Graphical abstract
Keywords: Coronavirus, COVID-19(SARS Cov2), Cytokine, Growth factors, Co-morbidity, BCG
Abbreviations: ARDS, acute respiratory distress syndrome; SARS-CoV, severe acute respiratory syndrome coronavirus; MERS-CoV, middle east respiratory syndrome coronavirus; COVID-19, coronavirus disease 2019; RNA, ribonucleic acid; HCoV, human coronavirus; ALI, acute lung injury; TGF-β, transforming growth factor-β; IFN, interferon; TNF, tumor necrosis factor; IL, interleukin; CCL, chemokine (C-C motif) ligand; IL-1RA, interleukin-1 receptor antagonist; RANTES, regulated on activation, normal T cell expressed and secreted; MCP-1, monocyte chemoattractant protein-1; MIP, macrophage inflammatory protein; IP-10, IFN-γ-inducible protein-10; CXCL, C-X-C motif chemokine; TH, T helper; NKT, natural killer T cells; COPD, chronic obstructive pulmonary disease; MIG, Monokine induced by gamma interferon; BALF, bronchoalveolar lavage fluid; bFGF, basic fibroblast growth factor; G-CSF/GCSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; PDGF, platelet-derived growth factor; VEGF, vascular endothelial growth factor; ACE2, angiotensin converting enzyme 2; DPP4, dipeptidyl peptidase 4; TMPRSS2, transmembrane protease serine 2; T2DM, type2 diabetes; GDM, gestational diabetes mellitus; BCG, bacillus calmette- guérin; BCG-CORONA, reducing health care workers absenteeism in COVID-19 pandemic by enhanced trained immune responses through bacillus calmette-guérin vaccination, a randomized controlled trial; BRACE, BCG vaccination to reduce the impact of COVID-19 in healthcare workers following coronavirus exposure; BADAS, BCG vaccine for health care workers as defense against COVID 19
Abstract
The seventh human coronavirus SARS-CoV2 belongs to the cluster of extremely pathogenic coronaviruses including SARS-CoV and MERS-CoV, which can cause fatal lower respiratory tract infection. Likewise, SARS-CoV2 infection can be fatal as the disease advances to pneumonia, followed by acute respiratory distress syndrome (ARDS). The development of lethal clinical symptons is associated with an exaggerated production of inflammatory cytokines, referred to as the cytokine storm, is a consequence of a hyperactivated immune response aginst the infection. In this article, we discuss the pathogenic consequences of the cytokine storm and its relationship with COVID-19 associated risk factors. The increased pro-inflammatory immune status in patients with risk factors (diabetes, hypertension, cardiovascular disease, COPD) exacerbates the Cytokine-storm of COVID-19 into a ‘Cytokine Super Cyclone’. We also evaluate the antiviral immune responses provided by BCG vaccination and the potential role of ‘trained immunity’ in early protection against SARS-CoV2.
1. Introduction
Coronaviruses are positive-sense, enveloped RNA virus with a genome of 26–32 Kb, and belong to the Coronaviridae family of viruses capable of infecting both humans and animals [1]. Human Coronavirus (HCoV) can be categorized into two groups, depending on their pathogenic capacity; HCoV−OC43, HCoV-NL63, HCoV-229E and HCoV-HKU are included in the low pathogenic hCoV group that causes upper respiratory tract infection and cold-like symptoms, while severe acute respiratory syndrome Coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV) and the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) are highly pathogenic human coronaviruses [2,3]. Unlike low pathogenic hCoV, the highly pathogenic viruses produce severe disease and death, as a result of lower respiratory tract infection leading to clinical pneumonia, acute lung injury (ALI) and ARDS [4]. Also, SARS CoV2 showed ∼79.0 % genome identity to SARS‐CoV and ∼50 % to MERS CoV [5]. Similar to MERS and SARS CoV, bats are considered the natural host of SARS-CoV2. The virus is then transmitted via intermediate host to human; the intermediate host for MERS CoV, SARS CoV and SARS CoV2 are dromedary camels, civets and pangolins respectively [6].
Viral entry into the host results in activation of innate immune mechanisms, together with the synthesis and secretion of inflammatory mediators. Interaction of specific viral components with pattern recognition receptors of the host immune system activates cellular signalling pathways that culminate in the production of various cytokines. These cytokines elicit host responses such as extravasation of leukocytes and activation, proliferation and cell differentiation of adaptive and innate responses [7,8]. Interleukin (IL)-1α/β, IL-18 and IL-6, tumor necrosis factor (TNF)-α, Interferon (IFN)-α/β, Transforming growth factor(TGF)-β are the major cytokines produced, while CCL-2/monocyte chemoattractant protein (MCP)-1, MCP-3, CCL-3/Macrophage Inflammatory Protein (MIP-1-α), CCL-5 (Chemokine (C-C motif) ligand 5)/RANTES (regulated on activation, normal T cell expressed and secreted) and IFN-γ-inducible protein-10 (IP-10)/CXCL10 (C-X-C motif chemokine 10) are the chemokines released during an influenza virus-induced infection [9,10]. These cytokines exert host antiviral responses in different ways. IFN-α/β can inhibit viral replication, thus inducing a potent antiviral state in the host [11], while TNF-α triggers the immune cell localization at infection site and activation of caspases to promote apoptosis [12]. IL-1 is a pleiotropic cytokine that enhances inflammatory cell infiltration by increasing membrane permeability through activation of mast cells and subsequent release of the vasolilator histamine [13]. IL-6 mediates monocyte differentiation to macrophages and enhances mononuclear cell infiltration to the inflammation loci [14]. Moreover, synergistically IL-6 and TGF-β induces maturation of naive helper T cells (CD4+) to T Helper 17 (TH17) cells which are potential effectors against pathogens. Interestingly, IL-6 also hinders the Treg expansion by TGF-β [15,16]. Enhancement of type 1 immunity and activation of natural killer T cells (NKT) are some of the additional biological actions of IL-18 during viral infection [17,18].
Although these cytokines are released as a component of the host response to infection, chronic, exacerbated cytokine release, the cytokine storm, adversely affects the host in both SARS and MERS infection, leading to severe complications and multiple organ failure [19]. A similar hypercytokinemia-cytokine storm also contributes to SARS-CoV2/COVID-19 infection [20]. In this review we highlight the pathogenesis of MERS CoV, SARS CoV and SARS CoV2, with a focus on the role of cytokines in pathology and co-morbidities of COVID-19 infection.
2. Pathogenesis of human coronaviruses
2.1. SARS CoV
The epidemic outbreak of SARS-CoV in 2002–2003 had a fatality ratio of 14%–15%, which was higher in an older age group (greater than 50 %) and less than 1% in younger age group (WHO:https://www.who.int/csr/sars/archive/2003_05_07a/en/). The reproductive number, an indicator of the ability to spread the viral infection to others was 3 (https://www.who.int/csr/sars/en/WHOconsensus.pdf?ua=1). SARS-CoV infected patients had clinical features such as malaise, fever, chills, cough and myalgia, along with laboratory findings of thrombocytopenia, elevated concentration of enzymes like alanine aminotransferase (ALT), lactate dehydrogenase (LDH) and creatine kinase (CK) and lymphocytopenia [21]. Autopsy of lungs from patients who died of SARS-CoV showed epithelial denudation, haemophagocytosis, macrophage infiltrates in alveoli and interstitium of the lung [22]. Chronic obstructive pulmonary disease (COPD), cardiac disease, diabetes and cancer in patients infected with SARS CoV were associated with adverse clinical results [23].
SARS CoV entry to the cells is mediated by ACE2 (Angiotensin converting enzyme 2) receptor that is highly expressed in lung, kidney, heart, liver and tongue. Viral Spike protein (S) binds to the ACE2 receptor, followed by viral fusion with the cell membrane, which involves priming of the S protein by proteases on the cell surface, and culminates in the entry and replication of the virus in the host cell cytoplasm [24]. It has been shown that ACE2 has protective role in ALI [25]. The ACE2 receptor plays a key role in renin-angiotensin system (RAS) that maintains blood pressure and water electrolyte balance. Decreased expression of ACE2 negatively affects the RAS system, leading to increased microvascular permeability and inflammation [26]. ACE2 expression has also been found to be down regulated by SARS CoV infection, which further augments the intensity of lung injury [27]. Moreover, increased viral load, excessive immune cell infiltration and increased pro-inflammatory cytokine release, further exacerbate lung injury in SARS, suggesting that both the cytokine storm and direct cytopathogenic effects of the virus contribute to the immunopathogensis observed in SARS CoV infection [28].
Profiling of systemic cytokine levels in individuals suffering from SARS-CoV disease showed elevated concentrations of T Helper 1 (TH1) cytokines such as IFN-γ, IL-2 and IL-12. Additionally, cytokines that promote inflammation like IL-6, IL-1, MCP-1, IP-10, CXCL9/MIG (monokine induced by gamma interferon), IL-18, TGF-β and IL-8 were also increased [[29], [30], [31], [32], [33]]. Interestingly, SARS patients showed no change in blood IL-10 and IL-4 levels [33]. Increased amounts of MIG, MCP-1, IP-10, IL‐6, IL‐8 and IL‐18 in blood correlated with disease severity in SARS patients [30,31]. In bronchoalveolar lavage (BAL) fluid, cytokine levels were also increased in patients with SARS infection. Higher concentrations of MCP-1, TNF-α, IL-8, IL-6 and RANTES in BAL could explain the clinical observation of alveolar infiltration of mononuclear cells of SARS-CoV patients [34]. Furthermore, SARS CoV delayed the induction of the IFN antiviral response and expression of interferon-stimulated genes [35]; the delayed-type 1 interferon signalling, coupled with increased secretion of pro-inflammatory mediators produced lung immunopathology in SARS CoV infected mice [36].
