Olfaction is the sense of smell. It is one of the chemical senses, involving the detection of chemical stimuli and conversion of these stimuli into electrical energy for perception via the central nervous system.1 Apart from playing a role in the determination of the flavor of food and beverages, olfaction has a role in acting as an early warning system to detect hazards. Reduction of olfactory function has been shown to be associated with loss of appetite, consumption of bad food, and, in many people, problems with cooking.2 It has been hypothesized that olfaction aids in possible avoidance of food poisoning.3 Olfaction also plays a significant role in the process of enjoyment of food. Most information regarding the flavor of food is thought to come from olfaction.4 Lack of proper olfaction also has been associated with weight loss and weight gain.5 , 6 The sense of smell is reported in the literature as connected to emotions, either positive or negative.7 Quality of life is reduced significantly in patients with olfactory disorders.8 Olfactory disorders have been reported as prominent features that can be possible early signs of neurodegenerative (ND) diseases.9 Loss of this sensation has been attributed to be one of the first manifesting symptoms in COVID-19.10 In our article, we highlight the basic principles underlying the physiology and pathophysiology of olfaction and its possible relationship with disease entities. We also look at the significance of olfaction as it relates to dentistry and orofacial pain.
Physiology of Olfaction
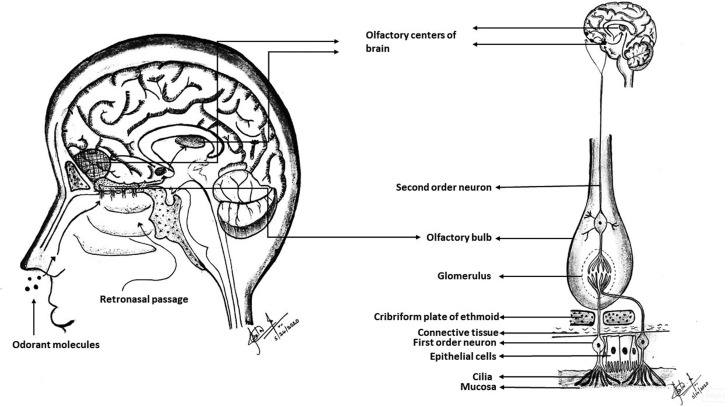
The chemosensation of olfaction is mediated via the cranial nerves CN I (olfactory) and CN V (trigeminal).11 CN I is responsible for olfaction, whereas CN V mediates general sensory innervation including chemosensation. The olfactory epithelium present in the superior part of the nostrils contains olfactory cells, which are the receptor cells for olfaction.12 An action potential is generated when the odorant molecule binds to the olfactory receptor.13 The action potential is carried via the axons of these primary afferent neurons to the olfactory bulb, where the synapsing with second-order neurons occurs.14 Anatomically, the olfactory bulb is positioned over the cribriform plate of the ethmoid bone. These second-order neurons form the olfactory tract carrying signals to the higher centers in the brain.15 These centers include the primary and secondary olfactory cortices.16 Figure 1 shows the gross structures involved in olfaction and olfactory pathways.
Figure 1.
Structures involved in olfaction and olfactory pathways. Drawing courtesy of Dr. Sita M. Baddireddy.
Disorders of Olfaction
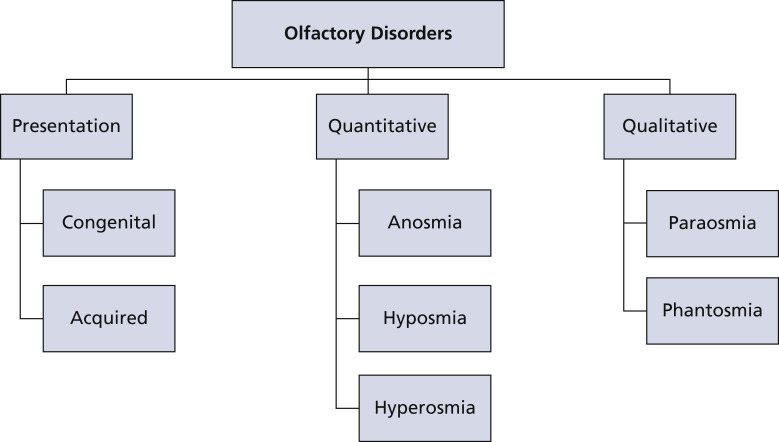
Olfaction disorders may be classified as congenital or acquired. Being born with an olfactory disorder is rare.8 Quantitatively, olfactory disorders can be divided into anosmia, hyposmia, and hyperosmia. Anosmia is the inability to perceive odors. It includes total anosmia, which is an inability to perceive all odors, and partial anosmia, which is an inability to perceive some but not all odors. Reduced ability to smell is termed hyposmia. Enhanced ability to smell is termed hyperosmia, which is relatively rare.17
Disorders of olfaction can be categorized qualitatively as paraosmia and phantosmia.18 Distorted smell perception in the presence of odorant is dysosmia or paraosmia.19 In particular, when this perception is fetid, it is referred to as cacosmia.16 Phantosmia refers to the perception of an odorant in the absence of one. It is a form of olfactory hallucination.20 Figure 2 shows the classification of olfactory disorders.
Figure 2.
Disorders of olfaction.
