Abstract
Background
Many adult drinkers consume far beyond the binge threshold. This “high-intensity drinking” (HID), defined as 2 (HID-2) and 3 (HID-3) times the binge threshold, is of public health interest due to its role in acute alcohol-related harms. Research on HID has mostly been limited to college-aged young adults, focused on contextual factors, and neglected the potential role of genetic influences on the propensity to engage in HID.
Methods
Structured diagnostic interviews assessing past-year alcohol involvement were conducted with 3,785 individuals (1,365 men, 2,420 women; Mage = 32, range = 21 to 46), including 3,314 twins and 471 nontwin siblings from the Australian Twin Registry. Multinomial logistic regression analyses were conducted to compare HID-2 and HID-3 to binge drinking on demographic correlates, drinking characteristics, and drinking-related consequences. Biometric modeling was conducted to estimate the role of genetic, common, and individual-specific environmental factors in HID propensity.
Results
Among past-year drinkers, the prevalence of HID-2 and HID-3 was both 22%, with men disproportionally represented. The frequencies of drinking, intoxication, and binge drinking significantly increased across the heavier drinking categories, which also evidenced higher average consumption quantities and higher rates of alcohol-related consequences. The propensity to engage in HID was significantly heritable (A = 37% [95% CI: 28 to 46%]), with individual-specific environmental influences accounting for the remainder of the variance.
Conclusions
This study convincingly demonstrates that HID is not restricted to college-aged young adults, but also can be highly prevalent among those of working age, and that the propensity to engage in HID is partially explained by genetic influences.
Keywords: High-Intensity Drinking, Alcohol, Binge Drinking, Twin Study
The definition of binge drinking as consuming 4 or 5 or more alcoholic drinks in 1 drinking episode for women and men, respectively (Wechsler et al., 1995), has proven to be an invaluable tool in the nearly 15 years since it was officially approved by the National Institute on Alcohol Abuse and Alcoholism (NIAAA; NIAAA 2004). There has recently been interest in reconceptualizing extreme alcohol use that surpasses the binge threshold (Patrick and Azar, 2018). High-intensity drinking (HID), also called “extreme binge drinking” and defined in 2 levels as 8 to 11/10 to 14 (i.e., 2 times the binge threshold for women/men) and 12+/15+ drinks (i.e., 3 times the binge threshold for women/men) in a single drinking episode, has been proposed to describe and understand this type of high-volume alcohol use (Patrick, 2016; Patrick and Azar, 2018).
The main impetus behind differentiating HID from binge drinking is that the 4+/5+ binge cutoff obscures very high-risk drinking, thereby failing to differentiate individuals who are at high risk for acute harm (Patrick, 2016). A dichotomous approach “assigns identical risk to all bingers regardless of how far they exceed the threshold” and, as a result, removes important information that is relevant to public health (Hingson et al., 2017, p. 717). For example, high-intensity drinkers are 3 times more likely to meet criteria for alcohol use disorder (AUD), and, among individuals with AUD, high-intensity drinkers are more likely to meet criteria for a moderate or severe disorder than individuals who do not exceed the binge threshold (Linden-Carmichael et al., 2017). Relative to binge drinking, HID is also associated with significantly more frequent and severe drinking-related consequences such as blacking out, emergency department visits, driving under the influence, and alcohol-related legal problems (Hingson et al., 2017; Patrick et al., 2016). Further, individuals drinking to the higher HID threshold have greater odds of injury, physical fights, and getting arrested as compared to individuals drinking to the lower HID threshold (Hingson et al., 2017).
Research on the determinants of HID has focused primarily on contextual factors (Patrick and Azar, 2018), with less attention paid to individual-level factors that might also contribute to the propensity to engage in HID. There is a small body of existing evidence from disparate literatures (twin, molecular genetic, and animal studies) that supports an important role for genetic factors in the etiology of HID. For example, a twin study of US male Vietnam veterans, 42 years of age on average, reported that genetic factors accounted for 32% of the variation in liability to consume 20 or more drinks on a single occasion (Slutske et al., 1999).
An existing measure of drinking that is closely aligned with HID, included in 2 twin studies (both reported in Dick et al., 2011), is “daily maximum drinks” (i.e., “max drinks”) consumed in the past year. Among 1,378 25-year-old Finnish twin pairs, the contributions of genetic and individual-specific environmental factors to past-year max drinks were 49 and 51%; among 1,766 US twin pairs, 36 years of age on average, these contributions were 56 and 44% (common environmental influences were dropped from models because they were nonsignificant; Dick et al., 2011). These results strongly support the notion that genes contribute to the amount of alcohol 1 is willing and able to consume on a single occasion. Indeed, gene identification efforts have successfully detected loci (Kuo et al., 2006; Saccone et al., 2000) and common polymorphisms associated with max drinks (Pan et al., 2013; Xu et al., 2015).
Of interest is the repeated finding from multivariate twin studies that different alcohol use phenotypes, such as the quantity and frequency of alcohol use and max drinks, have overlapping, but distinct, genetic architectures (Agrawal et al., 2009; Dick et al., 2011; Kendler et al., 2010). Consistent with this is recent genomic evidence demonstrating that max drinks are only modestly predicted from a polygenic risk score for weekly alcohol consumption derived in an independent sample (Johnson et al., 2019). These findings have also been mirrored in the animal literature. Early seminal studies indicated the importance of genetic influences on alcohol preference in mice (McClearn and Rodgers, 1961; Williams et al., 1949); more recent studies have led to the development of “high drinking in the dark” mice that have been selectively bred to drink to high blood alcohol concentrations (Barkley-Levenson and Crabbe, 2014; Crabbe et al., 2009). Research suggests that genetic influences for “high drinking in the dark” mice only partially overlap with genetic influences for alcohol preference (Crabbe et al., 2011; Rhodes et al., 2007). In sum, there is existing research in humans and mice on phenotypes that closely resemble HID (max drinks, “high drinking in the dark”), suggesting that there are important genetic influences for this aspect of drinking. It would be an oversight to not incorporate these lines of evidence into the emerging dialogue about the factors contributing to HID.
In addition, HID research has primarily been based on adolescent, college student, and young adult samples (Patrick et al., 2016; Patrick and Terry-McElrath, 2017; Patrick et al., 2016), with less attention paid to samples of community-based, working-age adults (Linden-Carmichael et al., 2017). The present study attempts to fill these gaps by examining HID in a genetically informative study of this understudied segment of the population. Specifically, we examined demographic correlates, drinking characteristics, and drinking-related consequences of HID in a community-based sample of working-age Australian adult twins; we also examined the contribution of genetic, common, and individual-specific environmental influences to HID propensity.
