-mediated receptor multimerization, since two JAKs are close enough for trans-phosphorylation [22]. The activated JAKs phosphorylate the receptors, activate and phosphorylate their main substrate STATs. Phosphorylated STAT dimerizes with other members of STAT family with conserved SH2 domains. Finally, the dimerized STATs in the cytoplasm are transferred into the nucleus and combine to specific DNA elements to regulate the expression of cytokine-responsive genes. Subsequently, cytokine storm was finally induced.
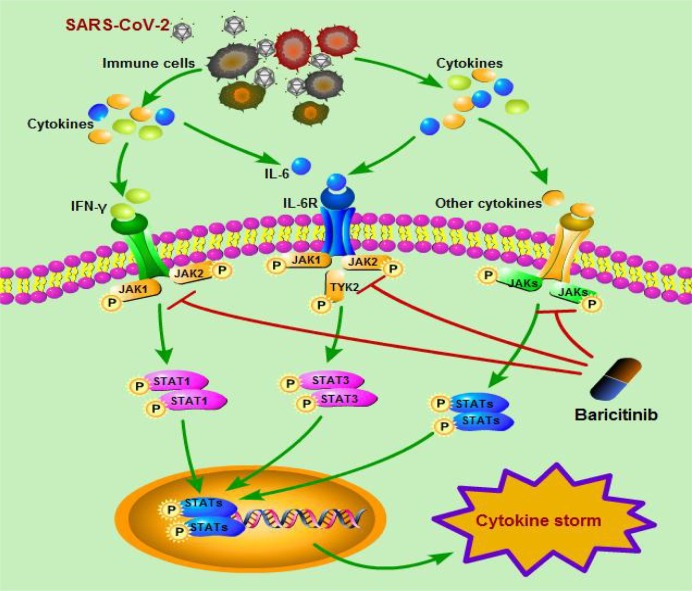
Baricitinib is an ATP competitive kinase inhibitor that inhibits selectively, effectively, and reversibly JAK1/JAK2. By suppressing JAK1/JAK2, baricitinib intracellularly inhibits the proinflammatory signal of several cytokines, such as IL-6, IL-12, IL-23 and IFN-γ [6]. It has been demonstrated clinical benefits for the patients with moderate or severe RA, including reducing the incidence of structural joint damage [18]. This drug might to lower the hyper inflammation, or so-called cytokine storm caused by SARS-CoV-2, that would prevent damage to the lungs and possibly other organs (see Fig. 1 ).
Fig. 1.
The possible mechanisms of baricitinib on cytokine storm caused by SARS-CoV-2.
When the human body is invaded by SARS-CoV-2, the immune system will release a large number of cytokines (such as IFN-γ, IL-6 etc.). Excessive amounts of cytokines bind to their receptors and activates JAKs, which occurs upon ligand-mediated receptor multimerization. The activated JAK phosphorylates the receptor, activates and phosphorylates its main substrate STATs. For example, IFN-γ signals via JAK1 and JAK2 which in turn activate STAT1, whereas IL-6 can signal via JAK1, JAK2, and/or TYK2 and activates STAT3. Phosphorylated STAT dimerizes with other members of STAT family. Then, the dimerized STATs in the cytoplasm are transferred into the nucleus and combine to specific DNA elements to regulate the expression of cytokine-responsive genes. Subsequently, cytokine storm was finally induced.
However, there are some different views on the theory of baricitinib to treat cytokine storms, which needs to be clarified. In certain pathological conditions, the inflammatory process is widely recognized as a localized protective response of the body when it is attacked by pathogens, such as SARS-CoV-2. The JAK-STAT is the primary signaling pathway regulated by cytokines and is crucial for initiating the innate immunity, orchestrating the adaptive immune mechanisms, and finally constraining the inflammatory and immune responses [16], [23]. It seems that using a JAK1/JAK2 inhibitor to treat a viral disease might appear illogical given that the antiviral effects of IFN are largely mediated by the JAK-STAT signalling pathway. So, it is speculated here that in early asymptomatic disease and stages of the disease not requiring admittance to hospital, approximately 80% of COVID-19 patients are able to clear the virus through endogenous antiviral mechanisms, certainly including the IFN. Therefore, it is not recommended that baricitinib or other JAK inhibitors be given to these individuals [24].