In a 2019 article, the author attempted to classify the degrees of anosmia and hyposmia as mild, moderate, severe (for hyposmia), and total among populations of patients who experienced hazardous events because of a smell disorder.21 It has been reported that the risk for hazardous events increased proportionately with an increase in the degree of olfactory impairment.22 On the basis of quantitative olfactory tests, the overall prevalence of olfactory dysfunctions has been reported variedly as approximately 20% through 25% in earlier studies.23 , 24 A 2017 study placed the overall prevalence at an average of 20% and prevalence in older people at an average of 40%.25
Etiology of Olfactory Disorders
On the basis of etiology, olfactory disorders can be categorized as local or systemic. Olfactory disturbances most commonly occur owing to local nasal diseases. These can prevent odorants from reaching the nasal epithelium owing to conductive and inflammatory issues. These issues include polyposis, seasonal rhinitis, allergic rhinitis, sinusitis, and trauma or malignancy of the nose, paranasal sinuses, and nasopharynx.26, 27, 28, 29, 30, 31 Numerous systemic factors are known to be associated with disorders of olfaction. Many bacterial, viral, and fungal infections have been known to be associated with smell disorders.32, 33, 34 Trauma involving the head has been associated with olfactory dysfunctions.35 , 36 Abnormalities of olfaction are involved in several neurologic conditions. Olfactory dysfunctions, including aura, have been documented with epilepsy.37, 38, 39 Literature has shown the presence of olfactory dysfunctions with migraine episodes.40 The association of osmophobia with migraine is well documented in the literature. Some odors have been reported to be triggers for migraines.41 , 42 To a lesser extent, migraine also can be associated with phantosmia and cacosmia.43
Olfactory disorders are seen in patients with multiple sclerosis.44 As the disease progresses, olfactory function impairment increases.45 ND diseases including Parkinson and Alzheimer have been associated with olfactory dysfunctions. Studies have suggested that olfactory disorders could be an early indicator of these ND diseases.46 , 47
Several endocrinologic disease entities have been documented to have an association with olfaction disorders. Diabetes, Addison disease, Cushing syndrome, and hypothyroidism are some of the endocrine diseases associated with smell dysfunctions.48, 49, 50, 51 Chronic renal disorders and their relation to dysfunctions of olfaction, including hyposmia and anosmia, have been documented in the literature.52 , 53 Olfactory functions are altered in liver diseases as well.54 Certain drugs such as antibiotics and antidepressants have been known to cause olfactory dysfunction.55, 56, 57, 58, 59 The box shows the etiology of olfactory disorders.
Box.
Etiology of olfactory disorders.
| Local |
| ▪ Polyposis |
| ▪ Seasonal rhinitis |
| ▪ Allergic rhinitis |
| ▪ Sinusitis |
| ▪ Trauma |
| ▪ Malignancy |
| Systemic |
| ▪ Infections: viral, bacterial, fungal |
| ▪ Endocrine: diabetes, Addison disease, Cushing syndrome, hypothyroidism |
| ▪ Trauma |
| ▪ Neurologic: epilepsy, migraine, multiple sclerosis, neurodegenerative diseases |
| ▪ Drugs |
| ▪ Renal disease |
| ▪ Kidney disease |
Relevance in Dentistry and Orofacial Pain
The sensory innervation to oral, perioral, and nasal structures comes predominantly from the trigeminal nerve.60 The trigeminal and olfactory systems are distinct yet interrelated entities in the nasal cavity.61 Most chemosensory stimulants on entering the nasal cavity produce olfactory and trigeminal sensations.62 The stimulation of the trigeminal nerve by such stimulants can produce different sensations like burning, prickling, and stinging.62 There is ample evidence that the trigeminal nerve works concurrently with the olfactory nerve to bring about the perception of smell.63 Variations in trigeminal nerve sensitivity also have been reported to coexist in patients with olfactory disorders.64
Relation to taste
Taste, smell, and touch contribute to providing the flavor of food and beverages.65 Olfaction plays a major role in the perception of flavors. A neurologic connection between smell and taste sensations is well documented in the literature.66 Olfaction via particular retronasal passages is involved in the perception of taste and flavor.2 , 67 In the process of mastication, volatile molecules from the food travel to the nasal cavity through the posterior part of the oral cavity. This is responsible for the phenomenon of retronasal olfaction.68 Dysgeusia is an altered perception of taste in response to a tastant stimulus. Phantogeusia is unpleasant taste due to a gustatory hallucination (in the absence of any stimulus).69 Olfactory dysfunctions have been known to affect gustatory function.70 Often, patients who have taste disorders have olfactory disorders rather than gustatory dysfunction.71 , 72 Patients may not be completely aware of the presence or severity of anosmia or hyposmia.73 Patients tend to consult the dentist if they perceive a disordered taste sensation. Dental practitioners must be aware of this fact.
Protective nature of olfaction
Compared with other mammals and other animals, humans have lost a good percentage of our strength of olfactory senses through evolution.74 It is conceivable that humans use olfaction to detect the relative quality of the food that is about to be consumed. We use the sense of smell as a sense of protection to check if the food is suitable for ingestion. Literature suggests approximately 50% of the patients with olfactory dysfunctions may eat rotten food and approximately 30% may eat burnt food, possibly causing food poisoning.3 This points to a possible protective nature of olfaction as related to sustenance of life. Olfaction alone might not serve this protective function fully, but it may do so in conjunction with other senses such as gustation. Anosmia or hyposmia has been known to induce potentially deleterious habits of consumption, such as excessive eating of sweet and high-fat food.