MATERIALS AND METHODS
Participants and Procedure
Participants were 3,785 individuals (3,314 twins and 471 nontwin siblings) from the Australian Twin Registry Cohort III (1,365 men, 2,420 women; Mage = 32, range = 21 to 46, twin range = 27 to 371). Notably, 97% of the sample was over age 28 and 94% was between the ages of 28 and 38, representing a unique age group in HID research. Participants were surveyed by computer-assisted telephone interview (CATI) in 2005 to 2009 (participation rate = 76%; Lynskey et al., 2012). Individuals who were lifetime abstainers (n = 48) or who did not drink in the past year (n = 311) were excluded from analyses, leaving a final sample size of 3,426 for the descriptive analyses. Data from 2,964 twins of known zygosity were included in the biometric analyses. This included 938 monozygotic (MZ) and 1,066 dizygotic (DZ) twins from complete pairs and 355 MZ and 605 DZ twins from incomplete pairs.
Potential sampling bias was examined in the twin sample by comparing the prevalence of HID among twins from pairs concordant for participation in the interview (“complete pairs”) to twins whose cotwin did not participate in the interview (“incomplete pairs”). Incomplete twin pairs provide a window into characteristics of non-participating twins (including those in which neither twin from a pair participated). That is, if twins with HID were systematically under- or over-sampled, lower or higher prevalences of HID would be expected among twins whose cotwin did not participate than among twins concordant for participation in the interview (assuming that HID is correlated in twin pairs). There was no evidence of sampling bias; twins from incomplete pairs were only slightly more likely to be HID drinkers than twins from complete pairs (men: 65.80% vs. 64.76%; women: 32.41% vs. 30.56%), and these differences were not statistically significant (t = 1.27, p = 0.20).
Measures
Demographics
As part of the CATI interview, participants reported their age, ancestry, educational attainment, and marital status. For ancestry, participants were asked to report the lineage of each of their 4 biological grandparents, with up to 2 ancestral lineages for each grandparent. United Kingdom (i.e., Britain, Scotland, Wales; 84%), Ireland (32%), Germany (15%), and Italy (5%) were the most common countries of descent; 7% reported no lineage from these most prevalent groups, less than 1% reported Asian ancestry, and slightly less than 2% of the sample had at least 1 grandparent of indigenous Australian ancestry. For educational attainment, participants were asked to report their highest educational level attained using a respondent booklet with a list of 10 possible response options (primary incomplete, primary completed, year 8 completed, year 9 completed, year 10 completed, year 11 completed, year 12 completed, technical college, undergraduate degree, and graduate degree). Due to low prevalence of the lower categories, they were collapsed into a “less than high school” group (24% of the sample); the rest of the sample was evenly distributed across technical college (28%), undergraduate degree (28%), and graduate degree (20%). Participants were asked if they were currently married, widowed, separated, divorced, or never married. Because the rates of being widowed, separated, and divorced were very low (5% combined), never married (41% of the sample) and married (53% of the sample) were used as comparison groups for analyses of marital status.
Alcohol Use
Assessment of alcohol use was based on the Australian version of the Semi-Structured Assessment of the Genetics of Alcoholism (Bucholz et al., 1994; Heath et al., 1997) and administered via CATI. Reports of past-year frequency of drinking, being drunk, and binge drinking, typical quantity of drinks per drinking episode, and maximum number of drinks consumed in a single 24-hour period (max drinks) were queried. Response options for frequency measures included 10 options ranging from “every day” to “never.” The categorical frequency of drinking measure was converted to a number of drinking days in the past-year variable by computing the number of days per year that corresponded to the category (e.g., every day = 365 days; 2 d/wk = 2*52 = 104 days; 2 to 3 d/month = 2.5*12 = 30 days). Typical quantity response options included 10 items ranging from 1 to 2 drinks to 31 or more drinks. For response options with a range, the mean of the lower and upper bound was taken and used as the quantity for that response (e.g., “3 to 4 drinks” translated to 3.5 drinks).
For the assessment of max drinks, participants were asked “what is the largest number of drinks you have ever had in a 24-hour period? By a drink I mean a can or a stubbie of beer, a glass of wine, or a nip of spirits.” Following this, participants were asked to report on the types (beer, wine, spirits, and other), strength, and quantity of alcohol consumed. This was converted by the interviewer into the number of standard drinks consumed, which was summed by the CATI system to create a lifetime max drinks variable. Participants were asked the first and last time that this amount of alcohol was consumed in a 24-hour period. For 15% of participants, the lifetime max drinks had occurred in the past 12 months; the remaining participants were asked about the largest number of drinks consumed in a 24-hour period in the past 12 months. Past-year max drinks were based on these 2 pieces of information. Previous studies have reported excellent reliabilities (r = 0.90) for retrospective reports of max drinks (Rutledge et al., 2008; Slutske et al., 1999).
HID
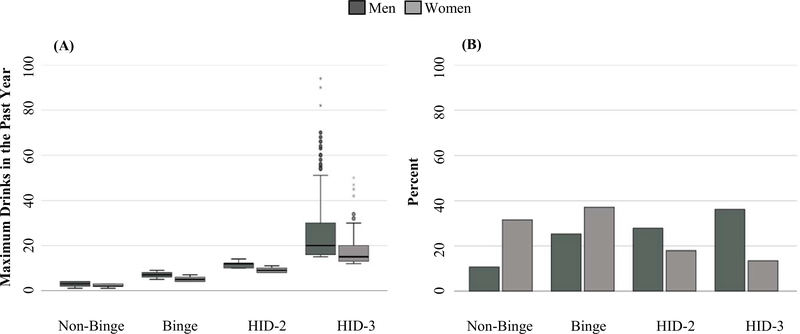
HID was derived from the maximum drinks in the past-year variable (Hingson et al., 2017). A 4-level ordinal drinking level variable was created according to the following thresholds: “Non-binge” included individuals who reported that their maximum drinks did not exceed 3 (for women) or 4 (for men), “binge” included individuals reporting 4 to 7 (for women) or 5 to 9 (for men), “high-intensity low” (HID-2) included individuals reporting 8 to 11 (for women) or 10 to 14 (for men), and “high-intensity high” (HID-3) included individuals who reported 12 or more (for women) or 15 or more (for men). The upper limit of the HID-3 drinking category was 50 for women and 94 for men (see Fig. 1A).
Fig. 1.
