Abstract
Recently, the Wang group at Soochow University and the Gu group at the University of California, Los Angeles demonstrated the targeting ability of platelet-derived extracellular vesicles to deliver anti-inflammatory drug [5-(p-fluorophenyl)-2-ureido] thiophene-3-carboxamide (TPCA-1) to pneumonia for calming the local cytokine storm in acute lung injury.
Recently, the Wang group at Soochow University and the Gu group at the University of California, Los Angeles demonstrated the targeting ability of platelet-derived extracellular vesicles to deliver anti-inflammatory drug [5-(p-fluorophenyl)-2-ureido] thiophene-3-carboxamide (TPCA-1) to pneumonia for calming the local cytokine storm in acute lung injury.
Main Text
The 2019 novel coronavirus (SARS-Cov-2) has infected over 8.5 million people and has killed more than 450,000 people around the world (https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6). On January 24, 2020, Lancet reported that the high mortality rate of severe patients (>60%) is closely related to the cytokine storm syndrome (CSS).1 CSS is characterized by a large number of cells releasing excessive amount of pro-inflammatory cytokines. This uncontrolled inflammatory process can cause multi-organ failure, elevated serum ferritin, and, if untreated, often death.2 Therefore, development of therapies to effectively control the cytokine storm is the key to reducing COVID-19 mortality.
Research shows that an inflammatory mediator, called interleukin-6 (IL-6), is one of the major pathologic effectors of CSS. Therefore, IL-6 receptor blockade and IL-6 neutralization are potential solutions for calming CSS. Following this scenario, in the last decades, two drugs—Tocilizumab (IL-6 receptor blockade) and corticosteroid—have been developed and proved to be effective. However, Tocilizumab has shown serious side effects among rheumatoid arthritis patients, including the infection of skin and soft tissue, the increase of serum cholesterol level, the transient decrease in neutrophil count, and abnormal liver function.3 On the other hand, corticosteroid may also cause avascular necrosis and/or osteoporosis among patients with severe acute respiratory syndromes (SARS).4 Biomimetic carriers, which take advantage of the inherent ability of cells to interact with their environments, can specifically target the disease sites and reduce side effects.5 Thus, developing a suitable biomimetic carrier for immune-modulating drugs may provide an alternative for effective treatment with reduce side effects.
The Wang group and the Gu group have published numerous works on platelet-mediated drug delivery.6 , 7 Platelets are small blood cells (1–3 μm) with excellent inflammatory targeting ability. However, using platelets as carriers may accelerate the inflammation and progression of pneumonia. To solve this conundrum, the two groups developed a platelet-derived extracellular vesicle (PEV) platform. PEVs are nano-sized membrane vesicles (100–150 nm) derived from platelets (Figure 1 ). The existence of CD41 on PEVs confirmed that the PEVs were derived from platelets, while some cytosolic proteins, such as actin, were lost in PEVs compared with platelets. PEVs inherit the inflammatory targeting ability of platelets8 but do not aggravate the inflammation and progression. Therefore, PEVs are promising biomimetic carriers for anti-inflammatory drug delivery.
Figure 1.
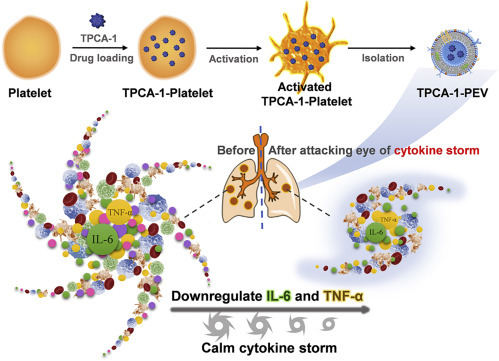
Biomimetic TPCA-1-PEV Nanodrugs Calm the Cytokine Storm in Pneumonia
(A) Schematic illustration of preparation of nanodrug TPCA-1-PEV from platelet-derived exosomes.
(B) TPCA-1-PEV calms cytokine storm arisen from pneumonia by downregulating related cytokines TNF-α and IL-6.