However, both COVID-19 and SARS are characterized by an over exuberant inflammatory response, akin to a so-called cytokine storm, and viral load is not correlated with the worsening of symptoms [9], [25]. The clinically severe phase of COVID-19 is accompanied by high levels of cytokine signalling, all of which signal through the JAK-STAT pathway. This finding suggests that when hospital care is required for patients with a pathogenic SARS-CoV-2 infection, JAK-STAT pathway inhibition might be a potential strategy. In patients with moderate disease requiring hospital care, the peak SARS-CoV-2 load occurs within approximately 7 days of symptom onset, and later, as the viral titre decreases in some patients, cytokine storm, causes the severe phase of the disease [26].
4. Progress in clinical trials of baricitinib for severe COVID-19
Baracitinib has shown favorable pharmacokinetic properties such as low plasma protein binding affinity, minimal interaction with CYP enzymes, and drug transporters [27]. Currently, in the COVID-19 outbreak, baricitinib is being combined with different antivirals, since this combination would decrease viral infectivity, viral replication, and control cytokine storm. As of June 2, 2020, we retrieved a total of 14 clinical trials of baricitinib in the treatment of COVID-19 worldwide through the US National Library of Medicine [28]. These trials were distributed in East Asia, Europe, North America and Southeast Asia (1, 9, 4 and 1, respectively). A Phase 2/3 clinical trial with the title of “Baricitinib Therapy in COVID-19: A Pilot Study on Safety and Clinical Impact” listed in Italy (NCT04358614) has been completed. In the trial, twelve patients with a median age of 63.5 (IQR: 57.7–72.2) years were enrolled. All consecutive hospitalized patients with moderate COVID-19 pneumonia were administered baricitinib 4 mg/day combined with lopinavir/ritonavir (200/50 mg bid) for 2 weeks. The last consecutive patients with moderate COVID-19 pneumonia receiving standard therapy (lopinavir/ritonavir 200/50 mg bid and hydroxychloroquine 400 mg/day for 2 weeks) admitted before the date of the first baricitinib-treated patient served as controls. Baricitinib-treated patients were compared with controls. Excitingly, the results showed that all clinical characteristics and respiratory function parameters significantly improved both at week 1 and week 2 in the baricitinib-treated group, compared to baseline. Fever, arterial oxygen saturation (SpO2), ratio arterial oxygen partial pressure/fractional inspired oxygen (PaO2/FiO2), CRP, and Modified Early Warning Score (MEWS) significantly improved in the baricitinib-treated group compared with controls (p: 0.000; 0.000; 0.017; 0.023; 0.016, respectively). In the control group, no significant changes were recorded at week 2 compared to baseline. ICU transfer was requested in 33% (4/12) of controls and in none of the baricitinib-treated patients. Discharge at week 2 occurred in 58% (7/12) of the baricitinib-treated patients vs 8% (1/12) of controls (p = 0.027). Baricitinib-treatment was well tolerated with no infections, cardiovascular and hematologic AEs occurred after 2 weeks treatment [29].
5. Clinical application of baricitinib
5.1. Previous clinical application of baricitinib
Baricitinib was approved by the European Union for the treatment of RA patients in 2017. According to the US FDA drug instructions, baricitinib is recommended for adult patients with moderately to severely active RA who have had an inadequate response to one or more TNF antagonist therapies. The recommended dose is 2 mg/d [30]. So far, there have been very few research reports about the extra-RA effects of baricitinib. As is known that targeting cytokines and cytokine receptors with biologics has revolutionized the treatment of many immune and inflammatory diseases. Wallace et al. found that baricitinib signifiantly improved the signs and symptoms of active systemic lupus erythematosus in patients who were not adequately controlled despite standard of care therapy [31]. Recent studies reported that baricitinib improved clinical signs and symptoms in patients with moderate-to-severe atopic dermatitis and induced rapid reduction of itch [32].