Panel (A): Box-and-whisker plots of the distributions of the max drinks variable by sex and drinking category. Upper limit max drinks for men = 94, upper limit max drinks for women = 50; within-box black line represents the group median of max drinks, filled box represents the interquartile range (scores 25% above and below the median are represented in their respective area of the filled box above and below the median line), upper and lower whiskers represent upper and lower quartiles (scores up to 1.5s times the interquartile range), circles represent outliers with scores between 1.5 and 3 times the interquartile range, asterisks represent outliers with scores more than 3 times the interquartile range. Panel (B): Percent of men and women in each drinking category. HID-2: 2 times sex-adjusted binge threshold, HID-3: 3 or more times sex-adjusted binge threshold.
Drinking-Related Consequences
Participants were queried about experiencing alcohol-related blackout (i.e., “drinking enough so that you could not remember things you had said or done”) and passing out (i.e., “falling asleep from drinking too much”) in the past year. Alcohol abuse and dependence were assessed using DSM-IV criteria and scored according to the DSM-5 criteria for AUD absent the criterion of craving, which was not included in the DSMIV. A past-year diagnosis of AUD was based on having 2 or more past-year symptoms.
Analytic Plan
Descriptive Analyses
Multinomial logistic regression analyses and chi-square cross-tabulations were conducted to compare the drinking categories (nonbinge, binge, HID-2, and HID-3) in the full sample and among men and women separately. All models included age as a covariate. The purpose of these descriptive analyses was 2-fold. First, we wanted to use additional measures of past-year drinking behaviors and consequences to verify that the measure of HID was identifying a pattern of behavior, despite the fact that the measure was based on a single drinking episode. With this verification, we then sought to examine whether there were differences between the 2 HID categories and binge drinking.
Drinking categories were compared on demographic characteristics, past-year drinking behavior, and drinking-related consequences. Three models were fitted for each variable: a model with the variable of interest as the sole predictor, then a model controlling for sex, and, finally, a model including a variable by sex interaction to probe for potential sex differences in the association between the variable of interest and drinking behavior. These analyses included the full sample of twins and siblings, and used survey data analysis procedures in SAS software version 9.4 (SAS Institute, 2015) that corrects for the nonindependence of twin and sibling pair observations.
Twin Analyses
Twin correlations in HID liability were estimated, and biometric modeling was conducted in Mplus (Muthén and Muthén, 2017). In all models, the thresholds (prevalences) for men and women were allowed to differ. Biometric models were fitted by the method of robust weighted least squares directly to the raw twin data, which uses data from incomplete as well as complete twin pairs. An assumption was made that there existed a latent liability continuum underlying the ordinal HID levels by employing a liability-threshold model (Kendler, 1993; Neale and Cardon, 1992). Biometric model-fitting partitioned the variation in HID liability (or propensity) into additive genetic, common environmental or nonadditive genetic, and individual-specific environmental influences (the latter also includes measurement error). Age was included as a covariate in all models. Quantitative sex differences, or differences in the proportion of genetic, common environmental, and individual-specific environmental factors, were examined by constraining parameter estimates for men and women to be equal; a significant decrease in model fit under these constraints would indicate the presence of quantitative sex differences. Qualitative sex differences, or different genetic sources of liability for men and women, were tested by constraining the genetic correlation for opposite-sex twin pairs to 0.5 (i.e., the genetic correlation for same-sex twin pairs). A significant reduction in model fit compared with an unconstrained model would indicate the presence of qualitative sex differences. Model comparisons were conducted with Wald tests. Bias-corrected bootstrapped confidence intervals around parameters were estimated.
RESULTS
Prevalence and Demographic Correlates of HID
Past-year HID was unexpectedly prevalent in this sample: 22% (N = 751) reported HID-3, 22% (N = 738) reported HID-2, 32% (N = 1110) reported binge-only consumption, and 24% (N = 827) reported nonbinge consumption. There were notable sex differences in rates of HID, F(6, 1988) = 59.15, p < 0.0001. Compared with binge, men were more likely than women to report HID-2 (OR = 2.31, 95% CI [1.90 to 2.81], p < 0.0001) and HID-3 (OR = 4.07, 95% CI [3.31, 5.01], p < 0.0001), whereas women were more likely than men to report nonbinge (OR = 2.05, 95% CI [1.61, 2.60], p < 0.0001; see Figure 1b). Age was associated with drinking category, F(3, 1995) = 17.40, p < 0.0001, such that being older decreased odds of HID-3 compared with binge. Educational attainment was differentially associated with drinking level, F(6, 1995) = 13.79, p < 0.0001, demonstrating a protective effect against HID-3 compared with both binge and HID-2. Marital status was also differentially associated with drinking level, F(6, 1940) = 28.04, p < 0.0001, with never married individuals being at higher risk for HID-2 and HID-3 as compared to binge. There was a significant interaction between marital status and sex, χ2 = 21.81, df = 3, p < 0.0001. Rates at which men engaged in HID-2 and HID-3 were not significantly different between those who were never married and those who were married (HID-2: 28.76% vs. 28.22%, HID-3: 40.10% vs. 30.85%; p = 0.08 to 0.53), whereas the rates at which women engaged in HID-2 and HID-3 were significantly higher among those who were never married than those who were married (HID-2: 25.15% vs. 12.44%, HID-3: 19.20% vs. 8.38%; p = 0.002 to 0.0004).
Drinking Correlates of HID
Comparison of drinking categories substantiated the hypothesis that past-year drinking behaviors would differ by drinking level (see Table 1). HID-3 drinkers, on average, consumed alcohol on roughly 40% of days (i.e., about 3 d/wk) and typically consumed, on average, approximately 4.5 drinks per drinking occasion; on average, HID-3 drinkers consumed at their respective gender-specific binge thresholds during a typical drinking episode. HID-2 drinkers, on average, consumed alcohol on roughly 30% of days (i.e., about 2 d/wk) and typically consumed, on average, approximately 3.5 drinks per drinking occasion. Binge-only drinkers, on average, consumed alcohol on roughly 20% of days (i.e., about 1.5 d/wk) and typically consumed, on average, approximately 2.5 drinks per drinking occasion. In sum, individuals reporting meeting or exceeding 3 times the binge threshold at least once in the past-year drank roughly twice as often and consumed roughly twice as much on a typical drinking occasion as compared to those who reported binge-only drinking in the past year.
Table 1.