Burning mouth syndrome
Burning mouth syndrome (BMS) is a pain condition classically described as coexisting with taste alterations. Taste disturbances are present in approximately 70% of patients with BMS.75 Literature suggests patients with BMS can exhibit varied taste disturbances such as dysgeusia and ageusia, which can occur as alteration on taste perception, persistently altered taste, or both.76 , 77 However, there are reports of concomitant alterations in olfaction as well. Studies have shown the olfactory threshold to be higher in patients with BMS than control patients.78 , 79 Patients with BMS are proposed to have reduced capability of identifying odors compared with control patients.80 Odors have been shown to induce changes in perception of taste.81 The interaction between trigeminal, olfactory, and gustatory systems also has been proposed in the literature with regard to the pathophysiology of BMS.79 It has been well documented in the literature that olfactory dysfunctions coexist with abnormalities in thresholds for pain, tactile sensation, temperature perception, and gustation.79 The brain center responsible for convergence of impulses from the olfactory and gustatory pathways is the orbitofrontal cortex.82 , 83 Pain syndromes such as BMS and dysfunctions of olfaction are similar because both these entities are affected or modulated via the same or similar centers in the brain.
Sjögren syndrome
Complex interactions exist between the immune and olfactory systems. Changes in the immune system can affect olfaction.84 Sjögren syndrome (SS) is an autoimmune disease affecting multiple organ systems, including salivary and lacrimal glands. Impaired smell and taste are common symptoms reported by patients with SS.80 , 85 In patients with SS, there is a reduction in olfactory acuity. By and large, this is related to generalized hyposmia in these patients. There is no definitive inability to recognize or detect any specific odor in these patients.86 Mechanisms involved in olfactory changes of SS include decreased mucin (an odorant carrier), recurrent rhinosinusitis, septal ulcerations, crustings, and immunologic mechanisms.80 , 84
Infections
Pathogens like bacteria and viruses are known to cause olfactory disturbances.87 , 88 The most common causes are local nasal infections and upper respiratory diseases. Patients with chronic sinusitis have symptoms of pain and persistent smell loss even after medical or surgical treatment.28 Viral infections are hypothesized to cause damage to the olfactory epithelium.89 , 90 Olfactory dysfunctions like anosmia and hyposmia are common during severe upper respiratory infections and persist long after the other symptoms have resolved.91 Detection of the virus in patients with postviral infections could indicate the role of the virus in olfactory dysfunction.92
The mechanism underlying olfactory dysfunction in infections is controversial. It could be the result of a conductive problem (preventing air from reaching the olfactory neuroepithelium), or it could be the result of nasal inflammation (inhibiting function).16 There is some evidence that postviral anosmia may be mediated centrally, with decreased metabolism of certain regions of the brain in which olfactory information is perceived.93 This postviral anosmia is also poorly responsive to treatment, although antiinflammatory medications or steroids are beneficial in some cases.91
COVID-19 and Anosmia
The COVID-19 pandemic has prompted researchers to look into the association of this new disease to anosmia. COVID-19 is caused by a new strain of coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).94 In addition to the common clinical features such as dry cough, fever, sore throat, shortness of breath, and headache, there are several reports of anosmia and hyposmia associated with this condition.95, 96, 97, 98 Much like as in viral infections discussed above, a study from 2020 showed the presence of olfactory dysfunctions in patients with COVID-19.97 The earliest reports of anosmia occurring in patients with COVID-19 came in February 2020.99 , 100 Since these reports, there have been others describing new-onset anosmia that occurs concomitantly with COVID-19. It is the belief of the scientific community that olfactory dysfunction is potentially an early symptom of COVID-19.101 The Centers for Disease Control and Prevention has added “new loss of taste or smell” to the symptom list (the symptoms starting within 2-14 days of COVID-19 exposure).102 The prevalence of olfactory dysfunction is reported to be an average of 50% in patients with COVID-19.103
Anosmia is being considered as a marker for COVID-19 by reputed international medical entities such as the British Association for Otorhinolaryngology.104 In addition, there is a suggestion that patients with new-onset anosmia, even if they are asymptomatic for COVID-19, quarantine themselves in anticipation of the possible onset of COVID-19. This might help in reducing the further community spread of the disease.105 It has been hypothesized that the 2 possible routes of entry of the COVID-19 virus into the central nervous system are through the circulation or across the cribriform plate of the ethmoid bone (the site of olfactory nerve entry).106
Two medications used in orofacial pain management, ibuprofen (for inflammatory pain) and renin-angiotensin system blockers (angiotensin-converting enzyme inhibitors for prophylactic treatment of migraine), have come into the literature with researchers putting forward the hypothesis that they facilitate or worsen the effects of COVID-19 infection.107 However, other researchers have questioned this and disputed that these drugs play any such role.108 Redeployment or reassignment of practicing pain physicians in the backdrop of the pandemic has put further significant constraints on access to care for patients with chronic pain. Remotely supported e-health pain management services are being deployed substantially in the United States and Europe in an attempt to address this problem.109 With the COVID-19 pandemic, health care systems are stretched thin, access to care for patients with chronic pain has been hampered, and clinicians in pain and orofacial pain management find it increasingly difficult to coordinate optimum pain management for their patients.110 Most articles published since the start of the pandemic refer to the latest guidelines such as consensus recommendations from expert pain management physician panels.111
Clinical pearls
Dental and orofacial pain practitioners must be aware of anosmia and hyposmia, associated entities such as BMS, and gustatory alterations when screening patients. The importance of this knowledge lies in the fact that subtle taste alterations and olfactory disorders may not be evident or noticed by the patient. A careful and complete history may enable astute dental clinicians to detect early signs of COVID-19. Traditionally, a new onset of anosmia or hyposmia would evoke suggestion of a possible onset of a ND disease or similar systemic entity or local causes. In the context of the global COVID-19 pandemic, clinicians should consider new-onset anosmia or hyposmia as a red flag for the infection. Dentists could be the first-line defense against COVID-19 by means of promptly screening routine patients for possible recent-onset anosmia or hyposmia.