5.2. Drug usage for treating COVID-19 with baricitinib and applicable patients
Baricitinib has not been approved for cytokine storm caused by SARS-CoV-2. However, As of April 29, 2020, 14 clinical trials of baricitinib in the treatment of COVID-19 have been registered for reference, and one of which has been completed with encouraging results. In these clinical trials, participants were administered baricitinib 2 mg or 4 mg po daily as monotherapy or in combination with antiviral drugs (such as Lopinavir/Ritonavir) for 7–14 days.
We can also refer to the patient eligibility criteria of current clinical trials to determine which COVID-19 patients are suitable for receiving baricitinib. However, eligibility criteria of study participants fail to reach agreement, because clinical trials of baricitinib are conducted under widely varying conditions [28]. The more widely adopted inclusion criteria is: Laboratory-confirmed SARS-CoV-2 infection; Inpatients diagnosed with moderate to severe COVID-19 and there is evidence of pneumonia; COVID-19 with risk factors (hypertension, diabetes, cardiac disease, chronic lung disease and obesity etc.); Aged ≥ 18 years. While the more widely adopted exclusion criteria is: History of thrombophlebitis; Patient with latent tuberculosis (TB) infection; Pregnancy and lactation; Current Herpes zoster infection; Evidence of concomitant bacterial infections; Treatment with other potent immunosuppressants concurrently; Absolute lymphocyte count(ALC) < 0.5 × 109/L; Absolute neutrophil count (ANC) < 1.0 × 109/L; Hemoglobin level (Hb) < 8 g/dL; eGFR < 60 mL/min/1.73 m2; AST or ALT > 5 times ULN.
On the other hand, it has been reported that baricitinib is not an ideal option for management of COVID-19 on the ground of some research reports on small samples [33]. An epidemiologic study reported that ALC in the non-survivors is 0.6 × 109cells/L (Inter-quartile range: 0.5–0.8 × 109 cells/L). Another study reported that ALC in patients receiving ICU Care is 0.4 × 109/L (Inter-quartile range: 0.2–0.8 × 109/L). Unfortunately, baricitinib is not recommended in patients with ALC < 0.5 × 109/L, ANC < 1.0 × 109/L. Based on the foregoing, baratinib is indeed not suitable for this group of patients receiving ICU Care. In fact, this did not conflict with the results of current clinical trials, which recommended baricitinib for moderate to severe COVID-19 patients.
5.3. Drug safety monitoring and pharmaceutical care of baricitinib
Based on drug instructions of the US FDA, the following adverse reactions need to be noted. ① Serious Infections The most common serious infections reported with baricitinib included pneumonia, herpes zoster, and urinary tract infection. ② Malignancy Clinical drug application research has shown that malignancies excluding non-melanoma skin cancers were reported in 2 patients treated with baricitinib 2 mg and 6 patients treated with baricitinib 4 mg during the 0 to 52 week treatment period. ③ Thrombosis Thrombosis, including deep venous thrombosis (DVT) and pulmonary embolism (PE) has been observed at an increased incidence in patients treated with baricitinib. ④ Laboratory Abnormalities Such as neutropenia, lymphopenia, anemia, thrombocytosis, liver enzyme elevations (ALT, AST), lipid elevations (total cholesterol, LDL, HDL) and elevation of creatine phosphokinase (CK).
According to recent research reports, baricitinib was generally well tolerated on the whole. Smolen JS analyzed 3492 patients with moderate to severe active RA who received baricitinib for 6637 total patient-years of exposure (median 2.1 yrs, maximum 5.5 yrs) and found no differences in rates of death, adverse events leading to drug discontinuation, malignancies, major adverse cardiovascular event, or serious infections were seen for baricitinib 4 mg/d versus placebo. However infections including herpes zoster were significantly more frequent for baricitinib 4 mg/d versus placebo [34]. Coincidentally, a meta-analysis of baricitinib in patients with RA showed that 4 mg of baricitinib increased herpes zoster infection as compared to placebo and 2 mg of baricitinib [35]. An integrated analysis showed that during placebo-controlled period through week 24, there was 6 events (1.4 per 100 person-years) reported in patients who received 4 mg baricitinib [36]. Another integrated analysis showed that older age, obesity, history of DVT/PE, and the use of selective COX-2 inhibitor were independent risk factors for venous thromboembolism [37]. In fact, 5 of the 6 patients who developed DVT/PE had some of these risk factors.