Past-Year Drinking Behavior by Drinking Category
| Nonbinge M (95% Cl) |
Binge M (95% CI) |
HID-2 M (95% CI) |
HID-3 M (95% CI) |
Wald F(3, 923–1995)a | |
|---|---|---|---|---|---|
| Drinking frequency | |||||
| Total | 31.28 (27.30 to 35.28)234 | 75.72 (70.83 to 80.62)134 | 110.76 (104.07 to 117.45)124 | 143.91 (135.72 to 151.11)123 | 53.20 |
| Men | 48.84 (34.43 to 62.25)2*34 | 88.60 (79.18 to 98.02)1*34 | 123.20 (113.02 to 133.39)124 | 160.48 (150.51 to 170.45)123 | 16.91 |
| Women | 27.78 (23.96 to 31.59)234 | 70.37 (64.67 to 76.06)134 | 98.97 (90.25 to 107.68)124† | 116.67 (103.75 to 129.60)123† | 30.36 |
| Drunk frequency | |||||
| Total | 0.31 (0.22 to 0.39)234 | 5.40 (4.61 to 6.19)134 | 17.68 (15.65 to 19.71)124 | 42.37 (37.81 to 46.93)123 | 21.48 |
| Men | 0.31 (0.08 to 0.54)2†3*4* | 6.52 (5.04 to 7.99)1†34 | 20.50 (17.13 to 23.87)1*24 | 47.26 (41.51 to 53.01)1*23 | 10.07 |
| Women | 0.31 (0.21 to 0.40)234 | 4.93 (3.99 to 5.87)134 | 15.01 (12.74 to 17.29)123 | 34.33 (27.02 to 41.64)124 | 10.29 |
| Binge frequency | |||||
| Total | 1.02 (0.29 to 1.75)234 | 11.45 (10.13 to 12.78)34 | 38.92 (35.18 to 42.66)124 | 78.18 (72.01 to 84.36)123 | 28.93 |
| Men | 0.68 (0.18 to 1.18)2*3*4 | 17.85 (15.03 to 20.66)1*34 | 46.94 (40.95 to 52.93)1*24 | 90.14 (82.37 to 97.91)1*23 | 18.95 |
| Women | 1.09 (0.22 to 1.96)2*3*4* | 8.79 (7.35 to 10.23)1*34 | 31.33 (26.46 to 35.73)1*24 | 58.52 (49.32 to 67.73) 1*23 | 10.04 |
| Typical quantity | |||||
| Total | 1.62 (1.59 to 1.66)234 | 2.47 (2.37 to 2.57)134 | 3.40 (3.24 to 3.56)124 | 4.74 (4.47 to 5.02)123 | 70.64 |
| Men | 1.80 (1.69 to 1.92)234 | 2.72 (2.53 to 2.90)134 | 3.58 (3.35 to 3.82)124 | 5.22 (4.84 to 5.61)123 | 31.13 |
| Women | 1.59 (1.56 to 1.63)234 | 2.37 (2.26 to 2.48)134 | 3.23 (3.02 to 3.44)124* | 3.96 (3.61 to 4.32)123* | 33.46 |
Frequency measured in 1-day units, quantity measured in standard drinks; all tests include age as a covariate
= differs from non-binge
= differs from binge
= differs from HID-2
= differs from HID-3
Bonferroni corrected p = 0.004; all drinking groups differ from all others on every measure at p < 0.0001 unless noted.
p < 0.004.
p < 0.05.
All significant at p < 0.0001.
Drinking-Related Consequences and HID
The rate and odds of past-year blackout, passing out, and AUD increased with each successive drinking category (see Table 2). Binge-only drinkers reported these consequences at relatively low rates; blackout was over 5 times as prevalent in HID-2 drinkers, and over 10 times as prevalent in HID-3 drinkers. Similarly, passing out was 4 times as prevalent among HID-2 drinkers, and over 6 times as prevalent among HID-3 drinkers compared to binge-only drinkers. Roughly 2% of binge-only drinkers met criteria for a past-year AUD of any severity (i.e., endorsed at least 2 criteria), compared with over 12% of HID-2 and almost 29% of HID-3 drinkers.
Table 2.
Prevalence of Past-Year Drinking-Related Consequences in Binge and High-Intensity Drinkers
| Binge M (95% CI) |
HID-2 M (95% CI) |
HID-3 M (95% CI) |
Rao Scott χ2 df = 2a |
|
|---|---|---|---|---|
| Blackout | ||||
| Total | 2.43 (1.52 to 3.34)23 | 12.20 (9.78 to 14.61)13 | 28.10 (24.79 to 31.41)12 | 49.34 |
| Men | 0.92 (0.00 to 1.96)23 | 10.59 (7.41 to 13.76)13 | 27.41 (23.31 to 31.51)12 | 16.88 |
| Women | 3.07 (1.86 to 4.27)23 | 13.72 (10.14 to 17.30)13 | 29.23 (23.74 to 34.71)12 | 29.80 |
| Pass out | ||||
| Total | 3.25 (2.15 to 4.34)23 | 13.01 (10.53 to 15.49)13 | 21.57 (18.58 to 24.56)12 | 29.05 |
| Men | 2.14 (0.57 to 3.73)23 | 11.42 (7.99 to 14.86)13* | 20.77 (17.12 to 24.42)12* | 13.66 |
| Women | 3.70 (2.30 to 5.11)23 | 14.51 (10.92 to 18.11)13† | 22.89 (17.91 to 27.87)12† | 17.71 |
| AUD | ||||
| Total | 2.16 (1.31 to 3.02)23 | 12.60 (10.21 to 15.00)13 | 28.76 (25.52 to 32.00)12 | 58.81 |
| Men | 2.45 (0.77 to 1.14)23 | 10.86 (7.64 to 14.09)13 | 33.40 (29.12 to 37.69)12 | 30.20 |
| Women | 2.04 (1.05 to 3.04)23 | 14.25 (10.72 to 17.77)1 | 21.13 (16.37 to 25.88)1 | 25.04 |
Prevalence relative to column category (e.g., 3.25% of binge drinkers passed out); non–binge-only drinkers excluded due to near-zero prevalence of consequences; all tests include age as a covariate
= differs from binge
= differs from HID-2
= differs from HID-3
Bonferroni-corrected significance level = 0.006; test significant at p < 0.0001 unless noted.
p < 0.006.
p < 0.05.
All significant at p < 0.0001.
There was a significant interaction between past-year AUD and sex, χ2 = 14.43, df = 2, p = 0.0007). Each successive drinking level increased odds of AUD; however, odds for HID-3 compared with HID-2 were substantially accelerated in men as compared to women. Men accounted for significantly more of the AUD diagnoses in the HID-3 group than did women, χ2 = 45.29, df = 1, p < 0.0001.
Twin Similarity for HID
Twin concordance for the 4 drinking levels along with the corresponding correlations in liability (polychoric correlations) is presented in Table 3. Examination of Table 3 revealed that there were many twin pairs that were concordant for HID-2 and HID-3. Remarkably, the most common type of concordant pair among men was HID-3; there were 28 MZ pairs and 21 DZ pairs in which both twins had consumed 15 or more drinks in a single day in the past year. This reinforces the idea that there is substantial meaningful variation above the binge threshold, and even above the HID-2 threshold. Cross-trait twin correlations of HID with other past-year alcohol consumption measures can be found in the supplemental materials (Table S1); these analyses demonstrate that HID is a unique construct genetically as well as phenotypically.