Conclusions
Although the exact mechanisms involved in the pathophysiology of anosmia are not clear, it is becoming increasingly evident in the literature that it could have an association with several local and systemic disease entities. Olfactory disorders range in etiology from a simple local infection to a brain lesion to a serious autoimmune or ND disorder. Anosmia or hyposmia can be a significant cause of change in the quality of life, especially if it is long lasting. There have been several case reports of patients describing anosmia and hyposmia as affecting their lives in an unusually negative manner. Developments in the global COVID-19 pandemic have brought this anomaly to the forefront of the scientific community. Even in an otherwise asymptomatic person, new-onset anosmia or hyposmia could be an indication of an underlying cause that could be life-altering for that patient. Dental professionals could be instrumental in screening for anosmia via asking simple questions, thereby facilitating a prompt referral to an appropriate medical professional as needed.
Biographies
Dr. Thomas is a clinical assistant professor, Center for Temporomandibular Disorders and Orofacial Pain, Department of Diagnostic Sciences, Rutgers School of Dental Medicine, 110 Bergen St, Newark, NJ 07103.
Dr. Baddireddy is a resident in orofacial pain, Center for Temporomandibular Disorders and Orofacial Pain, Department of Diagnostic Sciences, Rutgers School of Dental Medicine, Newark, NJ.
Dr. Kohli is a masters candidate in orofacial pain and oral biology, Center for Temporomandibular Disorders and Orofacial Pain, Department of Diagnostic Sciences, Rutgers School of Dental Medicine, Newark, NJ.
Footnotes
Disclosure. None of the authors reported any disclosures.
References
- 1.Costanzo L. 6th ed. Elsevier; Philadelphia, PA: 2017. Physiology. [Google Scholar]
- 2.Walliczek-Dworschak U., Hummel T. The human sense of olfaction. Facial Plast Surg. 2017;33(4):396–404. doi: 10.1055/s-0037-1603828. [DOI] [PubMed] [Google Scholar]
- 3.Temmel A.F., Quint C., Schickinger-Fischer B., Klimek L., Stoller E., Hummel T. Characteristics of olfactory disorders in relation to major causes of olfactory loss. Arch Otolaryngol Head Neck Surg. 2002;128(6):635–641. doi: 10.1001/archotol.128.6.635. [DOI] [PubMed] [Google Scholar]
- 4.Murphy C., Cain W.S., Bartoshuk L.M. Mutual action of taste and olfaction. Sens Processes. 1977;1(3):204–211. [PubMed] [Google Scholar]
- 5.Aschenbrenner K., Hummel C., Teszmer K. The influence of olfactory loss on dietary behaviors. Laryngoscope. 2008;118(1):135–144. doi: 10.1097/MLG.0b013e318155a4b9. [DOI] [PubMed] [Google Scholar]
- 6.Duffy V.B., Backstrand J.R., Ferris A.M. Olfactory dysfunction and related nutritional risk in free-living, elderly women. J Am Diet Assoc. 1995;95(8):879–884. doi: 10.1016/S0002-8223(95)00244-8. [DOI] [PubMed] [Google Scholar]
- 7.Croy I., Olgun S., Joraschky P. Basic emotions elicited by odors and pictures. Emotion. 2011;11(6):1331–1335. doi: 10.1037/a0024437. [DOI] [PubMed] [Google Scholar]
- 8.Croy I., Nordin S., Hummel T. Olfactory disorders and quality of life: an updated review. Chem Senses. 2014;39(3):185–194. doi: 10.1093/chemse/bjt072. [DOI] [PubMed] [Google Scholar]
- 9.Godoy M.D., Voegels R.L., Pinna Fde R., Imamura R., Farfel J.M. Olfaction in neurologic and neurodegenerative diseases: a literature review. Int Arch Otorhinolaryngol. 2015;19(2):176–179. doi: 10.1055/s-0034-1390136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haldrup M., Johansen M.I., Fjaeldstad A.W. Anosmia and ageusia as primary symptoms of COVID-19. Ugeskr Laeger. 2020;182(18):V04200205. [in Danish] [PubMed] [Google Scholar]
- 11.Pinto J.M. Olfaction. Proc Am Thorac Soc. 2011;8(1):46–52. doi: 10.1513/pats.201005-035RN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall J.E. 13th ed. Elsevier; Philadelphia, PA: 2016. Guyton and Hall Textbook of Medical Physiology. [Google Scholar]
- 13.Nagappan P.G., Subramaniam S., Wang D.Y. Olfaction as a soldier: a review of the physiology and its present and future use in the military. Mil Med Res. 2017;4:9. doi: 10.1186/s40779-017-0119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myers S.M.A. 2nd ed. Elsevier; Philadelphia, PA: 2015. Netter’s Essential Physiology. [Google Scholar]
- 15.Nagayama S., Homma R., Imamura F. Neuronal organization of olfactory bulb circuits. Front Neural Circuits. 2014;8:98. doi: 10.3389/fncir.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawkes C.H., Doty R.L. Cambridge University Press; Cambridge, MA: 2009. The Neurology of Olfaction. [Google Scholar]
- 17.Hummel T., Landis B.N., Huttenbrink K.B. Smell and taste disorders. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2011;10:Doc04. doi: 10.3205/cto000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philpott C.M., Boak D. The impact of olfactory disorders in the United Kingdom. Chem Senses. 2014;39(8):711–718. doi: 10.1093/chemse/bju043. [DOI] [PubMed] [Google Scholar]