2.2. MERS CoV
According to WHO, the Middle East respiratory syndrome (MERS) was observed in 2494 individuals, with a 34.4 % case-fatality rate as of November 2019. The R0 was estimated to be less than 1,indicating a relatively low transmissibility of virus [37]. MERS was reported in 27 countries globally, with 50-59 year old men as the highest risk group (WHO, 2019) (https://applications.emro.who.int/docs/EMRPUB-CSR-241-2019-EN.pdf?ua=1&ua=1&ua=1&ua=1&ua=1&ua=1). Similar to SARS-CoV, the early clinical indications of infection by MERS-CoV included cough, dyspnea, fever, and myalgia, followed by the development of pneumonia. In addition to symptoms associated with the respiratory system, patients infected with MERS-CoV also experienced diarrhea, vomiting, and abdominal pain. Interstitial infiltrates were observed in chest radiography of MERS-CoV infected individuals, while laboratory findings demonstrated increased concentrations of LDH and aspartate aminotransferase (AST), low eosinophil and platelet count and decreased albumin level [38].
Dipeptidyl peptidase 4 (DPP4 or CD26) is the functional receptor for MERS-CoV. Compared to other coronavirus, MERS-CoV exhibits a broad tropism because of the wide expression of DPP4 in different tissues, including epithelial cells in the kidney, small intestine, alveoli, prostate, liver, and activated T lymphocytes [39]. In lungs, DPP4 is expressed in multinucleated epithelial cells, submucosal gland cells and bronchial pneumocytes but not in upper airway epithelium [40]. Additionally, MERS-CoV can infect monocyte-derived-dendritic cells and macrophages when compared with SARS-CoV [28]. Ultra-structural findings in MERS infected patients showed the presence of viral particles in pneumocytes, pulmonary and skeletal muscle macrophages and renal proximal tubular epithelial cells [41]. Furthermore, up-regulation of Smad7 and FGF2 (fibroblast growth factor 2) expression in kidney and lung by MERS‐CoV resulted in cell death and tissue damage [42]. Infection of T cells of peripheral blood, spleen and tonsils with MERS CoV resulted in activation of extrinsic and intrinsic apoptosis pathways leading to cell death, thus supporting the observation of lymphopenia in MERS infection [43]. Moreover, patients with co-morbidities such as diabetes, cardiovascular diseases, end-stage renal disease, and hypertension were more prone to fatal conditions [44].
Blood cytokine analysis performed in severely infected MERS individuals demonstrated a marked increase in concentration of IL-6, IL-10, IL-15, IP-10, IL-17, TNF and IFN-γ /α2 in comparison to controls [44,45]. Additionally, significantly increased mRNA expression of IL-8, IL-12, IFN-γ, RANTES, IP-10, MCP-1 and MIP1-α were seen in human macrophages infected by MERS-CoV [46], suggesting that these cytokines are implicated in the infiltration of immune cell in lungs leading to hyper inflammation and lung injury.
2.3. SARS CoV2/COVID 19
The first documented case of COVID19 was in December 2019 in Wuhan China, (https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4) and as of 22 June 2020, 8,860,331 individuals were SARS-CoV-2 positive resulting in the death of 465,740 patients, with 216 countries and regional territories affected globally. Thus SARS-CoV-2 pandemic fit the very high-risk category of the earlier epidemics caused by SAR-CoV and MERS-CoV (https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200622-covid-19-sitrep-154.pdf?sfvrsn=d0249d8d_2). According to WHO, the estimated R0 was 2–2.5, but a later report estimated the R0 as 3.28, signifying high transmission capacity of virus [47].
Initially, the majority of patients presented with clinical indications like dry cough, myalgia, fever, fatigue, dyspnoea, headache, vomiting and diarrhoea. At later stages, severely infected patients showed clinical manifestations including pharyngeal pain, dizziness, abdominal pain, and anorexia. Laboratory analysis of the patient samples revealed leucopenia, lymphopenia, high pro-thrombin time, high levels of hepatic enzymes like AST, LDH, increased bio-markers of inflammation like erythrocyte sedimentation rate, serum C-reactive protein and ferritin. Elderly people and those patients with underlying diseases like COPD, hypertension, cardiovascular disease and diabetes belonged to the high-risk group, where the disease could advance to fatal acute respiratory distress syndrome (ARDS). Patients with COVID-19 pneumonia showed ground-glass opacity and segmental consolidation of both lungs, shown as patchy areas in computer-assisted tomography scan. Other major complications of SARS-Cov2 infection included shock, arrhythmia, acute cardiac injury and secondary infection which finally need assistance by mechanical ventilation [48,49].
Like SARSCoV, ACE2 is the primary cell entry receptor for SARS-CoV2. Upon priming of Spike protein by the cellular protease TMPRSS2 (transmembrane protease serine 2), viral fusion with the cell membrane is triggered [24]. Protein structural analysis demonstrated that the receptor binding domains (RBD) of S protein from SARSCoV2 and SARS CoV showed 72 % amino acid sequence identity, indicating a higher affinity of SARS-CoV2 to its receptor in comparison to SARS-CoV [50]. Furthermore, the furin-like cleavage site of SARS-CoV2 Spike protein also contributed to priming, making the virus more contagious. Interestingly this furin-like cleavage site was observed in human coronavirus OC43 and MERS but not in SARS-CoV [51]. Infection with a cytopathic virus like SARS-CoV-2 resulted in inflammasome formation and pyroptosis mediated by Caspase1 activation and associated inflammatory response [52].
COVID-19 infected patients showed higher concentrations of peripheral blood immune mediators including MIG, IL-6, CCL8 (MCP-2), IL-9, IL-1β, MIP2-α (CXCL2), TNF-α, CXCL16, IL-2, IL-1RA(interleukin-1 receptor antagonist), IL-7, MIP1-α, IP-10, IL-8, basic fibroblast growth factor (bFGF), MCP-1, Granulocyte-colony stimulating factor (G-CSF/GCSF), IFN-γ, GM-CSF(Granulocyte-macrophage colony-stimulating factor), MIP1-β, PDGF(Platelet-derived growth factor), IL-10, and VEGF(Vascular endothelial growth factor). IL-2, IL-6, IL-7, IP-10, IL-2R, IL-10, TNF-α, MIP1-α, MCP-1 and GSCF levels positively correlated with disease severity suggesting that hypercytokinemia can aggravate immunopathology and inflammation [48,[53], [54], [55]]
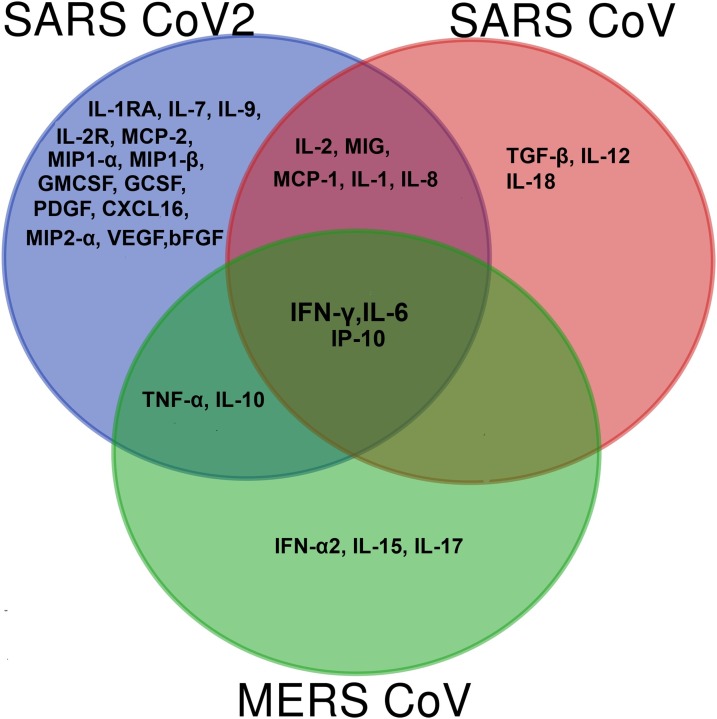
Only a few in vitro studies on COVID-19 and cytokine deregulation are currently available; based on in vitro data, there is a significant distinguishable pattern in the cytokine storm of COVID-19 with SARS-CoV and MERS-CoV infection (Fig. 1 ). Only three cytokines viz., IL-6, IP-10 and IFN-γ showed markedly elevated levels in all three highly pathogenic hCoV infections. In SARS patients, the level of IP -10 correlated with disease severity [31], where IP-10 induction in lungs led to immune cell infiltration and apoptosis causing acute lung injury in SARS [30]. Moreover, increased levels of IP-10 were also associated with disease progression and poor prognosis in MERS-CoV infection [44]. Increased expression of MIG and IP-10, chemo attractants that enlist activated T cells by chemokine receptor CXCR3 signalling was observed in inflammation, specifically in activated bronchial epithelium [56]. The SARS-CoV murine model showed up-regulation of IP-10, MIG and its receptor in the lungs, implicating the CXCR3 cascade in the development of ARDS in SARS CoV [57]. Enhanced CXCR3 signalling was previously reported with aetiology of pulmonary fibrosis [58]. A recent study on COVID-19 reported that elevated levels of nasopharyngeal IP-10 could be used as a biomarker for undiagnosed COVID-19 [59], and overall indicates the importance of IP-10 signalling in the progression to pneumonia and ultimately, the fatality of COVID-19 patients. Recent ex vivo experiments conducted in human lung tissue idenitifed high transmission and asymptomatic infection by SARS-CoV2, and further demonstrated increased replication compared to SARS-CoV, However, SARS-CoV2 did not induce significant levels of IFN(type I, II, III), or pro-inflammatory mediators except IP-10 in human lung tissues [60]. In another study, peripheral blood mononuclear cells (PBMC) and BAL transcriptome sequencing of SARS-CoV-2 infected patients revealed enrichment of MCP-1, IP-10, MIP1-A, and MIP-1B genes. Of note, SARS-CoV-2 also induced apoptosis and P53 signalling pathways, again implicating this laboratory finding of lymphopenia in COVID-19 patients [61].
Fig. 1.
Venn diagram showing peripheral cytokine profile of SARS CoV, MERS CoV and SARS CoV2 infection. IL-6, IFN-γ and IP-10 showed significantly higher levels in all three highly pathogenic hCoV infections. IL-8, MIG, IL-1, MCP-1 and IL-2 levels were altered in both SARS CoV and SARS CoV2 infected patients. TNF-α and IL-10 levels were increased in SARS CoV2 and MERS. Cytokines and chemokines like IL-1RA, IL-7, IL-9, IL-2R, GCSF, GMCSF, PDGF, CXCL16, MIP2- α, MCP-2, VEGF, bFGF, MIP1-α and MIP1-β cytokines were uniquely elevated in SARS CoV2 infection.