The above adverse reactions data was obtained from a series clinical study of long course treatment with baricitinib. However, short-term use of baricitinib (i.e., 7–14 days) might reactivate no latent infections, including herpesviruses or tuberculosis, in COVID-19 patients. It needs to be further demonstrated.
Based on the above evidence, pharmaceutical care of baricitinib mainly includes the following: ① Avoid use of baricitinib in patients with active, serious infection and active TB. ② Baricitinib is not recommended in patients with ALC < 0.5 × 109/L, ANC < 1.0 × 109/L or Hb < 8 g/dL. ③ Prompt investigation of the cause of liver enzyme elevation is recommended. If increases in ALT or AST are observed and drug-induced liver injury is suspected, interrupt baricitinib until this diagnosis is excluded. ④ Baricitinib should be used with caution in patients who have risk factors such as older age, obesity, history of DVT/PE, and the use of selective COX-2 inhibitor.
5.4. The use of baricitinib in specific populations
The following information is mainly from the drug instructions of the US FDA.
Pregnant women: The limited human data on use of baricitinib in pregnant women are not sufficient to inform a drug-associated risk for major birth defects or miscarriage. In animal embryo-fetal and post-natal development studies, oral baricitinib administration to pregnant rats and rabbits at exposures equal to and greater than approximately 20–84 times the maximum recommended human dose (MRHD), resulted in reduced fetal body weights, increased embryo lethality, skeletal malformations and reduction in pup viability. No developmental toxicity was observed in pregnant rats and rabbits treated with oral baricitinib during organogenesis at approximately 5 and 13 times the exposure at the MRHD, respectively.
Older adults: Baricitinib is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function.
Patients with hepatic impairment: No dose adjustment is necessary in patients with mild or moderate hepatic impairment. The use of baricitinib has not been studied in patients with severe hepatic impairment and is therefore not recommended.
Patients with renal impairment: The recommended dose of baricitinib in patients with moderate renal impairment (eGFR 30–60 mL/min/1.73 m2) is 1 mg once daily. Baricitinib is not recommended for use in patients with severe renal impairment (eGFR < 30 mL/min/1.73 m2).
6. Conclusion
As a cell surface protein, ACE2 is involved in receptor-mediated endocytosis for SARS-CoV-2 entry to the cells. Baricitinib, a JAK1/JAK2 inhibitor, might be a potential strategy. Baricitinib would interrupt the passage and intracellular assembly of SARS-CoV-2 into the target cells by binding to AAK1 and GAK. On the other hand, the JAK-STAT signaling pathway participates the cytokine storm in moderate to severe COVID-19 cases. Baricitinib is expected to treat cytokine storm caused by COVID-19 by suppressing JAK1/JAK2. And a number of clinical trials have been registered around the world, one of which has been completed with encouraging results. Universally, participants were administered baricitinib (2–4 mg, qd) for 1–2 weeks. We can refer to the current clinical trials to determine drug usage and applicable patients with COVID-19. We look forward to more announcement of the results of these trials. Baricitinib was generally well tolerated on the whole. The common and important adverse reactions are infections, abnormal blood routine, elevated liver enzymes and DVT/PE. whether the adverse reactions will occur during the short course treatment of COVID-19 needs to be further demonstrated. Baricitinib should be used with caution in patients who have the above risk factors. Human data on use of baricitinib in pregnant women are not sufficient. When using baricitinib in patients with renal insufficiency, attention should be paid to dose adjustment or discontinuation.
7. Authors’ contributions
Xiuhong Zhang and Zhigang Qi were involved in the design of the study and wrote the first draft of the manuscript. Yan Zhang was also involved in the design of the study and collected the literature. Weizhen Qiao and Ji Zhang critically revised the article.
Funding
The preparation of this review was supported by Wuxi medical and health guidance plan for scientific and technological development (NZ2019004), Jiangsu Pharmaceutical Association-Aosaikang Hospital Pharmacy Fund (A201729) and Jiangsu Pharmaceutical Association-Tianqing Hospital Pharmacy Fund (Q2019087).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
I certify that no individuals other than the listed co-authors contributed to this publication.