- 19.Nordin S., Bramerson A. Complaints of olfactory disorders: epidemiology, assessment and clinical implications. Curr Opin Allergy Clin Immunol. 2008;8(1):10–15. doi: 10.1097/ACI.0b013e3282f3f473. [DOI] [PubMed] [Google Scholar]
- 20.Henkin R.I., Potolicchio S.J., Levy L.M. Olfactory hallucinations without clinical motor activity: a comparison of unirhinal with birhinal phantosmia. Brain Sci. 2013;3(4):1483–1553. doi: 10.3390/brainsci3041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doty R.L. Epidemiology of smell and taste dysfunction. Handb Clin Neurol. 2019;164:3–13. doi: 10.1016/B978-0-444-63855-7.00001-0. [DOI] [PubMed] [Google Scholar]
- 22.Pence T.S., Reiter E.R., DiNardo L.J., Costanzo R.M. Risk factors for hazardous events in olfactory-impaired patients. JAMA Otolaryngol Head Neck Surg. 2014;140(10):951–955. doi: 10.1001/jamaoto.2014.1675. [DOI] [PubMed] [Google Scholar]
- 23.Doty R.L., Shaman P., Applebaum S.L., Giberson R., Siksorski L., Rosenberg L. Smell identification ability: changes with age. Science. 1984;226(4681):1441–1443. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- 24.Murphy C., Schubert C.R., Cruickshanks K.J., Klein B.E., Klein R., Nondahl D.M. Prevalence of olfactory impairment in older adults. JAMA. 2002;288(18):2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- 25.Seubert J., Laukka E.J., Rizzuto D. Prevalence and correlates of olfactory dysfunction in old age: a population-based study. J Gerontol A Biol Sci Med Sci. 2017;72(8):1072–1079. doi: 10.1093/gerona/glx054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dzaman K., Pleskacz W.A., Walkanis A., Rapiejko P., Jurkiewicz D. Taste and smell senses estimation in patients with nasal polyps. Otolaryngol Pol. 2007;61(5):831–837. doi: 10.1016/S0030-6657(07)70537-1. [in Polish] [DOI] [PubMed] [Google Scholar]
- 27.Becker S., Pflugbeil C., Gröger M. Olfactory dysfunction in seasonal and perennial allergic rhinitis. Acta Otolaryngol. 2012;132(7):763–768. doi: 10.3109/00016489.2012.656764. [DOI] [PubMed] [Google Scholar]
- 28.Raviv J.R., Kern R.C. Chronic sinusitis and olfactory dysfunction. Otolaryngol Clin North Am. 2004;37(6):1143–1157. doi: 10.1016/j.otc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Coelho D.H., Costanzo R.M. Posttraumatic olfactory dysfunction. Auris Nasus Larynx. 2016;43(2):137–143. doi: 10.1016/j.anl.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Kim D.H., Hong Y.K., Jeun S.S. Can tumor size be a predictive factor of olfactory dysfunction after endoscopic endonasal trans-sphenoidal approach? J Craniofac Surg. 2018;29(3):543–546. doi: 10.1097/SCS.0000000000004193. [DOI] [PubMed] [Google Scholar]
- 31.Bonfils P., Jankowski R., Faulcon P. Smell dysfunction in nasal and paranasal sinus disease: a review of the literature (II) Ann Otolaryngol Chir Cervicofac. 2001;118(3):143–155. [in French] [PubMed] [Google Scholar]
- 32.Welge-Lussen A., Wolfensberger M. Olfactory disorders following upper respiratory tract infections. Adv Otorhinolaryngol. 2006;63:125–132. doi: 10.1159/000093758. [DOI] [PubMed] [Google Scholar]
- 33.Qiao X.F., Wang G.P., Li X., Bai Y.H., Zheng W. Analysis of the clinical effect of olfactory training on olfactory dysfunction after upper respiratory tract infection. Acta Otolaryngol. 2019;139(7):643–646. doi: 10.1080/00016489.2019.1614224. [DOI] [PubMed] [Google Scholar]
- 34.Pulickal G.G., Navaratnam A.V., Nguyen T., Dragan A.D., Dziedzic M., Lingam R.K. Imaging sinonasal disease with MRI: providing insight over and above CT. Eur J Radiol. 2018;102:157–168. doi: 10.1016/j.ejrad.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 35.Howell J., Costanzo R.M., Reiter E.R. Head trauma and olfactory function. World J Otorhinolaryngol Head Neck Surg. 2018;4(1):39–45. doi: 10.1016/j.wjorl.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frasnelli J., Lague-Beauvais M., LeBlanc J. Olfactory function in acute traumatic brain injury. Clin Neurol Neurosurg. 2016;140:68–72. doi: 10.1016/j.clineuro.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Khurshid K., Crow A.J.D., Rupert P.E. A quantitative meta-analysis of olfactory dysfunction in epilepsy. Neuropsychol Rev. 2019;29(3):328–337. doi: 10.1007/s11065-019-09406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desai M., Agadi J.B., Karthik N., Praveenkumar S., Netto A.B. Olfactory abnormalities in temporal lobe epilepsy. J Clin Neurosci. 2015;22(10):1614–1618. doi: 10.1016/j.jocn.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 39.Espinosa-Jovel C., Toledano R., Jimenez-Huete A. Olfactory function in focal epilepsies: understanding mesial temporal lobe epilepsy beyond the hippocampus. Epilepsia Open. 2019;4(3):487–492. doi: 10.1002/epi4.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akturk T., Tanik N., Serin H.I., Sacmaci H., Inan L.E. Olfactory bulb atrophy in migraine patients. Neurol Sci. 2019;40(1):127–132. doi: 10.1007/s10072-018-3597-6. [DOI] [PubMed] [Google Scholar]