Another major cytokine that mediates organ damage in cytokine storm is IL-6. Excessive secretion of IL-6 results in activation of coagulation pathway, conversion of naive T cells, increase in vascular permeability and reduced cardiac function, contributing to the increased disease severity [62,63]. SARS patients also showed increased levels of IL-6 which correlated with disease progression [30]. Increased levels of Th17 CD4 T cells seen in COVID-19 patients can be explained by high levels of IL-6, which is implicated in T Helper 17 (TH17) cells development [16,64]. Interestingly, spike protein of SARS CoV induced activation of NF-κB signalling, resulting in IL-6 and TNF-α secretion in murine macrophages [65]. Another study reported that inhibiting NF-κB signalling was associated with decreased IL-6 levels and increased animal survival in SARS [66]. These studies implicate IL-6 as a key contributor to the patho-physiology of the cytokine-storm seen in both SARS CoV infections. One mechanism proposed for decreased T cells in COVID-19 infection implicated increased levels of IL-6 and increased interaction between Fas and its ligand. Moreover, an increased lymphocyte count was observed in patients SARS CoV2 infection when treated with tocilizumab, the IL-6 receptor antagonist [67,68]. Additional studies are required to understand the mechanisms of SARS Cov2 induced lymphopenia and other immunological features.
As depicted in Fig. 1, altered MCP-1, IL-2, MIG and IL-8 levels were observed in both SARS CoV and SARS CoV2 infected patients. Thus, the cytokine storm in SARS-CoV2 infection has been linked with both TH1 (mediated by IFN-γ, IP-10, IL-1 β, MCP-1, IL-2) and TH2 responses, mediated by IL-10 production [48]; in SARS-CoV infection, no change occurs in TH2 cytokines [33]. Compared to SARS-CoV, an increased range of cytokines and chemokines are involved in the immune pathology of COVID-19. Common cytokines increased in SARS-CoV2 and MERS infection included IL-10, IP-10, IL-6, and TNF-α, indicating that SARS-CoV2 displayed less similarity with MERS CoV than with SARS-CoV. Based on these observations, it is reasonable to suggest that an increased response of TH1 cytokines, TH2 cytokines and pro-inflammatory chemokines contributed to the immune pathology of COVID-19 infection, in contrast to SARS which was predominantly mediated by IFN-γ induced TH1 response [69]. Another study also demonstrated that COVID-19 patients display defective interferon activation and enhanced NFκB mediated TNF-α and IL-6 production [70]. Moreover, SARS CoV2 infection was also associated with defective IFN1 expression, as seen with SARS-COV. Blanco-Melo et al., using data from ferret models, infected cell lines, primary bronchial cells and serum cytokine profiling, demonstrated delayed IFN (type1 and 111) expression, along with increased levels of chemokines [55]. Single-cell sequencing (scRNA-seq) of COVID-19 patients showed impaired immune response by activation of pro-inflammatory signalling pathways in lung samples [71]. These findings suggest that the combined action of exaggerated cytokine levels and defective antiviral response by interferons resulted in the deterioration of SARS-CoV2 infected patients. Interferon therapy for COVID-19 patients has already been suggested [72] and findings of a clinical trial with Interferon-α2b have shown promising results [73]. Further studies will have to be performed to reveal the actual immune mechanisms contributing to the cytokine storm-induced hyper-inflammation during disease. Comparative analysis of SARS-CoV, MERS-CoV and SARS-CoV2 is summarised in Table 1 .
Table 1.
Comparative analysis of SARS CoV2, MERS CoV2 and SARS CoV2.
| SARS CoV | MERS CoV | SARS CoV2 | |
|---|---|---|---|
| Origin | Bat | Bat | Bat |
| Intermediate Host | Civet | Camel | Pangolin? |
| Receptor | ACE2 | DPP4 | ACE2 |
| Clinical manifestations | Malaise, fever, cough, myalgia, Chills | Fever, dyspnea, cough, myalgia, diarrhoea, vomiting, and abdominal pain | Fever, dry cough, myalgia, fatigue, dyspnoea, headache, vomiting and diarrhoea |
| Lung Pathology | Epithelial denudation, haemophagocytosis, macrophage infiltrates in alveoli and interstitium of the lung | Interstitial infiltrates were observed in chest radiography | Ground glass opacity and segmental consolidation of both lungs seen as patches in chest CT scan. |
| Major Laboratory Findings | Increased levels of ALT, CK and LDH lymphocytopenia and thrombocytopenia | Thrombocytopenia, low eosinophil count, decreased albumin level, higher levels of LDH and AST. | Leucopenia, lymphopenia, high pro-thrombin time, high levels of AST, LDH, increased inflammatory markers like C-reactive protein, serum ferritin and erythrocyte sedimentation rate |
| Cytokine Profile | MCP-1, IL-8, MIG, IL-2, TGF-β, IL-6, IP10, IL-1, IFN-γ, IL-18, and IL-12 | TNF-α, IP-10, IL-6, IL-17, IL-10, IL-15, IFN-α2, and IFN- γ | MCP-1, TNF-α, IP-10, IL-2, MIG, IL-8, IL-2R, IL-10, IL-1β, IFN-γ, IL-7, IL-1RA, IL-9, GM-CSF, basic FGF, G-CSF, MIP1-α, MIP1-β, PDGF, IL-6, and VEGF |
| Associated Risk Factors | Cancer, diabetes, cardiac disease, COPD | Diabetes, cardiovascular diseases, end stage renal disease and hypertension | COPD, Diabetes, hypertension and cardiovascular disease |
| Reference | (6,21−24,29−33) | (6,38,39,44,45) | (6,24,48,49,53−55) |
3. Relation of risk factors of COVID-19 and cytokine pattern
Like SARS and MERS, major co-morbidities related with COVID-19 patients include diabetes, hypertension, cardiovascular disease and COPD which increase the mortality rate in COVID-19 patients [48]. Several possible mechanisms may explain why patients with these diseases are more prone to COVID-19 mortality.
3.1. COPD and COVID-19
Although the occurrence of COPD in COVID-19 patients was reported as 3.2 % [74], disease severity and mortality rate was markedly higher (60 % and 63 % respectively [75],increasing the adverse effects of COVID-19 by 5.9 fold [76], although this data needs to be stratified based on cause and stages of COPD. Interestingly, current smokers tend to develop more COVID-19 associated complications compared to non-smokers; the collective frequency of smokers with COVID-19 is 9% [75]. Chronic exposure to environmental pollution, smoking and other irritants eventually result in chronic obstructive pulmonary disease (COPD), the main features of which are inflammation of the lung parenchyma, obstruction in airflow, pulmonary emphysema and chronic bronchitis [77]. Damage to epithelial cells resulted in the excessive infiltration of monocytes, neutrophils, and cytotoxic T lymphocytes (CD8+ T-cell) to peripheral airways and lung parenchyma, together with the release of inflammatory mediators [78]. Release of these inflammatory mediators from airway inflammation to systemic circulation further resulted in systemic inflammation and associated manifestations. A complex network of cytokines has been implicated in the inflammatory process of COPD; prominent cytokines elevated in both plasma and sputum samples of COPD patients were IL-18, VEGF, IL-6, IL-1β, TNF-α and IL8 [[79], [80], [81], [82]]
Protein-Protein interaction (PPI) network analysis identified CXCR4, TNF, CCL5, CCL2, IFN-γ, CXCL8, IL-6, IL-10, ICAM1, CXCL1 as the major cytokine genes involved in high risk COPD group of COVID-19 patients [83]. Additionally, the ACE2 receptor was upregulated in the lower airway epithelium of smokers and COPD patients, perhaps reflecting increased infectability of these patients [84]. Taken together, the dysregulated immune function, including increased cytokine production and subsequent inflammation in COPD, when superimposed on SARS-CoV2 infection, further aggravated the course of disease in the COPD risk group of COVID-19 patients.
3.2. Hypertension and COVID-19
Hypertension was shown to increase mortality in COVID-19 infected hypertensive patients by 2.5 fold [85]. Triggers such as angiotensin II, excessive salt in the diet etc., have been shown to affect immune cells in the kidney, heart and blood vessels, culminating in vascular injury and inflammation [86]. Several studies identified the role of IL-1β, IL-6, IL-8, IL-17, IL-18, IL-23, IFN-γ, TNF-α and TGF-β in the inflammatory pathogenesis of hypertension [87]. An increased concentration of IL-2, IFN-γ, IL-8, IL-1α, MCP-1, VEGF, epidermal growth factor, and TNF-α was observed in the serum of hypertensive patients [88]. Additionally, IL-4 and IL-10 (anti-inflammatory cytokine) concentrations were notably lower in hypertensive patients in comparison to control subjects, suggesting a skewed TH1, TH2 and TH17 immune response, stemming from hypertension [88,89]. As discussed earlier, COVID-19 patients also displayed high levels of IL-1, MCP-1, IL-6, IL-2, TNF-α and IL-8, suggesting an interrelated role between hypertension, inflammatory mediator release and deterioration of COVID‐19 patients.
Moreover, the role of the RAS in pathology of hypertension is well described and type 1 (AT1) angiotensin II (ANG II) receptor blockers (ARBs) and angiotensin-converting enzyme (ACE) inhibitors are given to hypertensive patients as first line therapies [90]. In addition to the increased ACE2 expression, decreased viral clearance also contributes to the intensified pathological scenario in COVID-19 infections [91]. Computational network analysis identified CXCL8, VEGFA, CCL2, IL-10, IL-6, MMP-9, TNF, as key genes in hypertension high-risk COVID-19 patients [83]. Thus the low-grade chronic inflammation observed in hypertensive patients facilitated the cytokine storm, culminating in ARDS and death in COVID-19.