References
- 1.Y. Zhao, Z.X. Zhao, Y.J. Wang, et al. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov, BioRxiv (2020) https://doi.org/10.1101/2020.01.26.919985 (access 3 Jun 2020).
- 2.W.J. Guan, Z.Y. Ni, Y. Hu, et al., Clinical characteristics of 2019 novel coronavirus infection in China, MedRxiv (2020) https://doi.org/10.1101/2020.02.06.20020974 (access 20 Feb 2020).
- 3.K.J. Xu, H.L. Cai, Y.H. Shen, et al., Management of corona virus disease-19 (COVID-19): the Zhejiang experience, Zhejiang Da Xue Xue Bao (Yi Xue Ban) (2020) http://kns.cnki.net/kcms/detail/33.1248.R.20200222.1417.002.html (in Chinese) (access 3 Mar 2020).
- 4.J.W. Zhang, X. Hu, P.F. Jin, Cytokine storm induced by SARS-CoV-2 and the drug therapy. Zhongguo Yao Xue Za Zhi (2020) http://kns.cnki.net/kcms/detail/11.2162.R.20200225.1052.002.html (in Chinese) (access 9 Feb 2020).
- 5.S.Y. Zhang, L. Li, A.Z. Shen, et al., Rational use of tocilizumab in the treatment of novel coronavirus pneumonia, Clin. Drug Investig. (2020) https://doi.org/10.1007/s40261-020-00917-3 (access 29 Apr 2020). [DOI] [PMC free article] [PubMed]
- 6.Keystone E.C., Taylor P.C., Drescher E., et al. Safety and efficacy of baricitinib at 24 weeks in patients with rheumatoid arthritis who have had an inadequate response to methotrexate. Ann. Rheum. Dis. 2015;74(2):333–340. doi: 10.1136/annrheumdis-2014-206478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fei Z.Y., Dong H., Qian Q.J., et al. Analysis of pathogens and risk factors for death in elderly patients with severe pneumonia. Zhong Hua Yi Yuan Gan Ran Xue Za Zhi. 2019;29(3):380–383. (in Chinese) [Google Scholar]
- 8.Ma Y.P., Yan Y.H., Sun X.D., et al. Correlation between inflammatory factor expression level in severe pneumococcal alveolar lavage fluid and prognosis. Zhong Hua Yi Yuan Gan Ran Xue Za Zhi. 2019;29(7):1007–1010. (in Chinese) [Google Scholar]
- 9.Huang C.L., Wang Y.M., Li X.W., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.L. Chen, H.G. Liu, W. Liu, et al., Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhong Hua Jie He He Hu Xi Za Zhi (2020) https://doi.org/10.3760/cma.j.issn.1001-0939.2020.0005 (in Chinese) (access 15 Feb 2020).
- 11.S.X. Wan, Q.J. Yi, S.B. Fan, et al., Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). MedRxiv (2020) https://doi.org/10.1101/2020.02.10.20021832 (access 20 Feb 2020).