- 41.Silva-Neto R.P., Peres M.F., Valenca M.M. Odorant substances that trigger headaches in migraine patients. Cephalalgia. 2014;34(1):14–21. doi: 10.1177/0333102413495969. [DOI] [PubMed] [Google Scholar]
- 42.Tai M.S., Yet S.X.E., Lim T.C., Pow Z.Y., Goh C.B. Geographical differences in trigger factors of tension-type headaches and migraines. Curr Pain Headache Rep. 2019;23(2):12. doi: 10.1007/s11916-019-0760-6. [DOI] [PubMed] [Google Scholar]
- 43.Fornazieri M.A., Neto A.R., de Rezende Pinna F. Olfactory symptoms reported by migraineurs with and without auras. Headache. 2016;56(10):1608–1616. doi: 10.1111/head.12973. [DOI] [PubMed] [Google Scholar]
- 44.Atalar A.Ç., Erdal Y., Tekin B., Yıldız M., Akdogan Ö., Emrev U. Olfactory dysfunction in multiple sclerosis. Mult Scler Relat Disord. 2018;21:92–96. doi: 10.1016/j.msard.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 45.Li L.M., Yang L.N., Zhang L.J. Olfactory dysfunction in patients with multiple sclerosis. J Neurol Sci. 2016;365:34–39. doi: 10.1016/j.jns.2016.03.045. [DOI] [PubMed] [Google Scholar]
- 46.Marin C., Vilas D., Langdon C. Olfactory dysfunction in neurodegenerative diseases. Curr Allergy Asthma Rep. 2018;18(8):42. doi: 10.1007/s11882-018-0796-4. [DOI] [PubMed] [Google Scholar]
- 47.Huttenbrink K.B., Hummel T., Berg D., Gasser T., Hahner A. Olfactory dysfunction: common in later life and early warning of neurodegenerative disease. Dtsch Arztebl Int. 2013;110(1-2):1–7, e1. doi: 10.3238/arztebl.2013.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z., Zhang B., Wang X. Olfactory dysfunction mediates adiposity in cognitive impairment of type 2 diabetes: insights from clinical and functional neuroimaging studies. Diabetes Care. 2019;42(7):1274–1283. doi: 10.2337/dc18-2584. [DOI] [PubMed] [Google Scholar]
- 49.Pruszewicz A., Kosowicz J. Quantitative and qualitative studies of the taste and smell in adrenal insufficiency. Endokrynol Pol. 1966;17(3):321–327. [in Polish] [PubMed] [Google Scholar]
- 50.Nores J.M., Biacabe B., Bonfils P. Olfactory disorders and general pathology: analysis and review of the literature. Rev Med Interne. 2000;21(1):95–104. doi: 10.1016/s0248-8663(00)87235-5. [in French] [DOI] [PubMed] [Google Scholar]
- 51.Dwivedi S., Aggarwal R. Modified shoe for adjusting hard stuffy and smelly sole: an uncommon accompaniment of hypothyroidism. J Midlife Health. 2012;3(1):45–46. doi: 10.4103/0976-7800.98819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frasnelli J.A., Temmel A.F., Quint C., Oberbauer R., Hummel T. Olfactory function in chronic renal failure. Am J Rhinol. 2002;16(5):275–279. [PubMed] [Google Scholar]
- 53.Nigwekar S.U., Weiser J.M., Kalim S. Characterization and correction of olfactory deficits in kidney disease. J Am Soc Nephrol. 2017;28(11):3395–3403. doi: 10.1681/ASN.2016121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heiser C., Haller B., Sohn M. Olfactory function is affected in patients with cirrhosis depending on the severity of hepatic encephalopathy. Ann Hepatol. 2018;17(5):822–829. doi: 10.5604/01.3001.0012.3143. [DOI] [PubMed] [Google Scholar]
- 55.Che X., Li Y., Fang Y., Reis C., Wang H. Antiarrhythmic drug-induced smell and taste disturbances: a case report and literature review. Medicine (Baltimore) 2018;97(29):e11112. doi: 10.1097/MD.0000000000011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshida K., Fukuchi T., Sugawara H. Dysosmia and dysgeusia associated with duloxetine. BMJ Case Rep. 2017 doi: 10.1136/bcr-2017-222470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schiffman S.S. Influence of medications on taste and smell. World J Otorhinolaryngol. Head Neck Surg. 2018;4(1):84–91. doi: 10.1016/j.wjorl.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ottaviano G., Savietto E., Scarpa B. Influence of number of drugs on olfaction in the elderly. Rhinology. 2018;56(4):351–357. doi: 10.4193/Rhin17.152. [DOI] [PubMed] [Google Scholar]
- 59.Doty R.L., Bromley S.M. Effects of drugs on olfaction and taste. Otolaryngol Clin North Am. 2004;37(6):1229–1254. doi: 10.1016/j.otc.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 60.Leston J.M. Functional anatomy of the trigeminal nerve. Neurochirurgie. 2009;55(2):99–112. doi: 10.1016/j.neuchi.2009.01.001. [in French] [DOI] [PubMed] [Google Scholar]
- 61.Tremblay C., Frasnelli J. Olfactory and trigeminal systems interact in the periphery. Chem Senses. 2018;43(8):611–616. doi: 10.1093/chemse/bjy049. [DOI] [PubMed] [Google Scholar]