3.3. Cardiovascular disease and COVID-19
The death rate of COVID-19 patients with CVD was 10.5 % [92]. High blood pressure, hyperlipidemia, insulin resistance and other risk factors activate complex inflammatory cascades leading to atherosclerosis. Chronic endothelial injury results in endothelial dysfunction with augmented vascular permeability followed by extravasation of T lymphocytes, monocytes and mononuclear leukocytes. This finally leads to macrophage activation, foam cells formation and plaque development [93,94]. Cytokines such as IL-1β, IL-6, PDGF, IL-8, IFN-γ, TNF-α, and MCP-1, released from smooth muscle cells, epithelial cells and immune cells of atherosclerotic plaques, modulated smooth muscle cell activity and increased cell death [95,96]. High levels of IL-18, IL-1β, TNF-α, IL-8, IFN-γ, IL-6, PDGF, and MCP-1 are known to modulate inflammatory angiogenesis in cardiovascular disease [95], and moreover, systemic levels of IL-6 and IL-18 are considered a predictor of increased mortality in CVD patients [[97], [98], [99]]. Overall, the hyperinflammation observed in CVD likewise appears to negatively influence the course of SARS-CoV2 infection in these patients.
3.4. Diabetes and COVID-19
Altered glucose homeostasis and immune imbalance contributes to the increased susceptibility of diabetic patients to various microbial pathogens [100]. SARS CoV2 infected patients with diabetes had a 2 times increased risk of disease and mortality, compared to individuals without diabetes [101]. Hyper immune activation and increased pro-inflammatory markers are associated with type2 diabetes (T2DM) and its microvascular complications such as retinopathy, nephropathy etc [102]. These inflammatory mediators alter glucose metabolism, insulin resistance and insulin sensitivity, enhanced liver acute phase proteins synthesis and secretion and other inflammatory markers [103]. TNF-α, MCP-1, IL-6 and IL-1β are prevalent cytokines that participate in the immune pathologic process of type 2 diabetes [104]. Additionally, levels of IL-4, TNF-β, IL-7, GMCSF, IL-15, IFN- α/γ, IL-1α/β, TNF-α, IL-12p70, MIP1-α, IL-17A, IL-6, IP-10, IL-10, MIP1-β, MCP-1, IL-8, IL-13 and IL-9 are increased in type 2 diabetic patients [105,106].
An immune-mediated inflammatory cascade is a crucial event in type1 diabetes, which involves the activation and infiltration of T lymphocytes, macrophages and other immune cells, as well as production of inflammatory mediators in the pancreas [107]. Increased levels of IL-1α, TNF-α, IL-23, IL-1β, IL-10, IL-17A, and IL-6 were seen in type1 diabetic patients [[108], [109], [110]] and in a non-obese diabetic murine model [111].
SARS CoV2 infection resulted in high levels of inflammatory cytokines as described above, and contributed to the deterioration of COVID‐19 patients with diabetes. Patients with type 1 or type 2 diabetes are treated with ARBs and ACE inhibitors, which may contribute to increased ACE2 expression in diabetic patients with SARS-CoV2 [90]. TNF, CXCL8, IL-10, CCL2, ICAM1, IFN-γ, IL-2, CXCR4 were the key genes identified by PPI network analysis for the COVID-19 diabetic high-risk group [83]. Hyperglycemia, increased coagulation rate, and elevated release of pro-inflammatory cytokines [112] all facilitate the severity of COVID‐19 in diabetic patients.
Gestational Diabetes Mellitus (GDM) as a comorbidity has been observed in two pregnant women, who died of COVID-19 infection [113]. As described above, GDM is associated with increased cytokine levels that further enhanced maternal insulin resistance, as well as increased the high risk of pregnancy outcome and neonatal complications [114]. Pro-inflammatory cytokine such as IL-6, TNF-α, IL-8, and IL-18 were elevated in GDM patients, together with a decreased expressionof IL-10 [115].
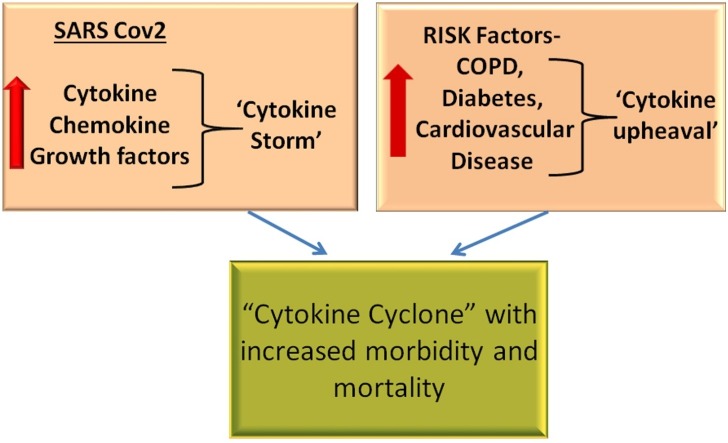
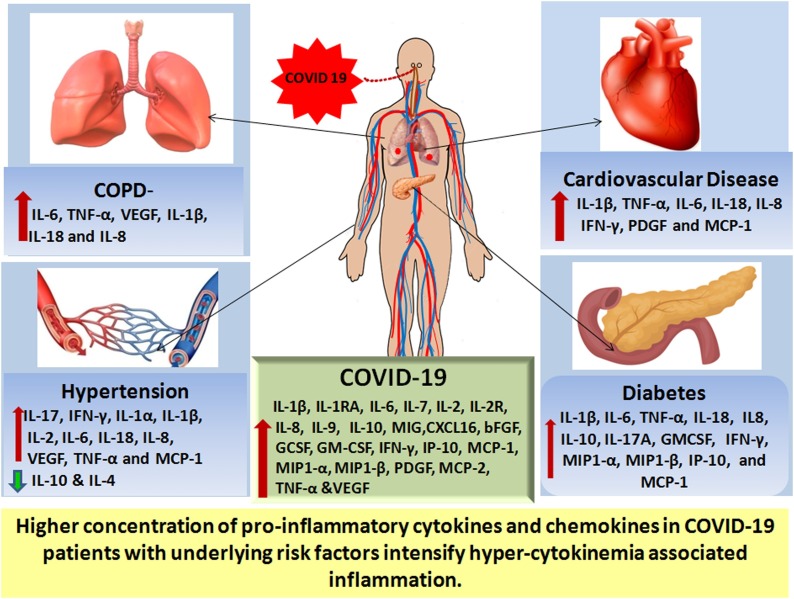
On the basis of the above mentioned data, one can envisage that elevated concentrations of these pro-inflammatory chemokines and cytokines in COVID-19 patient with underlying risk factors, further intensifies cytokine storm-associated inflammation associated with SARS CoV2, ultimately converting the cytokine-storm of COVID-19 into a ‘Cytokine Super Cyclone’ (Fig. 2 ). Importantly, co-morbidities associated with COVID-19 share an overlapping hyperinflammatory cytokine profile that may further indicate the importance of anti-cytokine therapy strategies in the management of these co-morbidities. Further studies are necessary to evaluate risk factors like COPD, hypertension, CVD and diabetes in COVID-19 disease management.
Fig. 2.
Schematic diagram representing higher pro-inflammatory cytokines and chemokines in COVID-19 patients with underlying risk factors, with hyper-cytokinemia associated inflammation culminating in lethal complications in SARS-CoV2 infection.
4. BCG Vaccination as a strategy to control the Cytokine Super cyclone in COVID-19 patients with risk factors
Bacillus Calmette-Guérin (BCG), a vaccine given to protect against Tuberculosis (TB) and other non-tuberculous mycobacteria (NTM) infections such as Buruli ulcer, is administered in new-born [116]. This live attenuated vaccine was developed at Pasteur Institute in Paris by Albert Calmette and Camille Guérin. The research work started in 1908 and took over 13 years for the first trial in human. During this long period that spans the First World War, they successfully sub-cultured Mycobacterium bovis isolated from cow for 231 times and generated a less virulent strain of M bovis. Since then, BCG is the only vaccine used against TB and more interestingly, it also confers protection against leprosy [117,118]. WHO recommends a single dose of BCG for infants in TB endemic as well as leprosy high risk countries and globally almost 100 million infants are vaccinated yearly [119]. The strains of Mycobacterium bovis used as BCG vaccine currently in different countries are generated by a number of passages from actual Paris strain. Pasteur 1173 P2, the Glaxo 1077, the Danish 1331, the Russian BCG-I, the Moreau RDJ and the Tokyo 172-1 strains are commonly used BCG strains and they differ both phenotypically and genotypically [117,120]. Additionally, the use of BCG for intravesical treatment in bladder cancer is also reported [121].
Based on several clinical trials and observational studies, it has been shown that BCG vaccination reduced the neonatal, as well as childhood death rate [122,123]. Retrospective cross-sectional studies conducted in sub-Saharan African children below 5 years of age demonstrated a positive association between BCG vaccination and lower risk of malaria [124]. Additionally, epidemiological studies also established the protective role of BCG against respiratory tract infections in children [125,126]. Vaccination with BCG enhanced IFN-γ and IL-10 level, thus providing immunity against respiratory tract infection even in an elderly age group [127].