Table 3.
Twin pair concordance and polychoric correlations for levels of past-year binge and high-intensity drinking
| Twin 2 |
Twin 2 |
|||||||
| Monozygotic men (155 pairs) |
Monozygotic women (314 pairs) |
|||||||
| Twin 1 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| 1) Nonbinge | 5 | 3 | 3 | 2 | 46 | 43 | 12 | 6 |
| 2) Binge | 3 | 15 | 15 | 10 | 38 | 45 | 19 | 16 |
| 3) High-intensity 2 | 1 | 11 | 13 | 17 | 3 | 19 | 16 | 10 |
| 4) High-intensity 3 | 3 | 9 | 17 | 28 | 6 | 12 | 12 | 11 |
| r = 0.38, p < 0.001 | r = 0.41, p < 0.001 | |||||||
| Twin 2 |
Twin 2 |
|||||||
| Dizygotic men (109 pairs) |
Dizygotic women (237 pairs) |
|||||||
| Twin 1 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| 1) Nonbinge | 6 | 2 | 0 | 4 | 31 | 17 | 11 | 8 |
| 2) Binge | 0 | 3 | 9 | 6 | 24 | 38 | 16 | 13 |
| 3) High-intensity 2 | 2 | 11 | 9 | 13 | 8 | 20 | 10 | 10 |
| 4) High-intensity 3 | 3 | 5 | 15 | 21 | 4 | 15 | 6 | 6 |
| r = 0.19, p = 0.009 | r = 0.20, p < 0.001 | |||||||
| Male twin |
||||||||
| Dizygotic opposite-sex (187 pairs) |
||||||||
| Female twin | 1 | 2 | 3 | 4 | ||||
| 1) Nonbinge | 4 | 20 | 18 | 20 | ||||
| 2) Binge | 5 | 17 | 17 | 18 | ||||
| 3) High-intensity 2 | 5 | 13 | 14 | 13 | ||||
| 4) High-intensity 3 | 2 | 6 | 5 | 10 | ||||
| r < 0.01, p = 0.89 | ||||||||
Correlations are age-adjusted. Data from 355 MZ (216 women, 319 men) and 605 DZ twins from incomplete pairs (169 women from same-sex pairs, 136 men from same-sex pairs, and 199 women and 101 men from opposite-sex pairs) were also included in the biometric modeling.
Dark gray diagnoal boxes indicate twin pairs concordant for drinking category.
The MZ and DZ twin correlations for HID significantly differed among women (Wald χ2 = 4.74, df = 1, p = 0.03) but not among men (Wald χ2 = 2.25, df = 1, p = 0.13), suggesting that there may be genetic influences on HID propensity in women, but not men. However, the correlations within zygosity did not differ for men and women among the MZ pairs (Wald χ2 = 0.07, df = 1, p = 0.80) or the DZ pairs (Wald χ2 = 0.01, df = 1, p = 0.93), suggesting that there were no quantitative sex differences. Finally, there was no significant difference between the opposite-sex and same-sex DZ pairs (Wald χ2 = 3.59, df = 2, p = 0.17), suggesting that there were also no qualitative sex differences.
Genetic and Environmental Influences on HID Propensity
Biometric model-fitting suggested that there was a genetic contribution to past-year HID propensity in both men and women, and no evidence for a significant contribution of the common environment in either sex (see Table 4, model 1). Because constraining the parameter estimates in men and women did not result in a significant decrease in model fit (Wald χ2 = 1.04, df = 2, p = 0.59; see Table 4, model 3), the hypothesis of quantitative sex differences in the proportion of variation in HID propensity attributable to genetic, common, or individual-specific environmental factors was rejected. Evidence for qualitative sex differences was examined by testing whether the genetic correlation for opposite-sex twin pairs significantly differed from 0.5; it did not (Wald χ2 = 1.64, df = 1, p = 0.20; see Table 4, model 4). In sum, there was no evidence for quantitative or qualitative sex differences in the genetic and environmental influences on HID propensity. A final model assuming no quantitative or qualitative sex differences suggested that the propensity to engage in HID was significantly heritable, with estimates of genetic and individual-specific environmental influences of 37 and 63%, respectively (Table 4, model 4).
Table 4.
Results From Biometric Models Partitioning the Variation in High-Intensity Drinking Propensity Into Genetic (A), Common (C), and Individual-Specific (E) Environmental Factors
| Men |
Women |
Model fit |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | rg | A2 | C2 | E2 | A2 | C2 | E2 | χ2 | df | p |
| 1) ACE free, rg DZO free | ||||||||||
| Estimate | 0.01 | 0.38 | 0.00 | 0.62 | 0.41 | 0.00 | 0.59 | 36.25 | 33 | .32 |
| 95% CI | 0, 0.50 | 0.01, 0.55 | 0.00, 0.47 | 0.47, 0.76 | 0.17, 0.54 | 0.00, 0.36 | 0.48, 0.70 | |||
| 2) ACE free, rg DZO fixed | ||||||||||
| Estimate | 0.50 | 0.34 | 0.00 | 0.65 | 0.39 | 0.00 | 0.61 | 39.22 | 34 | .25 |
| 95% CI | Fixed | 0.00, 0.52 | 0.00, 0.50 | 0.50, 0.82 | 0.00, 0.50 | 0.00, 0.44 | 0.50, 0.73 | |||
| 3) ACE fixed, rg DZO free | ||||||||||
| Estimate | 0.01 | 0.40 | 0.00 | 0.60 | 0.40 | 0.00 | 0.60 | 37.29 | 35 | .36 |
| 95% CI | 0, 0.50 | 0.28, 0.50 | 0.00, 0.15 | 0.51, 0.69 | 0.28, 0.50 | 0.00, 0.15 | 0.51, 0.69 | |||
| 4) ACE fixed, rg DZO fixed | ||||||||||
| Estimate | 0.50 | 0.37 | 0.00 | 0.63 | 0.37 | 0.00 | 0.63 | 40.34 | 36 | .28 |
| 95% CI | Fixed | 0.28, 0.46 | 0.00, 0.00 | 0.54, 0.72 | 0.28, 0.46 | 0.00, 0.00 | 0.54, 0.72 | |||
All models included age as a covariate. Bold indicates significant parameter estimate. CI = confidence interval, DZO = opposite-sex dizygotic twins, rg = correlation between genetic influences in opposite-sex twin pairs.