- 62.Jacquot L., Monnin J., Brand G. Influence of nasal trigeminal stimuli on olfactory sensitivity. C R Biol. 2004;327(4):305–311. doi: 10.1016/j.crvi.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 63.Hummel T., Livermore A. Intranasal chemosensory function of the trigeminal nerve and aspects of its relation to olfaction. Int Arch Occup Environ Health. 2002;75(5):305–313. doi: 10.1007/s00420-002-0315-7. [DOI] [PubMed] [Google Scholar]
- 64.Hummel T., Frasnelli J. The intranasal trigeminal system. Handb Clin Neurol. 2019;164:119–134. doi: 10.1016/B978-0-444-63855-7.00008-3. [DOI] [PubMed] [Google Scholar]
- 65.Stevenson R.J. Flavor binding: its nature and cause. Psychol Bull. 2014;140(2):487–510. doi: 10.1037/a0033473. [DOI] [PubMed] [Google Scholar]
- 66.Bakalar N. Sensory science: partners in flavour. Nature. 2012;486(7403):S4–S5. doi: 10.1038/486S4a. [DOI] [PubMed] [Google Scholar]
- 67.Small D.M. Flavor is in the brain. Physiol Behav. 2012;107(4):540–552. doi: 10.1016/j.physbeh.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 68.Bojanowski V., Hummel T. Retronasal perception of odors. Physiol Behav. 2012;107(4):484–487. doi: 10.1016/j.physbeh.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 69.Lafreniere D. Overview of taste and olfactory disorders in adults. https://www.uptodate.com/contents/overview-of-taste-and-olfactory-disorders-in-adults Available at:
- 70.Zang Y., Han P., Burghardt S., Knaapila A., Schriever V., Hummel T. Influence of olfactory dysfunction on the perception of food. Eur Arch Otorhinolaryngol. 2019;276(10):2811–2817. doi: 10.1007/s00405-019-05558-7. [DOI] [PubMed] [Google Scholar]
- 71.Bromley S.M., Doty R.L. Olfaction in dentistry. Oral Dis. 2010;16(3):221–232. doi: 10.1111/j.1601-0825.2009.01616.x. [DOI] [PubMed] [Google Scholar]
- 72.Vissink A., Jager-Wittenaar H., Visser A., Spijkervet F.K., van Weissenbruch R., van Nieuw Amerongen A. Oral medicine 3: anatomy, physiology and diagnostic considerations of taste and smell disorders. Ned Tijdschr Tandheelkd. 2013;120(1):34–39. [in Dutch] [PubMed] [Google Scholar]
- 73.Dzaman K., Jadczak M., Rapiejko P., Syrylo A., Jurkiewicz D. Assessment of the correlation between taste and smell functioning. Pol Merkur Lekarski. 2005;19(111):280–282. [in Polish] [PubMed] [Google Scholar]
- 74.Rowe T.B., Shepherd G.M. Role of ortho-retronasal olfaction in mammalian cortical evolution. J Comp Neurol. 2016;524(3):471–495. doi: 10.1002/cne.23802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Forssell H., Jaaskelainen S., List T., Svensson P., Baad-Hansen L. An update on pathophysiological mechanisms related to idiopathic oro-facial pain conditions with implications for management. J Oral Rehabil. 2015;42(4):300–322. doi: 10.1111/joor.12256. [DOI] [PubMed] [Google Scholar]
- 76.Suarez P., Clark G.T. Burning mouth syndrome: an update on diagnosis and treatment methods. J Calif Dent Assoc. 2006;34(8):611–622. [PubMed] [Google Scholar]
- 77.Klasser G.D., Grushka M., Su N. Burning mouth syndrome. Oral Maxillofac Surg Clin North Am. 2016;28(3):381–396. doi: 10.1016/j.coms.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 78.Siviero M., Teixeira M.J., de Siqueira J.T., Siqueira S.R. Somesthetic, gustatory, olfactory function and salivary flow in patients with neuropathic trigeminal pain. Oral Dis. 2010;16(5):482–487. doi: 10.1111/j.1601-0825.2010.01660.x. [DOI] [PubMed] [Google Scholar]
- 79.Siviero M., Teixeira M.J., Siqueira J.T., Siqueira S.R. Central mechanisms in burning mouth syndrome involving the olfactory nerve: a preliminary study. Clinics (Sao Paulo) 2011;66(3):509–512. doi: 10.1590/S1807-59322011000300026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Su N., Poon R., Grushka M. Does Sjogren’s syndrome affect odor identification abilities? Eur Arch Otorhinolaryngol. 2015;272(3):773–774. doi: 10.1007/s00405-014-3208-y. [DOI] [PubMed] [Google Scholar]
- 81.Djordjevic J., Zatorre R.J., Jones-Gotman M. Odor-induced changes in taste perception. Exp Brain Res. 2004;159(3):405–408. doi: 10.1007/s00221-004-2103-y. [DOI] [PubMed] [Google Scholar]
- 82.Rolls E.T. Functions of the orbitofrontal and pregenual cingulate cortex in taste, olfaction, appetite and emotion. Acta Physiol Hung. 2008;95(2):131–164. doi: 10.1556/APhysiol.95.2008.2.1. [DOI] [PubMed] [Google Scholar]
- 83.Rolls E.T. Taste and smell processing in the brain. Handb Clin Neurol. 2019;164:97–118. doi: 10.1016/B978-0-444-63855-7.00007-1. [DOI] [PubMed] [Google Scholar]
- 84.Perricone C., Shoenfeld N., Agmon-Levin N., de Carolis C., Perricone R., Shoenfeld Y. Smell and autoimmunity: a comprehensive review. Clin Rev Allergy Immunol. 2013;45(1):87–96. doi: 10.1007/s12016-012-8343-x. [DOI] [PubMed] [Google Scholar]
- 85.Al-Ezzi M.Y., Pathak N., Tappuni A.R., Khan K.S. Primary Sjogren’s syndrome impact on smell, taste, sexuality and quality of life in female patients: a systematic review and meta-analysis. Mod Rheumatol. 2017;27(4):623–629. doi: 10.1080/14397595.2016.1249538. [DOI] [PubMed] [Google Scholar]