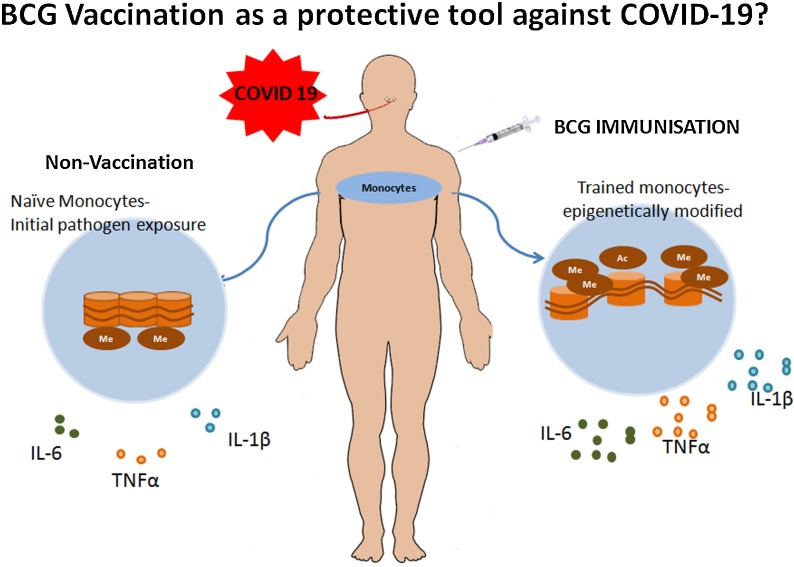
BCG also provides non-specific innate immunity against pathogens such as viruses and parasites, through an innate immune mechanism termed ‘trained immunity’ which is defined as the immunological recall of the innate immune system via epigenetic reprogramming [128]. Innate immune cells such as monocytes, NK cells and macrophages undergo epigenetic modification of histones that include methylation and acetylation, and enhance expression of IL-6, TNF-α and IL-1β after BCG vaccination. Thus, the trained phenotype of innate immune cells results in an increased secretion of pro-inflammatory cytokines on exposure to pathogens, thereby enhancing host protection [129,130]. Healthy subjects immunized with BCG showed increased production of TNF-α and IL-1β in response to bacterial and non-bacterial stimulants. Molecular alterations included increased H3K4me3 (trimethylation of histone H3 at lysine 4) at exposed promoter regions of these pro-inflammatory cytokines, leading to their increased expression [131]. In another study, BCG vaccination reduced viremia in an experimental yellow fever virus infection model by inducing trained immunity; epigenetic modification of monocytes resulted in increased production of IL-1β [132]. Moreover, BCG enhanced heterologous TH1/TH17 responses by inducing the long-lasting release of IL-22, IFN-γ, and IL-17, thereby attaining non-specific immune response to infections [133]. BCG also promotes host defence by enhancing adaptive T cell and B cell immune response mediated by CD8, CD4 T cells and antibodies respectively [134]. In line with this, several in vivo studies in murine models were also reported. BCG immunized mice expressed antimicrobial peptides and bestowed protection against malarial parasitemia [135]. Additionally, prior BCG vaccination in influenza virus challenged mice were found to have considerable protection via secretion of antibody or activation of cell-mediated immunity [136]. An in vivo murine study conducted to understand the effect of BCG against various viral infections such herpes simplex type 1, influenza A2 and encephalomyocarditis viruses demonstrated increased resistance of BCG immunised mice against these viruses compared to control mice [137]; also BCG vaccinated newborn mice were shown to be more resistant to Herpes Simplex Type 2 Infection [138].
Based on these observations, BCG vaccination has the potential to act as a protective agent against SARS CoV2, as it provides antiviral immunity [139]. SARS-CoV2 virus enhances the generation of IL-6, IFN-γ, TNF-α and IL-1β in mild to severely infected patients [48]. As discussed earlier, trained immunity provided by BCG vaccination involved cytokine release, thus generating an antiviral state and protection against SARS-CoV2 (Fig. 3 ). Ozdemir et al. has already documented a marked decrease in the number of cases per population, death per population and deaths per cases ratio of COVID-19 in BCG vaccinated countries compared to BCG-non-vaccinated countries [140]. Another observational study conducted in 25 level 4 European countries found a positive correlation between BCG vaccination and decreased death rate in COVID 19, but found no association between pneumococcal vaccine and seasonal influenza vaccination even after adjusting several covariates, such as days of lockdown, life-expectancy, net migration, median age, case-fatality rate, etc [141].
Fig. 3.
Antiviral immunity provided by BCG vaccine through trained immunity involves the discharge of IL-1β, TNF-α and IL-6 from epigenetically modified monocytes which confer early protection against SARS-Cov2.
Moreover, BCG vaccination protocols and bacteria strains used are different among BCG vaccinated countries [142]. Furthermore, it will be important to study the impact of COVID-19 infection in patients with co-morbidities like diabetes, hypertension, COPD and cardiovascular disease in BCG vaccinated and non-vaccinated countries. Although multiple studies support the hypothesis of a non-specific innate immune role of BCG vaccination against the COVID 19 [143,144], controversies have also been reported [145,146]. Because each study used different data extraction techniques, statistical analysis and correction for confounding variables, they cannot give ultimate evidence of causality and underscore the importance of clinical trials to determine the association between BCG-mediated protection and severity of COVID-19 infection.
Currently, a total of 17 clinical trials are registered with ClinicalTrials.gov to evaluate BCG induced trained immunity against SARS-CoV2 (https://clinicaltrials.gov/ct2/results?cond=COVID&term=bcg&cntry=&state=&city=&dist=). Out of 17, only 8 studies have started recruitment of participants. Among these, BCG-CORONA (Reducing Health Care Workers Absenteeism in COVID-19 Pandemic by Enhanced Trained Immune Responses Through Bacillus Calmette-Guérin Vaccination, a Randomized Controlled Trial), BRACE (BCG Vaccination to Reduce the Impact of COVID-19 in Healthcare Workers Following Coronavirus Exposure), BCG Vaccine for Health Care Workers as Defense Against COVID 19 (BADAS) and BCG vaccination for Healthcare Workers in COVID-19 Pandemic are conducting trials in healthcare workers, because of their vulnerability to COVID 19 infection. The primary objective of BRACE, BADAS and BCG Vaccination for Healthcare Workers in COVID-19 Pandemic is to analyse the occurrence and severity of COVID-19 in BCG vaccinated and non vaccinated groups. The BCG-CORONA trial is estimated to involve 1500 Health workers and aims to assess whether unplanned absenteeism of these participants can be reduced by BCG vaccination in the COVID-19 pandemic. Additionally, another study group in Germany is assessing if VPM1002, the genetically modified vaccine strain of BCG can be used as a protective agent against SARS CoV2 infection in the elderly and health care workers. WHO has not yet advised the use of BCG immunization for SARS-CoV2 infection (https://www.who.int/news-room/commentaries/detail/bacille-calmette-gu%C3%A9rin-(bcg)-vaccination-and-covid-19). In addition to clinical trials, more epidemiological studies are required to understand the status of co-morbidities and associated death in BCG vaccinated COVID-19 cases; these data are important to evaluate the potential benefit of BCG vaccinination and the trained immune response against COVID-19 in high risk cases. In conclusion, boosting the innate immune response by BCG vaccination via trained immunity/immune memory by BCG vaccination represents a important potential strategy to manage the immune consequences of COVID-19 – at least until a specific vaccine against SARS-CoV2 becomes available.
5. Conclusion
Uncontrolled secretion of cytokines in COVID 19 patients is associated with hyper-inflammation, increased disease severity and development of acute respiratory distress syndrome (ARDS). In high-risk patients with co-morbidities, COVID-19 infection and the ensuing ‘cytokine storm’ can exacerbate severe clinical manifestations, culminating in high mortality amongst patients with associated risk factors. On the other hand, vaccination with BCG and the subsequent regulated secretion of IFN and other cytokines including IL-1β, TNF-α and IL-6 can contribute to the emergence of trained innate immunity, with the potential to generate early protection against SARS-Cov2. Ongoing clinical studies are addressing these important issues with regard to management of COVID-19.
Funding
Dr. Malini Laloraya is supported by Department of Biotechnology (DBT)-Rajiv Gandhi Centre for Biotechnology. BSJ supported by Research Fellowship from Department of Science & Technology (DST/ INSPIRE Fellowship/2015/1F150361).
CRediT authorship contribution statement
Betcy Susan Johnson: Formal analysis, Writing - original draft. Malini Laloraya: Conceptualization, Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Biographies

Betcy Susan Johnson Completed her post-graduation degree on Medical Biochemistry in 2013 from Calicut University, India. She is currently pursuing PhD at Female Reproduction and Metabolic Syndrome Laboratory, Rajiv Gandhi Centre for Biotechnology. Her research interest is on understanding the pathogenesis involved in Polycystic Ovary Syndrome.

Malini Laloraya obtained her Ph.D. degree at the University of Indore(Devi Ahilya Vishwavidyalaya-DAVV) in 1989 with her thesis focussed on understanding the Role of free-radicals in luteal steroidogenesis and implantation. She completed her post-graduate degree in Life Sciences from the University of Indore (DAVV) in 1986. At present she is a Senior Scientist at Rajiv Gandhi Centre for Biotechnology and is the Group Leader for the Female Reproduction and Metabolic Syndrome Laboratory. She has several awards and fellowships to her credit. Some of them are MP Young Scientist Award, International Post-Doctoral Fellowship award of The Rockefeller Foundation, USA, Visiting fellowship under Healthy Babies program of NIH, USA, Raine Visiting Professorship The University of Western Australia in 2006/ 2008 by Raine Medical Research Foundation, Australia. She is a recipient of Labshetwar Award (2014) and Prof. G. P. Talwar Gold Medal Award (2020) of Indian Society for Study of Reproduction & Fertility. She is an elected fellow of The National Academy of Sciences, India (NASI) and was Conferred Fellowship in Reproduction and Endocrinology, SRBCE (2019). Her main research interests are understanding the molecular mechanisms involved in embryo implantation, involving adhesion, decidualization and immune tolerance during pregnancy, Polycystic Ovary Syndrome pathogenesis and immune aspect involved in PCOS and Type1 Diabetes.