DISCUSSION
HID was surprisingly prevalent in this community-based cohort of working-age adults and was notably higher than in past examinations of HID in the US (Hingson et al., 2017). This might be explained by Australian drinking culture (Midford, 2005) and the higher per capita alcohol consumption in Australia than in the US (World Health Organization, 2011). This cannot be explained by the low cost of alcohol, as alcohol is actually more expensive in Australia than in the US (Blecher et al., 2018; World Health Organization, 2011). One potentially relevant difference between Australian and US national samples is their racial and ethnic compositions. This sample was primarily of Northern European ancestry—a group that tends to engage in alcohol use and heavy drinking at a higher rate than other racial and ethnic groups (Chartier and Caetano, 2010). We suspect that this, coupled with few participants from lower-drinking racial and ethnic groups (e.g., those of East Asian and African descent) in the sample, may have contributed to the unexpectedly high rate of HID.
The rates of blackout, passing out, and AUD highlight the importance of differentiating drinkers who consume above the binge threshold, as the acute physiological consequences of alcohol only began to appear substantially at the HID level. Given that binge-only drinkers experience alcohol-related consequences at low rates and experience a somewhat different set of drinking-related consequences (Hingson et al., 2017), the unique experiences of heavier drinkers may be obscured by combining all heavy drinkers into a single category.
HID was especially prevalent among men, which mirrors past findings (Hingson et al., 2017) and the larger literature showing that men drink at higher rates, in higher quantities, and experience more alcohol-related problems compared with women (Wilsnack et al., 2000). The sex difference in the prevalence of HID observed in this mid-adult sample may be more pronounced than that found among younger samples. For example, the magnitude of sex differences in consuming at least 60 g of ethanol in a day (equivalent to 5 standard drinks, similar to the definition of “binge drinking” used in the present study) in the past year was substantially higher in older than in younger adults in samples from Australia and the US (Wilsnack et al., 2009), and the magnitude of sex differences in risky drinking was consistently higher in older than younger age groups across 5 national Australian surveys (Livingston et al., 2018).
The greater sex difference in HID prevalence among working-age than college-aged adults might be explained by cultural and societal norms, as well as age and developmental considerations more pointedly relevant to a mid-adult sample. For example, issues of homemaking and parenthood could play a role in reduced rates of HID among adult women who typically shoulder domestic responsibilities and are expected to abstain from alcohol while pregnant or breastfeeding (Laborde and Mair, 2012; Lyons and Willott, 2008). This was reflected in the present sample, with rates of HID being higher among unmarried women while not differing across marital status for men. Interestingly, previous studies have found that romantic partnership/marriage serves as a protective factor for heavy drinking (Barr et al., 2017) and AUD (Kendler et al., 2016) for men as well as women.
The propensity to engage in HID was significantly heritable. The heritability estimate of 37% was similar to the estimate from a previous study that examined consuming 20 or more drinks on a single occasion among middle-aged men (Slutske et al., 1999). Similar to the previous studies of max drinks conducted in the US and Finland, there was no evidence for common environmental factors, and the remaining variation was explained by individual-specific environmental factors. An important next step will be to determine the high-risk environments that apply to working-age adults, because most of the environments that have been identified in college-aged adults (Patrick and Azar, 2018) do not apply to this older age group. For example, the present study identified 2 potential individual-specific environmental factors contributing to HID propensity in working-age adults: lower educational attainment, and, among women, never having married.2 In addition, previous genetically informed studies have demonstrated that involvement in romantic partnerships can constrain the influence of genetic propensity for heavy drinking (Barr et al., 2017; Heath et al., 1989). This suggests that being in a committed relationship may have a protective influence by muting the genetic propensity to engage in heavy drinking. The protective influence of being in a committed relationship will be an important area of future research on the genetic epidemiology of HID.
There was no evidence for quantitative or qualitative sex differences in the genetic contribution to HID. In the full unconstrained biometric model, the heritability estimates for HID propensity for men and women were quite similar: 38% among men and 41% among women. Although the estimate of the genetic correlation of 0.01 among opposite-sex twin pairs appeared quite disparate from the correlation of 0.50 among same-sex pairs, the difference was not significant; this lack of evidence for significant qualitative sex differences could be due to low statistical power. To date, the only previous human study of HID was conducted among men (Slutske et al., 1999), and previous studies of max drinks were not able to examine qualitative sex differences because they did not include opposite-sex twin pairs (Dick et al., 2011; Kendler et al., 2010). Future research should continue to explore potential sex differences in the sources of genetic influences on HID-related phenotypes, particularly given the recent evidence of sex-specific genetic effects for the “high drinking in the dark” phenotype in mice (Iancu et al., 2018).
Limitations
This study has some limitations. Although the maximum drink proxy was bolstered by corroborating patterns of past-year drinking, future research should also query the frequency of HID directly. The development of psychometrically sound assessments focused specifically on HID will be important given that individuals drinking at this level are more likely to experience memory impairment and blackout, and to therefore be less reliable reporters of their drinking behavior (Northcote and Livingston, 2011).
Given the superior psychometric properties of continuous measures (Markon et al., 2011), the use of a categorical measure of alcohol use might be questioned. However, a useful property of categorical measures is that they better align with the categorical treatment decisions made by clinicians and that are also used by public health workers to gauge the treatment needs of a population (Kessler, 2002). The single-item categorical measure of binge drinking has been embraced by the NIAAA for just this reason and is now being routinely used in primary care (NIAAA 2007; Saitz, Cheng, Allensworth-Davies, Winter, and Smith, 2014). By dividing the binary construct of binge drinking into 3 levels of severity, HID has some of the benefits of both continuous and categorical approaches to measurement.
Although 1 of the goals of this study was to study HID in an understudied segment of the population, the generalizability of the findings might be limited by focusing on working-age adults. In particular, there is evidence from twin studies of alcohol involvement that the contribution of genetic and common environmental influences change across development (e.g., Deutsch, Wood, and Slutske, 2017; Kendler et al., 2008, 2012). In particular, the proportions of variation in HID propensity due to common environmental influences would likely be higher among adolescents and college-aged young adults than in the present study. Additionally, per capita alcohol use in Australia was particularly high during the time period when these data were collected, and therefore may not reflect the current state of alcohol consumption patterns (Australian Institute of Health and Welfare, 2016). Importantly, the birth cohort represented in this sample has been found to evidence higher drinking rates than more recent cohorts born in the 1990s, leaving open the possibility that the prevalence rates presented here may be due to a cohort effect (Livingston et al., 2016). Nonetheless, such a HID-enriched sample also represents a strength in the effort to better understand the factors associated with the engagement in HID.