- 86.Henkin R.I., Talal N., Larson A.L., Mattern C.F. Abnormalities of taste and smell in Sjogren’s syndrome. Ann Intern Med. 1972;76(3):375–383. doi: 10.7326/0003-4819-76-3-375. [DOI] [PubMed] [Google Scholar]
- 87.Rombaux P., Martinage S., Huart C., Collet S. Post-infectious olfactory loss: a cohort study and update. B-ENT. 2009;5(suppl 13):89–95. [PubMed] [Google Scholar]
- 88.Jung Y.G., Lee J.S., Park G.C. Does post-infectious olfactory loss affect mood more severely than chronic sinusitis with olfactory loss? Laryngoscope. 2014;124(11):2456–2460. doi: 10.1002/lary.24691. [DOI] [PubMed] [Google Scholar]
- 89.Seiden A.M. Postviral olfactory loss. Otolaryngol Clin North Am. 2004;37(6):1159–1166. doi: 10.1016/j.otc.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 90.Moran D.T., Jafek B.W., Eller P.M., Rowley J.C., 3rd Ultrastructural histopathology of human olfactory dysfunction. Microsc Res Tech. 1992;23(2):103–110. doi: 10.1002/jemt.1070230202. [DOI] [PubMed] [Google Scholar]
- 91.Gaines A. Olfactory disorders. Am J Rhinol Allergy. 2013;27(suppl 1):S45–S47. doi: 10.2500/ajra.2013.27.3898. [DOI] [PubMed] [Google Scholar]
- 92.Suzuki M., Saito K., Min W.P. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–277. doi: 10.1097/01.mlg.0000249922.37381.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim Y.K., Hong S.L., Yoon E.J., Kim S.E., Kim J.W. Central presentation of postviral olfactory loss evaluated by positron emission tomography scan: a pilot study. Am J Rhinol Allergy. 2012;26(3):204–208. doi: 10.2500/ajra.2012.26.3759. [DOI] [PubMed] [Google Scholar]
- 94.Coronaviridae Study Group of the International Committee on Taxonomy of V The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutierrez-Ocampo E. Latin American Network of Coronavirus Disease 2019-COVID-19 Research (LANCOVID-19). Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ge H., Wang X., Yuan X. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis. 2020;39(6):1011–1019. doi: 10.1007/s10096-020-03874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reinhard A., Ikonomidis C., Broome M., Gorostidi F. Anosmia and COVID-19. Rev Med Suisse. 2020;16(691-692):849–851. [in French] [PubMed] [Google Scholar]
- 98.Benezit F., Le Turnier P., Declerck C. RAN COVID Study Group. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30297-8. S1473-3099(20)30297-30298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mao L., Jin H., Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bagheri SHR, Asghari AM, Farhadi M, et al. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak. Med J Islam Repub Iran. 2020;34 (1):1-12. [DOI] [PMC free article] [PubMed]
- 101.Villalba N.L., Maouche Y., Ortiz M.B.A. Anosmia and dysgeusia in the absence of other respiratory diseases: should COVID-19 infection be considered? Eur J Case Rep Intern Med. 2020;7(4) doi: 10.12890/2020_001641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Centers for Disease Control and Prevention Symptoms of coronavirus. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html Available at:
- 103.Tong J.Y., Wong A., Zhu D., Fastenberg J.H., Tham T. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2020;163(1):3–11. doi: 10.1177/0194599820926473. [DOI] [PubMed] [Google Scholar]
- 104.Hopkins C. Loss of sense of smell as marker of COVID-19 infection. https://www.entuk.org/sites/default/files/files/Loss%20of%20sense%20of%20smell%20as%20marker%20of%20COVID.pdf Available at: Accessed May 20, 2020.
- 105.Pallanti S. Importance of SARs-Cov-2 anosmia: from phenomenology to neurobiology. Compr Psychiatry. 2020;100:152184. doi: 10.1016/j.comppsych.2020.152184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 107.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.MaassenVanDenBrink A., de Vries T., Danser A.H.J. Headache medication and the COVID-19 pandemic. J Headache Pain. 2020;21(1):38. doi: 10.1186/s10194-020-01106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eccleston C., Blyth F.M., Dear B.F. Managing patients with chronic pain during the COVID-19 outbreak: considerations for the rapid introduction of remotely supported (eHealth) pain management services. Pain. 2020;161(5):889–893. doi: 10.1097/j.pain.0000000000001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cohen S.P., Baber Z.B., Buvanendran A. Pain management best practices from multispecialty organizations during the COVID-19 pandemic and public health crises. Pain Med. 2020;21(7):1331–1346. doi: 10.1093/pm/pnaa127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shanthanna H., Strand N.H., Provenzano D.A. Caring for patients with pain during the COVID-19 pandemic: consensus recommendations from an international expert panel. Anaesthesia. 2020;75(7):935–944. doi: 10.1111/anae.15076. [DOI] [PMC free article] [PubMed] [Google Scholar]