References
- 1.Masters P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses2020 The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Opriessnig T., Huang Y. Coronavirus disease 2019 (COVID–19) outbreak: Could pigs be vectors for human infections? Xenotransplantation. 2020;27 doi: 10.1111/xen.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi Y., Lagniton P.N.P., Ye S., Li E., Xu R.H. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int. J. Biol. Sci. 2020;16:1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimura H., Yoshizumi M., Ishii H., Oishi K., Ryo A. Cytokine production and signaling pathways in respiratory virus infection. Front. Microbiol. 2013;4:276. doi: 10.3389/fmicb.2013.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mogensen T.H., Paludan S.R. Molecular pathways in virus-induced cytokine production. Microbiol. Mol. Biol. Rev. 2001;65:131–150. doi: 10.1128/MMBR.65.1.131-150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofmann P., Sprenger H., Kaufmann A., Bender A., Hasse C., Nain M., Gemsa D. Susceptibility of mononuclear phagocytes to influenza A virus infection and possible role in the antiviral response. J. Leukoc. Biol. 1997;61:408–414. doi: 10.1002/jlb.61.4.408. [DOI] [PubMed] [Google Scholar]
- 10.Schultz-Cherry S., Hinshaw V.S. Influenza virus neuraminidase activates latent transforming growth factor beta. J. Virol. 1996;70:8624–8629. doi: 10.1128/jvi.70.12.8624-8629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samuel C.E. Antiviral actions of interferons. Clin. Microbiol. Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L., Du F., Wang X. TNF-A¦ induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 13.Subramanian N.A.T.A., Bray M.A. Interleukin 1 releases histamine from human basophils and mast cells in vitro. J. Immunol. 1987;138:271–275. [PubMed] [Google Scholar]
- 14.Chomarat P., Banchereau J., Davoust J., Palucka A.K. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat. Immunol. 2000;1:510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 15.Schett G. Physiological effects of modulating the interleukin-6 axis. Rheumatology. 2018;57:ii43–ii50. doi: 10.1093/rheumatology/kex513. [DOI] [PubMed] [Google Scholar]
- 16.Kimura A., Kishimoto T. IL6: regulator of Treg/Th17 balance. Eur. J. Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 17.Nakanishi K. Unique action of interleukin-18 on T cells and other immune cells. Front. Immunol. 2018;9:763. doi: 10.3389/fimmu.2018.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu B., Mori I., Hossain M.J., Dong L., Takeda K., Kimura Y. Interleukin-18 improves the early defence system against influenza virus infection by augmenting natural killer cell-mediated cytotoxicity. J. Gen. Virol. 2004;85:423–428. doi: 10.1099/vir.0.19596-0. [DOI] [PubMed] [Google Scholar]
- 19.Channappanavar R., Perlman S. 39 ed. Springer; 2017. Pathogenic Human Coronavirus Infections: Causes and Consequences of Cytokine Storm and Immunopathology; pp. 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020 doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peiris J.S.M., Guan Y., Yuen K.Y. Severe acute respiratory syndrome. Nat. Med. 2004;10:S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholls J.M., Poon L.L., Lee K.C., Ng W.F., Lai S.T., Leung C.Y., Chu C.M., Hui P.K., Mak K.L., Lim W. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A., Walmsley S.L., Mazzulli T., Avendano M., Derkach P. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. Jama. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 24.Kai H., Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitorsΓÇölessons from available evidence and insights into COVID-19. Hypertens. Res. 2020:1–7. doi: 10.1038/s41440-020-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuba K., Imai Y., Penninger J.M. Angiotensin-converting enzyme 2 in lung diseases. Curr. Opin. Pharmacol. 2006;6:271–276. doi: 10.1016/j.coph.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J., Zheng X., Tong Q., Li W., Wang B., Sutter K., Trilling M., Lu M., Dittmer U., Yang D. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARSΓÇÉCoV, MERSΓÇÉCoV, and 2019ΓÇÉnCoV. J. Med. Virol. 2020;92:491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang N.L.-S., Chan P.K.-S., Wong C.K., To K.F., Wu A.K.-L., Sung Y.M., Hui D.S.-C., Sung J.J.-Y., Lam C.W.-K. Early enhanced expression of interferon-inducible protein-10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clin. Chem. 2005;51:2333–2340. doi: 10.1373/clinchem.2005.054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang Y., Xu J., Zhou C., Wu Z., Zhong S., Liu J., Luo W., Chen T., Qin Q., Deng P. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2005;171:850–857. doi: 10.1164/rccm.200407-857OC. [DOI] [PubMed] [Google Scholar]
- 31.Huang K., Su I., Theron M., Wu Y., Lai S., Liu C., Lei H. An interferon γrelated cytokine storm in SARS patients. J. Med. Virol. 2005;75:185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CHIEN J., HSUEH P., CHENG W., YU C., YANG P. Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology. 2006;11:715–722. doi: 10.1111/j.1440-1843.2006.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong C.K., Lam C.W.K., Wu A.K.L., Ip W.K., Lee N.L.S., Chan I.H.S., Lit L.C.W., Hui D.S.C., Chan M.H.M., Chung S.S.C. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C.H., Liu C.Y., Wan Y.L., Chou C.L., Huang K.H., Lin H.C., Lin S.M., Lin T.Y., Chung K.F., Kuo H.P. Persistence of lung inflammation and lung cytokines with high-resolution CT abnormalities during recovery from SARS. Respir. Res. 2005;6:42. doi: 10.1186/1465-9921-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menachery V.D., Eisfeld A.J., Sch+ñfer A., Josset L., Sims A.C., Proll S., Fan S., Li C., Neumann G., Tilton S.C. Pathogenic influenza viruses and coronaviruses utilize similar and contrasting approaches to control interferon-stimulated gene responses. MBio. 2014;5:e01174. doi: 10.1128/mBio.01174-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauch C.T., Oraby T. Assessing the pandemic potential of MERS-CoV. Lancet. 2013;382:662–664. doi: 10.1016/S0140-6736(13)61504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saad M., Omrani A.S., Baig K., Bahloul A., Elzein F., Matin M.A., Selim M.A., Al Mutairi M., Al Nakhli D., Al Aidaroos A.Y. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int. J. Infect. Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song Z., Xu Y., Bao L., Zhang L., Yu P., Qu Y., Zhu H., Zhao W., Han Y., Qin C. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11:59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Widagdo W., Raj V.S., Schipper D., Kolijn K., van Leenders G.J., Bosch B.J., Bensaid A., et al. Differential expression of the Middle East respiratory syndrome coronavirus receptor in the upper respiratory tracts of humans and dromedary camels. J. Virol. 2016;90:4838–4842. doi: 10.1128/JVI.02994-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alsaad K.O., Hajeer A.H., Al Balwi M., Al Moaiqel M., Al Oudah N., Al Ajlan A., AlJohani S., Alsolamy S., Gmati G.E., Balkhy H. Histopathology of Middle East respiratory syndrome coronovirus (MERSΓÇÉCoV) infectionΓÇôclinicopathological and ultrastructural study. Histopathology. 2018;72:516–524. doi: 10.1111/his.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeung M.L., Yao Y., Jia L., Chan J.F., Chan K.H., Cheung K.F., Chen H., Poon V.K., Tsang A.K., To K.K. MERS coronavirus induces apoptosis in kidney and lung by upregulating Smad7 and FGF2. Nat. Microbiol. 2016;1:1–8. doi: 10.1038/nmicrobiol.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chu H., Zhou J., Wong B.H.-Y., Li C., Chan J.F.-W., Cheng Z.S., Yang D., Wang D., Lee A.C.-Y., Li C. Middle East respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J. Infect. Dis. 2016;213:904–914. doi: 10.1093/infdis/jiv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim E.S., Choe P.G., Park W.B., Oh H.S., Kim E.J., Nam E.Y., Na S.H., Kim M., Song K.H., Bang J.H. Clinical progression and cytokine profiles of Middle East respiratory syndrome coronavirus infection. J. Korean Med. Sci. 2016;31:1717–1725. doi: 10.3346/jkms.2016.31.11.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahallawi W.H., Khabour O.F., Zhang Q., Makhdoum H.M., Suliman B.A. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi: 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou J., Chu H., Li C., Wong B.H.-Y., Cheng Z.S., Poon V.K.-M., Sun T., Lau C.C.-Y., Wong K.K.-Y., Chan J.Y.-W. Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis. J. Infect. Dis. 2014;209:1331–1342. doi: 10.1093/infdis/jit504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y., Gayle A.A., Wilder-Smith A., RocklÃv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020;27 doi: 10.1093/jtm/taaa021. taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y., Guo Y., Pan Y., Zhao Z.J. Structure analysis of the receptor binding of 2019-nCoV. Biochem. Biophys. Res. Commun. 2020;525:135–140. doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coutard B., Valle C., de L., Canard X.B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176 doi: 10.1016/j.antiviral.2020.104742. doi: 10.1016/j.antiviral.2020.104742.Epub;%2020Feb 10.: 104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yap J.K.Y., Moriyama M., Iwasaki A. Inflammasomes and Pyroptosis as therapeutic targets for COVID-19. J. Immunol. 2020 doi: 10.4049/jimmunol.2000513. ji2000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sauty A., Dziejman M., Taha R.A., Iarossi A.S., Neote K., Garcia-Zepeda E.A., Hamid Q., Luster A.D. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J. Immunol. 1999;162:3549–3558. [PubMed] [Google Scholar]
- 57.Glass W.G., Subbarao K., Murphy B., Murphy P.M. Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J. Immunol. 2004;173:4030–4039. doi: 10.4049/jimmunol.173.6.4030. [DOI] [PubMed] [Google Scholar]
- 58.Jiang D., Liang J., Hodge J., Lu B., Zhu Z., Yu S., Fan J., Gao Y., Yin Z., Homer R., Gerard C., Noble P.W. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J. Clin. Invest. 2004;114:291–299. doi: 10.1172/JCI16861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheemarla N.R., Brito A.F., Fauver J.R., Alpert T., Vogels C.B., Omer S.B., Ko A., Grubaugh N.D., Landry M.L., Foxman E.F. Host response-based screening to identify undiagnosed cases of COVID-19 and expand testing capacity. medRxiv. 2020 [Google Scholar]