There may also be limits to the generalizability of this study owing to the sample being Australians of primarily northern European ancestry. The similarity of the estimates of genetic and environmental influences to the previous twin studies of HID-like measures conducted in the US and Finland suggests that the biometric results are not specific to the Australian context. However, the US and Finnish samples are also predominantly of European ancestry (>90%; Dick et al., 2011; Henderson et al., 1990). It is critically important that future research includes more racially and ethnically diverse samples.
CONCLUSIONS
This study makes 2 important contributions to the literature. First, it convincingly demonstrates that HID is not restricted to college-aged young adults, but also can be highly prevalent among working-age adults. Whether there are other settings where the prevalence of HID among post–college-aged adults is as high as in the present study remains an important question for future research. Furthermore, the high rates with which adults engage in this high-risk drinking behavior highlight the importance of developing approaches to more effectively mitigate the harms of HID in this population. Second, it demonstrates that there are important genetic influences that may increase the propensity to engage in HID. Moving forward, it will be important to be mindful of the role of genetic predisposition as well as contextual factors in our emerging understanding of the factors that influence the occurrence of HID.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIDA DA18267 and NIAAA T32AA013526. We thank Richard Parker, Soad Hancock, Judith Moir, Sally Rodda, Pieta-Maree Shertock, Heather Park, Jill Wood, Pam Barton, Fran Husband, and Adele Somerville, who worked on this project, and the twins and their siblings for participating. Preliminary results from this study were presented as a poster at the 2018 Research Society on Alcoholism Annual Scientific Meeting. This manuscript has not been published or simultaneously submitted elsewhere.
Footnotes
Two twins from a pair were 40 years of age.
Cross-twin cross-trait correlations of educational attainment and marital status with HID were nearly all modest and nonsignificant, suggesting that educational attainment and marital status represent unique environmental characteristics that contribute to HID independent of genetic or shared environmental infiuences (see Table S2).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Contributor Information
Genevieve F. Dash, Department of Psychological Sciences, University of Missouri- Columbia, Columbia, Missouri
Christal N. Davis, Department of Psychological Sciences, University of Missouri- Columbia, Columbia, Missouri
Nicholas G. Martin, Queensland Institute of Medical Research (QIMR) Berghofer, Brisbane, QLD, Australia
Dixie J. Statham, Department of Psychology, Federation University, Ballarat, Vic., Australia
Michael T. Lynskey, Department of Addictions, King’s College London Institute of Psychiatry, Psychology and Neuroscience, London, UK.
Wendy S. Slutske, Department of Psychological Sciences, University of Missouri- Columbia, Columbia, Missouri
REFERENCES
- Agrawal A, Grant JD, Littlefield A, Waldron M, Pergadia ML, Lynskey MT, Madden PA, Todorov A, Trull T, Bucholz KK, Todd RD (2009) Developing a quantitative measure of alcohol consumption for genomic studies on prospective cohorts. J Stud Alcohol Drugs 70:157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Institute of Health and Welfare (2016) Trends in alcohol availability, use and treatment 2003–2004 to 2014–2015. Retrieved from https://www.aihw.gov.au/reports/alcohol/trends-in-alcohol-availabilityuse-and-treatment-2003-04-to-2014-15
- Barkley-Levenson AM, Crabbe JC (2014) High drinking in the dark mice: a genetic model of drinking to intoxication. Alcohol 48:217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr PB, Salvatore JE, Maes HH, Korhonen T, Latvala A, Aliev F, Viken R, Rose RJ, Kaprio J, Dick DM (2017) Social relationships moderate genetic influences on heavy drinking in young adulthood. J Stud Alcohol Drugs 78:817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blecher E, Liber A, Van Walbeek C, Rossouw L (2018) An international analysis of the price and affordability of beer. PLoS ONE 13:e0208831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI Jr, Reich T, Schmidt I, Schuckit MA (1994) A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol 55:149–158. [DOI] [PubMed] [Google Scholar]
- Chartier K, Caetano R (2010) Ethnicity and health disparities in alcohol research. Alcohol Res Health: the journal of the National Institute on Alcohol Abuse and Alcoholism, 33:152–160. [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Rhodes JS, Yu CH, Brown LL, Phillips TJ, Finn DA (2009) A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. Biol Psychiat 65:662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Spence SE, Brown LL, Metten P (2011) Alcohol preference drinking in a mouse line selectively bred for high drinking in the dark. Alcohol 45:427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch AR, Wood PK, Slutske WS (2017) Developmental etiologies of alcohol use and their relations to parent and peer influences over adolescence and young adulthood: a genetically informed approach. Alcohol Clin Exp Res 41:2151–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Meyers JL, Rose RJ, Kaprio J, Kendler KS (2011) Measures of current alcohol consumption and problems: two independent twin studies suggest a complex genetic architecture. Alcohol Clin Exp Res 35:2152–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PAF, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG (1997) Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med 27:1381–1396. [DOI] [PubMed] [Google Scholar]
- Heath AC, Jardine R, Martin NG (1989) Interactive effects of genotype and social environment on alcohol consumption in female twins. J Stud Alcohol 50:38–48. [DOI] [PubMed] [Google Scholar]
- Henderson WG, Eisen S, Goldberg J, True WR, Barnes JE, Vitek ME (1990) The Vietnam Era Twin Registry: A resource for medical research. Public Health Rep 105:368–373. [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Zha W, White AM (2017) Drinking beyond the binge threshold: predictors, consequences, and changes in the US. Am J Prev Med 52:717–727. [DOI] [PubMed] [Google Scholar]
- Iancu OD, Colville AM, Wilmot B, Searles R, Darakjian P, Zheng C, McWeeney S, Kawane S, Crabbe JC, Metten P, Oberbeck D (2018) Gender-specific effects of selection for drinking in the dark on the network roles of coding and noncoding RNAs. Alcohol Clin Exp Res 42:1454–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, St. Pierre CL, Meyers JL, Aliev F, McCutcheon VV, Lai D, Dick DM, Goate AM, Kramer J, Kuperman S, Nurnberger JI Jr (2019) The genetic relationship between alcohol consumption and aspects of problem drinking in an ascertained sample. Alcohol Clin Exp Res.43:1113–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS (1993) Twin studies of psychiatric illness: current status and future directions. Arch Gen Psychiatry 50:905–915. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Chen X, Dick D, Maes H, Gillespie N, Neale MC, Riley B (2012) Recent advances in the genetic epidemiology and molecular genetics of substance use disorders. Nat Neurosci 15:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Lönn SL, Salvatore J, Sundquist J, Sundquist K (2016) Effect of marriage on risk for onset of alcohol use disorder: a longitudinal and co-relative analysis in a Swedish national sample. Am J Psychiatry 173:911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Dick D, Prescott CA (2010) The relationship between genetic influences on alcohol dependence and on patterns of alcohol consumption. Alcohol Clin Exp Res 34:1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, Prescott CA (2008) Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch Gen Psychiatry 65:674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC (2002) The categorical versus dimensional assessment controversy in the sociology of mental illness. J Health Soc Behav 43:171–188. [PubMed] [Google Scholar]