- 12.J. Liu, S.M. Li, J. Liu, et al., Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. MedRxiv (2020) https://doi.org/10.1101/2020.02.16.20023671 (access 3 Mar 2020). [DOI] [PMC free article] [PubMed]
- 13.P. Conti, G. Ronconi, A. Caraffa, et al., Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents (2020) https://doi.org/10.23812/CONTI-E (access 29 Apr 2020). [DOI] [PubMed]
- 14.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zegeye M.M., Madelene L., Knut F., et al. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated pro-inflammatory response in human vascular endothelial cells. Cell Commun. Signal. 2018;16(1):55. doi: 10.1186/s12964-018-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Shea J.J., Schwartz D.M., Villarino A.V., et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaoka K., Saharinen P., Pesu M., et al. The Janus kinases (Jaks) Genome Biol. 2004;5(12):253. doi: 10.1186/gb-2004-5-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor P.C., Keystone E.C., van der Heijde Désirée, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N. Engl. J. Med. 2017;376(7):652–662. doi: 10.1056/NEJMoa1608345. [DOI] [PubMed] [Google Scholar]
- 19.Gadina M., Johnson C., Schwartz D., et al. Translational and clinical advances in JAK-STAT biology: the present and future of jakinibs. J. Leukoc. Biol. 2018;104(3):499–514. doi: 10.1002/JLB.5RI0218-084R. [DOI] [PubMed] [Google Scholar]
- 20.Lu R.J., Zhao X., Li J., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorrell F.J., Szklarz M., Abdul Azeez K.R., et al. Family-wide structural analysis of human numb-associated protein kinases. Structure. 2016;24(3):401–411. doi: 10.1016/j.str.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marrero M.B. Introduction to JAK/STAT signaling and the vasculature. Vasc. Pharmacol. 2005;43(5):307–309. doi: 10.1016/j.vph.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Benveniste E.N., Liu Y., Mcfarland B.C., et al. Involvement of the janus kinase/signal transducer and activator of transcription signaling pathway in multiple sclerosis and the animal model of experimental autoimmune encephalomyelitis. J. Interferon. Cytok. Res. 2014;34(8):577–588. doi: 10.1089/jir.2014.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.P.J. Richardson, M. Corbellino, J. Stebbing, Baricitinib for COVID-19: a suitable treatment? - Authors' reply, Lancet Infect. Dis. (2020) https://doi.org/10.1016/S1473-3099(20)30270-X (access 29 Apr 2020). [DOI] [PMC free article] [PubMed]
- 25.Peiris J.S.M., Chu C.M., Cheng V.C.C., et al. Clinical progression and viral load in a community outbreak or coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stebbing J., Phelan A., Griffin I., et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020;20(4):400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US National Library of Medicine. https://clinicaltrials.gov/ct2/home. (access 29 Apr 2020).
- 29.F. Cantini, L. Niccoli, D. Matarrese, et al., Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact, J. Infect. (2020) https://doi.org/10.1016/j.jinf.2020.04.017 (access 3 Jun 2020). [DOI] [PMC free article] [PubMed]
- 30.The U.S. Food and Drug Administration. Information for Olumiant (baricitinib) tablets, for oral use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/207924Orig1s000lbl.Pdf (access 25 Apr 2020).
- 31.Wallace D.J., Furie R.A., Yoshiya T., et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392(10143):222–231. doi: 10.1016/S0140-6736(18)31363-1. [DOI] [PubMed] [Google Scholar]
- 32.E.L. Simpson, J.P. Lacour, L. Spelman, et al., Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials, Br. J. Dermatol. (2020) https://doi.org/10.1111/BJD.18898 (access 2 Jun 2020). [DOI] [PubMed]
- 33.D. Praveen, R.C. Puvvada, M. Vijey Aanandhi, Baricitinib-a januase kinase inhibitor-not an ideal option for management of COVID-19, Int. J. Antimicrob. Ag. (2020) https://doi.org/10.1016/j.ijantimicag.2020.105967 (access 3 Jun 2020). [DOI] [PMC free article] [PubMed]
- 34.Smolen J.S., Genovese M.C., Takeuchi T., et al. Safety profile of baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J. Rheumatol. 2019;46(1):7–18. doi: 10.3899/jrheum.171361. [DOI] [PubMed] [Google Scholar]
- 35.Kunwar S., Collins C.E., Constantinescu F. Baricitinib, a Janus kinase inhibitor, in the treatment of rheumatoid arthritis: a systematic literature review and meta-analysis of randomized controlled trials. Clin. Rheumatol. 2018;37(10):2611–2620. doi: 10.1007/s10067-018-4199-7. [DOI] [PubMed] [Google Scholar]
- 36.Genovese M.C., Smolen J.S., Takeuchi T., et al. THU0078 safety profile of baricitinib for the treatment of rheumatoid arthritis up to 7 years: an updated integrated safety analysis. Ann. Rheum. Dis. 2019;78(Suppl 2):308.2-309. [Google Scholar]
- 37.Taylor P.C., Weinblatt M.E., Burmester G.R., et al. Cardiovascular safety during treatment with baricitinib in rheumatoid arthritis. Arthritis Rheumatol. 2019;71(7):1042–1055. doi: 10.1002/art.40841. [DOI] [PMC free article] [PubMed] [Google Scholar]