- 60.Chu H., Chan J.F., Wang Y., Yuen T.T., Chai Y., Hou Y., Shuai H., Yang D., Hu B., Huang X., Zhang X., Cai J.P., Zhou J., Yuan S., Kok K.H., To K.K., Chan I.H., Zhang A.J., Sit K.Y., Au W.K., Yuen K.Y. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa410. ciaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., Guo D., Hu W., Yang J., Tang Z., Wu H., Lin Y., Zhang M., Zhang Q., Shi M., Liu Y., Zhou Y., Lan K., Chen Y. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020;9:761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020;111 doi: 10.1016/j.jaut.2020.102452. doi: 10.1016/j.jaut.2020.102452. Epub;%2020 Apr 10.: 102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanaka T., Narazaki M., Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8:959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 64.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang W., Ye L., Ye L., Li B., Gao B., Zeng Y., Kong L., Fang X., Zheng H., Wu Z., She Y. Up-regulation of IL-6 and TNF-alpha induced by SARS-coronavirus spike protein in murine macrophages via NF-kappaB pathway. Virus Res. 2007;128:1–8. doi: 10.1016/j.virusres.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DeDiego M.L., Nieto-Torres J.L., Regla-Nava J.A., Jimenez-GuardeÃo J.M., Fernandez-Delgado R., Fett C., CastaÃo-Rodriguez C., Perlman S., Enjuanes L. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J. Virol. 2014;88:913–924. doi: 10.1128/JVI.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., Damoraki G., Gkavogianni T., Adami M.E., Katsaounou P., Ntaganou M., Kyriakopoulou M., Dimopoulos G., Koutsodimitropoulos I., Velissaris D., Koufargyris P., Karageorgos A., Katrini K., Lekakis V., Lupse M., Kotsaki A., Renieris G., Theodoulou D., Panou V., Koukaki E., Koulouris N., Gogos C., Koutsoukou A. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., Levantovsky R., et al. Immunology of COVID-19: current state of the science. Immunity. 2020;10 doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cameron M.J., Ran L., Xu L., Danesh A., Bermejo-Martin J.F., Cameron C.M., Muller M.P., Gold W.L., Richardson S.E., Poutanen S.M., Willey B.M., DeVries M.E., Fang Y., Seneviratne C., Bosinger S.E., Persad D., Wilkinson P., Greller L.D., Somogyi R., Humar A., Keshavjee S., Louie M., Loeb M.B., Brunton J., McGeer A.J., Kelvin D.J. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J. Virol. 2007;81:8692–8706. doi: 10.1128/JVI.00527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Pere H., Charbit B., Bondet V., Chenevier-Gobeaux C., Breillat P. Impaired type I interferon activity and exacerbated inflammatory responses in severe Covid-19 patients. medRxiv. 2020 doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen H., Liu W., Liu D., Zhao L., Yu J. SARS-CoV-2 activates lung epithelia cell proinflammatory signaling and leads to immune dysregulation in COVID-19 patients by single-cell sequencing. medRxiv. 2020 doi: 10.1016/j.ebiom.2021.103500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sallard E., Lescure F.X., Yazdanpanah Y., Mentre F., Peiffer-Smadja N., Florence A.D.E.R., Yazdanpanah Y., Mentre F., Lescure F.X., Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020 doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou Q., Chen V., Shannon C.P., Wei X.S., Xiang X., Wang X., Wang Z.H., Tebbutt S.J., Kollmann T.R., Fish E.N. Interferon alpha 2b treatment for COVID-19. Front. Immunol. 2020;11:1061. doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alqahtani J.S., Oyelade T., Aldhahir A.M., Alghamdi S.M., Almehmadi M., Alqahtani A.S., Quaderi S., Mandal S., Hurst J.R. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang B., Li R., Lu Z., Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY). 2020;12:6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Di S.A., Caramori G., Ricciardolo F.L., Capelli A., Adcock I.M., Donner C.F. Cellular and molecular mechanisms in chronic obstructive pulmonary disease: an overview. Clin. Exp. Allergy. 2004;34:1156–1167. doi: 10.1111/j.1365-2222.2004.02030.x. [DOI] [PubMed] [Google Scholar]
- 78.Moermans C., Heinen V., Nguyen M., Henket M., Sele J., Manise M., Corhay J.L., Louis R. Local and systemic cellular inflammation and cytokine release in chronic obstructive pulmonary disease. Cytokine. 2011;56:298–304. doi: 10.1016/j.cyto.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 79.Barnes P.J. The cytokine network in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2009;41:631–638. doi: 10.1165/rcmb.2009-0220TR. [DOI] [PubMed] [Google Scholar]
- 80.Franciosi L.G., Page C.P., Celli B.R., Cazzola M., Walker M.J., Danhof M., Rabe K.F., Della Pasqua O.E. Markers of disease severity in chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2006;19:189–199. doi: 10.1016/j.pupt.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 81.Barnes P.J., Celli B.R. Systemic manifestations and comorbidities of COPD. Eur. Respir. J. 2009;33:1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 82.Selvarajah S., Todd I., Tighe P.J., John M., Bolton C.E., Harrison T., Fairclough L.C. Multiple circulating cytokines are coelevated in chronic obstructive pulmonary disease. Mediators Inflamm. 2016;2016:3604842. doi: 10.1155/2016/3604842. doi: 10.1155/2016/3604842. Epub;%2016 Jul 25.: 3604842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kumar S. 2020. COVID-19: A Drug Repurposing and Biomarker Identification by Using Comprehensive Gene-disease Associations Through protein-protein Interaction Network Analysis. [Google Scholar]
- 84.Leung J.M., Yang C.X., Tam A., Shaipanich T., Hackett T.L., Singhera G.K., Dorscheid D.R., Sin D.D. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur. Respir. J. 2020;55:2000688–2002020. doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lippi G., Wong J., Henry B.M. Hypertension and its severity or mortality in Coronavirus Disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020;130:304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 86.Wenzel U., Turner J.E., Krebs C., Kurts C., Harrison D.G., Ehmke H. Immune mechanisms in arterial hypertension. J. Am. Soc. Nephrol. 2016;27:677–686. doi: 10.1681/ASN.2015050562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tanase D.M., Gosav E.M., Radu S., Ouatu A., Rezus C., Ciocoiu M., Costea C.F., Floria M. Arterial hypertension and interleukins: potential therapeutic target or future diagnostic marker? Int. J. Hypertens. 2019;2019:3159283. doi: 10.1155/2019/3159283. doi: 10.1155/2019/3159283. eCollection;%2019.: 3159283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mirhafez S.R., Mohebati M., Feiz D.M., Saberi K.M., Ebrahimi M., Avan A., Eslami S., Pasdar A., Rooki H., Esmaeili H., Ferns G.A., Ghayour-Mobarhan M. An imbalance in serum concentrations of inflammatory and anti-inflammatory cytokines in hypertension. J. Am. Soc. Hypertens. 2014;8:614–623. doi: 10.1016/j.jash.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 89.Ji Q., Cheng G., Ma N., Huang Y., Lin Y., Zhou Q., Que B., Dong J., Zhou Y., Nie S. Circulating Th1, Th2, and Th17 levels in hypertensive patients. Dis. Markers. 2017;2017:7146290. doi: 10.1155/2017/7146290. doi: 10.1155/2017/7146290. Epub;%2017 Jul 5.: 7146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8:e21–2600. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen X., Hu W., Ling J., Mo P., Zhang Y., Jiang Q., Ma Z., Cao Q., Deng L., Song S. Hypertension and diabetes delay the viral clearance in COVID-19 patients. medRxiv. 2020 [Google Scholar]
- 92.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., Madhur M.S., Tomaszewski M., Maffia P., D’Acquisto F., Nicklin S.A., Marian A.J., Nosalski R., Murray E.C., Guzik B., Berry C., Touyz R.M., Kreutz R., Wang D.W., Bhella D., Sagliocco O., Crea F., Thomson E.C., McInnes I.B. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020 doi: 10.1093/cvr/cvaa106. cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rafieian-Kopaei M., Setorki M., Doudi M., Baradaran A., Nasri H. Atherosclerosis: process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014;5:927–946. [PMC free article] [PubMed] [Google Scholar]
- 94.Williams J.W., Huang L.H., Randolph G.J. Cytokine circuits in cardiovascular disease. Immunity. 2019;50:941–954. doi: 10.1016/j.immuni.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mehra V.C., Ramgolam V.S., Bender J.R. Cytokines and cardiovascular disease. J. Leukoc. Biol. 2005;78:805–818. doi: 10.1189/jlb.0405182. [DOI] [PubMed] [Google Scholar]
- 96.Kofler S., Nickel T., Weis M. Role of cytokines in cardiovascular diseases: a focus on endothelial responses to inflammation. Clin. Sci. 2005;108:205–213. doi: 10.1042/CS20040174. [DOI] [PubMed] [Google Scholar]
- 97.Lindmark E., Diderholm E., Wallentin L., Siegbahn A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: effects of an early invasive or noninvasive strategy. JAMA. 2001;286:2107–2113. doi: 10.1001/jama.286.17.2107. [DOI] [PubMed] [Google Scholar]
- 98.Blankenberg S., Tiret L., Bickel C., Peetz D., Cambien F., Meyer J., Rupprecht H.J. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation. 2002;106:24–30. doi: 10.1161/01.cir.0000020546.30940.92. [DOI] [PubMed] [Google Scholar]
- 99.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;10 doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 100.Ferlita S., Yegiazaryan A., Noori N., Lal G., Nguyen T., To K., Venketaraman V. Type 2 diabetes mellitus and altered immune system leading to susceptibility to pathogens, especially Mycobacterium tuberculosis. J. Clin. Med. 2019;8:2219. doi: 10.3390/jcm8122219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kumar A., Arora A., Sharma P., Anikhindi S.A., Bansal N., Singla V., Khare S., Srivastava A. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab. Syndr. 2020;14:535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Navarro J.F., Mora C. Role of inflammation in diabetic complications. Nephrol. Dial. Transplant. 2005;20:2601–2604. doi: 10.1093/ndt/gfi155. [DOI] [PubMed] [Google Scholar]
- 103.Pickup J.C., Crook M.A. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–1248. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 104.Alexandraki K., Piperi C., Kalofoutis C., Singh J., Alaveras A., Kalofoutis A. Inflammatory process in type 2 diabetes: the role of cytokines. Ann. N. Y. Acad. Sci. 2006;1084:89–117. doi: 10.1196/annals.1372.039.89-117. [DOI] [PubMed] [Google Scholar]