- Kuo PH, Neale MC, Riley BP, Webb BT, Sullivan PF, Vittum J, Patterson DG, Thiselton DL, Van Den Oord EJ, Walsh D, Kendler KS (2006) Identification of susceptibility loci for alcohol-related traits in the Irish Affected Sib Pair Study of Alcohol Dependence. Alcohol Clin Exp Res 30:1807–1816. [DOI] [PubMed] [Google Scholar]
- Laborde ND, Mair C (2012) Alcohol use patterns among postpartum women. Matern Child Health J 16:1810–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden-Carmichael AN, Vasilenko SA, Lanza ST, Maggs JL (2017) High-intensity drinking versus heavy episodic drinking: Prevalence rates and relative odds of alcohol use disorder across adulthood. Alcohol Clin Exp Res 41:1754–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston M, Callinan S, Dietze P, Stanesby O, Kuntsche E (2018) Is there gender convergence in risky drinking when taking birth cohorts into account? Evidence from an Australian national survey 2001–13. Addiction 113:2019–2028. [DOI] [PubMed] [Google Scholar]
- Livingston M, Raninen J, Slade T, Swift W, Lloyd B, Dietze P (2016) Understanding trends in Australian alcohol consumption—an age–period–cohort model. Addiction 111:1590–1598. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Agrawal A, Henders A, Nelson EC, Madden PA, Martin NG (2012) An Australian twin study of cannabis and other illicit drug use and misuse, and other psychopathology. Twin Res Hum Genet 15:631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons AC, Willott SA (2008) Alcohol consumption, gender identities and women’s changing social positions. Sex Roles 59:694–712. [Google Scholar]
- Markon KE, Chmielewski M, Miller CJ (2011) The reliability and validity of discrete and continuous measures of psychopathology: a quantitative review. Psychol Bull 137:856–879. [DOI] [PubMed] [Google Scholar]
- McClearn GE, Rodgers DA (1961) Genetic factors in alcohol preference of laboratory mice. J Comp Physiol Psychol 54:116–119. [Google Scholar]
- Midford R (2005) Australia and alcohol: living down the legend. Addiction 100:891–896. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO (2017) Mplus. Muthén & Muthén, Los Angeles. National Institute on Alcohol Abuse and Alcoholism (2004) NIAAA Council approves definition of binge drinking. NIAAA Newsl; 3:003. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (2007) Helping Patients who Drink Too Much: A Clinician’s Guide: Updated 2005 Edition (No. 7) US Department of Health and Human Services, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism; Retrieved from https://www.paramounthealthcare.com/assets/documents/clinicalpractice-guidelines/Alcohol-Adultguideline-USPSTF.pdf [Google Scholar]
- Neale MC, Cardon LR (1992) Methodology for Genetic Studies of Twins and Families. Springer, Dordrecht. [Google Scholar]
- Northcote J, Livingston M (2011) Accuracy of self-reported drinking: observational verification of ‘last occasion’ drink estimates of young adults. Alcohol Alcohol 46:709–713. [DOI] [PubMed] [Google Scholar]
- Pan Y, Luo X, Liu X, Wu LY, Zhang Q, Wang L, Wang W, Zuo L, Wang KS (2013) Genome-wide association studies of maximum number of drinks. J Psychiatr Res 47:1717–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME (2016) A call for research on high-intensity alcohol use. Alcohol Clin Exp Res 40:256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Azar B (2018) High-intensity drinking. Alcohol Res Curr Rev 39:49–55. [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Cronce JM, Fairlie AM, Atkins DC, Lee CM (2016) Day-to-day variations in high-intensity drinking, expectancies, and positive and negative alcohol-related consequences. Addict Behav 58:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Terry-McElrath YM (2017) High-intensity drinking by underage young adults in the United States. Addiction 112:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Terry-McElrath YM, Kloska DD, Schulenberg JE (2016) High-intensity drinking among young adults in the United States: prevalence, frequency, and developmental change. Alcohol Clin Exp Res 40:1905–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge PC, Park A, Sher KJ (2008) 21st birthday drinking: extremely extreme. J Consult Clin Psychol. 76:511–516. 10.1037/0022006X.76.3.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T Jr, Crabbe JC (2007) Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav 6:1–18. [DOI] [PubMed] [Google Scholar]
- Saccone NL, Kwon JM, Corbett J, Goate A, Rochberg N, Edenberg HJ, Foroud T, Li TK, Begleiter H, Reich T, Rice JP (2000) A genome screen of maximum number of drinks as an alcoholism phenotype. Am J Med Genet Part A 96:632–637. [DOI] [PubMed] [Google Scholar]
- Saitz R, Cheng DM, Allensworth-Davies D, Winter MR, Smith PC (2014) The ability of single screening questions for unhealthy alcohol and other drug use to identify substance dependence in primary care. J Stud Alcohol Drugs 75:153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, True WR, Scherrer JF, Heath AC, Bucholz KK, Eisen SA, Goldberg J, Lyons MJ, Tsuang MT (1999) The heritability of alcoholism symptoms: “Indicators of genetic and environmental influence in alcohol-dependent individuals” revisited. Alcohol Clin Exp Res 23:759–769. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Dowdall GW, Davenport A, Rimm EB (1995) A gender-specific measure of binge drinking among college students. Am J Public Health 85:982–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RJ, Berry LJ, Berkstetcher E Jr (1949) Biochemical individuality. 3. Genetotrophic factors in the etiology of alcoholism. Arch Biochem 23:275–290. [PubMed] [Google Scholar]
- Wilsnack RW, Vogeltanz ND, Wilsnack SC, Harris TR (2000) Gender differences in alcohol consumption and adverse drinking consequences: cross-cultural patterns. Addiction 95:251–265. [DOI] [PubMed] [Google Scholar]
- Wilsnack RW, Wilsnack SC, Kristjanson AF, Vogeltanz-Holm ND, Gmel G (2009) Gender and alcohol consumption: patterns from the multinational GENACIS project. Addiction 104:1487–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2011) Global Status Report on Alcohol and Health. World Health Organization, Le Mont-sur-Lausanne, Switzerland. [Google Scholar]
- Xu K, Kranzler HR, Sherva R, Sartor CE, Almasy L, Koesterer R, Zhao H, Farrer LA, Gelernter J (2015) Genomewide association study for maximum number of alcoholic drinks in European Americans and African Americans. Alcohol Clin Exp Res 39:1137–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.