In the work of Wang and Gu, researchers first verified the targeting ability of PEVs to inflammatory lungs in vivo.9 They injected DiD-labeled PEVs intravenously into acute lung injury (ALI) mice. Near-infrared (NIR) fluorescence imaging showed that PEVs recognized inflammatory M1-type macrophages and other inflammation-associated cells. On the contrary, PEVs did not bind to healthy lung tissue, indicating an excellent inflammation-targeting ability of PEVs.
Researchers then evaluated the performance of PEVs as drug carriers. They loaded PEVs with an anti-inflammatory drug TPCA-1 (TPCA-1-PEVs) to calm CSS by downregulating IL-6 from monocytes. Enzyme linked immunosorbent assay showed that the levels of IL-6 and tumor necrosis factor alpha (TNF-α) in the ALI mice were significantly lowered at 20 h after intravenous injection of TPCA-1-PEVs (1 mg/kg of TPCA-1). Furthermore, the inflammatory cell infiltration of mice receiving TPCA-1-PEVs was significantly reduced compared with that of untreated ALI group and free drug treatment. Thus, TPCA-1-PEVs stand a chance of treating patients with CSS, including those severe cases infected by SARS-Cov-2.
The work of Wang and Gu revealed PEV as a useful biomimetic platform for targeting inflammatory sites and PEV-based drug delivery approach for treating CSS. Regarding SARS-Cov-2, the PEV-based nanodrug might offer an attractive alternative to the treatments in use or under development (e.g., serum of human donors recently recovered from SARS-Cov-2 infection, monoclonal antibodies, vaccines, and antiviral small molecules). PEV-based nanodrugs may also have potential to treat various inflammatory diseases such as atherosclerotic plaque, rheumatoid arthritis, and skin wound. One can also envision its use to control cytokine release syndrome (similar to CSS), one of the side effects in CAR-T therapy.10
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2017YFC1309100 and 2017YFA0205200), National Natural Science Foundation of China (81671753), and the Intramural Research Program (IRP), National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH).
Contributor Information
Zhongliang Wang, Email: wangzl@xidian.edu.cn.
Xiaoyuan Chen, Email: shawn.chen@nih.gov.
Web Resources
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University, https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6
Template:COVID-19 pandemic data, https://en.wikipedia.org/wiki/Template:COVID-19_pandemic_data
References
- 1.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrens E.M., Koretzky G.A. Review: Cytokine Storm Syndrome: Looking Toward the Precision Medicine Era. Arthritis Rheumatol. 2017;69:1135–1143. doi: 10.1002/art.40071. [DOI] [PubMed] [Google Scholar]
- 3.Jones G., Ding C. Tocilizumab: a review of its safety and efficacy in rheumatoid arthritis. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2010;3:81–89. doi: 10.4137/CMAMD.S4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang R.H., Kroll A.V., Gao W., Zhang L. Cell Membrane Coating Nanotechnology. Adv. Mater. 2018;30:e1706759. doi: 10.1002/adma.201706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C., Sun W., Ye Y., Hu Q., Bomba H.N., Gu Z. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nat Biomed Eng. 2017;1:0011. [Google Scholar]
- 7.Hu Q., Sun W., Wang J., Ruan H., Zhang X., Ye Y., Shen S., Wang C., Lu W., Cheng K. Conjugation of haematopoietic stem cells and platelets decorated with anti-PD-1 antibodies augments anti-leukaemia efficacy. Nat. Biomed. Eng. 2018;2:831–840. doi: 10.1038/s41551-018-0310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo S.C., Tao S.C., Yin W.J., Qi X., Yuan T., Zhang C.Q. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017;7:81–96. doi: 10.7150/thno.16803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Q., Fan Q., Xu J., Bai J., Han X., Dong Z., Zhou X., Liu Z., Gu Z., Wang C. Calming Cytokine Storm in Pneumonia by Targeted Delivery of TPCA-1 Using Platelet-Derived Extracellular Vesicles. Matter. 2020;3:287–301. doi: 10.1016/j.matt.2020.05.017. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadelain M., Rivière I., Riddell S. Therapeutic T cell engineering. Nature. 2017;545:423–431. doi: 10.1038/nature22395. [DOI] [PMC free article] [PubMed] [Google Scholar]