- 105.Randeria S.N., Thomson G.J.A., Nell T.A., Roberts T., Pretorius E. Inflammatory cytokines in type 2 diabetes mellitus as facilitators of hypercoagulation and abnormal clot formation. Cardiovasc. Diabetol. 2019;18:72–0870. doi: 10.1186/s12933-019-0870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hang H., Yuan S., Yang Q., Yuan D., Liu Q. Multiplex bead array assay of plasma cytokines in type 2 diabetes mellitus with diabetic retinopathy. Mol. Vis. 2014;20:1137–1145. eCollection;%2014.: 1137-1145. [PMC free article] [PubMed] [Google Scholar]
- 107.Szablewski L. Role of immune system in type 1 diabetes mellitus pathogenesis. Int. Immunopharmacol. 2014;22:182–191. doi: 10.1016/j.intimp.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 108.Fatima N., Faisal S.M., Zubair S., Ajmal M., Siddiqui S.S., Moin S., Owais M. Role of pro-inflammatory cytokines and biochemical markers in the pathogenesis of type 1 diabetes: correlation with age and glycemic condition in diabetic human subjects. PLoS One. 2016;11 doi: 10.1371/journal.pone.0161548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Purohit S., Sharma A., Zhi W., Bai S., Hopkins D., Steed L., Bode B., Anderson S.W., Reed J.C., Steed R.D., She J.X. Proteins of TNF-α and IL6 Pathways Are Elevated in Serum of Type-1 Diabetes Patients with Microalbuminuria. Front. Immunol. 2018;9:154. doi: 10.3389/fimmu.2018.00154. doi: 10.3389/fimmu.2018.00154. eCollection;%2018.: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gouda W., Mageed L., El Dayem S.M.A., Ashour E., Afify M. Evaluation of pro-inflammatory and anti-inflammatory cytokines in type 1 diabetes mellitus. Bulletin of the National Research Centre. 2018;42:14. [Google Scholar]
- 111.Iglesias M., Arun A., Chicco M., Lam B., Talbot C.C., Jr., Ivanova V., Lee W.P.A., Brandacher G., Raimondi G. Type-I interferons inhibit Interleukin-10 signaling and favor type 1 diabetes development in nonobese diabetic mice. Front. Immunol. 2018;9:1565. doi: 10.3389/fimmu.2018.01565. doi: 10.3389/fimmu.2018.01565. eCollection;%2018.: 1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hussain A., Bhowmik B., do Vale Moreira N.C. COVID-19 and diabetes: knowledge in progress. Diabetes Res. Clin. Pract. 2020;162:108142. doi: 10.1016/j.diabres.2020.108142. . doi: 10.1016/j.diabres.2020.108142. Epub;%2020 Apr 9.: 108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hantoushzadeh S., Shamshirsaz A.A., Aleyasin A., Seferovic M.D., Aski S.K., Arian S.E., Pooransari P., Ghotbizadeh F., Aalipour S., Soleimani Z., Naemi M., Molaei B., Ahangari R., Salehi M., Oskoei A.D., Pirozan P., Darkhaneh R.F., Laki M.G., Farani A.K., Atrak S., Miri M.M., Kouchek M., Shojaei S., Hadavand F., Keikha F., Hosseini M.S., Borna S., Ariana S., Shariat M., Fatemi A., Nouri B., Nekooghadam S.M., Aagaard K. Maternal death due to COVID-19. Am. J. Obstet. Gynecol. 2020:10. doi: 10.1016/j.ajog.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wedekind L., Belkacemi L. Altered cytokine network in gestational diabetes mellitus affects maternal insulin and placental-fetal development. J. Diabetes Complications. 2016;30:1393–1400. doi: 10.1016/j.jdiacomp.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 115.Kuzmicki M., Telejko B., Zonenberg A., Szamatowicz J., Kretowski A., Nikolajuk A., Laudanski P., Gorska M. Circulating pro- and anti-inflammatory cytokines in Polish women with gestational diabetes. Horm. Metab. Res. 2008;40:556–560. doi: 10.1055/s-2008-1073166. [DOI] [PubMed] [Google Scholar]
- 116.Zimmermann P., Finn Curtis A.N. Does BCG vaccination protect against nontuberculous mycobacterial infection? A systematic review and meta-analysis. J. Infect. Dis. 2018;218:679–687. doi: 10.1093/infdis/jiy207. [DOI] [PubMed] [Google Scholar]
- 117.Luca S., Mihaescu T. History of BCG Vaccine. Maedica. 2013;8:53–58. [PMC free article] [PubMed] [Google Scholar]
- 118.Fine P.E.M. BCG vaccination against tuberculosis and leprosy. Br. Med. Bull. 1988;44:691–703. doi: 10.1093/oxfordjournals.bmb.a072277. [DOI] [PubMed] [Google Scholar]
- 119.World Health Organization BCG vaccine: WHO position paper, February 2018GÇôrecommendations. Vaccine. 2018;36:3408–3410. doi: 10.1016/j.vaccine.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 120.World Health Organization Information sheet observed rate of vaccine reactions Bacille Calmette-Guérin (Bcg) Global Vaccine Saf. Immun. Vaccines Biol. 2012;20 [Google Scholar]
- 121.Alhunaidi O., Zlotta A.R. The use of intravesical BCG in urothelial carcinoma of the bladder. Ecancermedicalscience. 2019;13 doi: 10.3332/ecancer.2019.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aaby P., Roth A., Ravn H., Napirna B.M., Rodrigues A., Lisse I.M., Stensballe L., Diness B.R., Lausch K.R., Lund N. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J. Infec. Dis. 2011;204:245–252. doi: 10.1093/infdis/jir240. [DOI] [PubMed] [Google Scholar]
- 123.Higgins J.P., Soares-Weiser K., López-López J.A., Kakourou A., Chaplin K., Christensen H., Martin N.K., Sterne J.A., Reingold A.L. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. 2016:355. doi: 10.1136/bmj.i5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Berendsen M.L., van Gijzel S.W., Smits J., de M.Q., Aaby P., Benn C.S., Netea M.G., van der Ven A.J. BCG vaccination is associated with reduced malaria prevalence in children under the age of 5 years in sub-Saharan Africa. BMJ Glob. Health. 2019;4:e001862. doi: 10.1136/bmjgh-2019-001862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stensballe L.G., Nante E., Jensen I.P., Kofoed P.E., Poulsen A., Jensen H., Newport M., Marchant A., Aaby P. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea-Bissau: a beneficial effect of BCG vaccination for girls community based case-control study. Vaccine. 2005;23:1251–1257. doi: 10.1016/j.vaccine.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 126.Hollm-Delgado M.G., Stuart E.A., Black R.E. Acute lower respiratory infection among Bacille Calmette-Guérin (BCG)-vaccinated children. Pediatrics. 2014;133:e73–e81. doi: 10.1542/peds.2013-2218. [DOI] [PubMed] [Google Scholar]
- 127.Wardhana, Datau E.A., Sultana A., Mandang V.V., Jim E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med. Indones. 2011;43:185–190. [PubMed] [Google Scholar]
- 128.Netea M.G., van C.R. BCG-induced protection: effects on innate immune memory. Semin. Immunol. 2014;26:512–517. doi: 10.1016/j.smim.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 129.O'Neill L.A.J., Netea M.G. BCG-induced trained immunity: can it offer protection against COVID-19? Nat. Rev. Immunol. 2020;20:335–337. doi: 10.1038/s41577-020-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Covián C., Fernández-Fierro A., Retamal-Díaz A., Díaz F.E., Vasquez A.E., Lay M.K., Riedel C.A., González P.A., Bueno S.M., Kalergis A.M. BCG-induced cross-protection and development of trained immunity: implication for vaccine design. Front Immunol. 2019;10:2806. doi: 10.3389/fimmu.2019.02806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kleinnijenhuis J., Quintin J., Preijers F., Joosten L.A., Ifrim D.C., Saeed S., Jacobs C., van L.J., de J.D., Stunnenberg H.G., Xavier R.J., van der Meer J.W., van C.R., Netea M.G. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. U. S. A. 2012;109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Arts R.J.W., Moorlag S.J.C.F., Novakovic B., Li Y., Wang S.Y., Oosting M., Kumar V., Xavier R.J., Wijmenga C., Joosten L.A.B., Reusken C.B.E.M., Benn C.S., Aaby P., Koopmans M.P., Stunnenberg H.G., van C.R., Netea M.G. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23:89–100. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 133.Kleinnijenhuis J., Quintin J., Preijers F., Benn C.S., Joosten L.A., Jacobs C., van L.J., Xavier R.J., Aaby P., van der Meer J.W., van C.R., Netea M.G. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2014;6:152–158. doi: 10.1159/000355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moliva J.I., Turner Torrelles J.J.B. Immune responses to bacillus Calmette-Guérin vaccination: why do they fail to protect against Mycobacterium tuberculosis? Front. Immunol. 2017;8:407. doi: 10.3389/fimmu.2017.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Parra M., Liu X., Derrick S.C., Yang A., Tian J., Kolibab K., Kumar S., Morris S.L. Molecular analysis of non-specific protection against murine malaria induced by BCG vaccination. PLoS One. 2013;8:e66115. doi: 10.1371/journal.pone.0066115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Spencer J.C., Ganguly R., Waldman R.H. Nonspecific protection of mice against influenza virus infection by local or systemic immunization with Bacille Calmette-Guérin. J. Infect. Dis. 1977;136:171–175. doi: 10.1093/infdis/136.2.171. [DOI] [PubMed] [Google Scholar]
- 137.Floc'h F., Werner G.H. Increased resistance to virus infections of mice inoculated with BCG (Bacillus calmette-guérin) Ann. Immunol. (Paris) 1976;127:173–186. [PubMed] [Google Scholar]
- 138.Starr S.E., Visintine A.M., Tomeh M.O., Nahmias A.J. Effects of immunostimulants on resistance of newborn mice to herpes simplex type 2 infection. Proc. Soc. Exp. Biol. Med. 1976;152:57–60. doi: 10.3181/00379727-152-39327. [DOI] [PubMed] [Google Scholar]
- 139.Redelman-Sidi G. Could BCG be used to protect against COVID-19? Nat. Rev. Urol. 2020;17:316–317. doi: 10.1038/s41585-020-0325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ozdemir C., Kucuksezer U.C., Tamay Z.U. Is BCG vaccination affecting the spread and severity of COVID -19? Allergy. 2020;10 doi: 10.1111/all.14344. [DOI] [PubMed] [Google Scholar]
- 141.Gallagher J., Watson C., Ledwidge M. Association of Bacille Calmette-Guérin (BCG), adult pneumococcal and adult seasonal influenza vaccines with Covid-19 adjusted mortality rates in level 4 European countries. medRxiv. 2020 [Google Scholar]
- 142.Zwerling A., Behr M.A., Verma A., Brewer T.F., Menzies D., Pai M. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8:e1001012. doi: 10.1371/journal.pmed.1001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Miller A., Reandelar M.J., Fasciglione K., Roumenova V., Li Y., Otazu G.H. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. medRxiv. 2020 [Google Scholar]
- 144.Klinger D., Blass I., Rappoport N., Linial M. Significantly improved COVID-19 outcomes in countries with higher BCG vaccination coverage: a multivariable analysis. medRxiv. 2020 doi: 10.3390/vaccines8030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Singh S. BCG vaccines may not reduce COVID-19 mortality rates. medRxiv. 2020 [Google Scholar]
- 146.Fukui M., Kawaguchi K., Matsuura H. Does TB vaccination reduce COVID-19 infection?: no evidence from a regression discontinuity analysis. No Evidence Regression Discontinuity Anal. 2020 (April 9, 2020) [Google Scholar]