The macrophage-rich core of advanced human atheroma has been shown to be hypoxic, which may have implications for plaque stability.1 Recently, we showed that the hypoxia PET imaging agent 64Cu-ATSM could be used for visualizing hypoxic cells in atherosclerosis in animal models.2 In this study, we investigated the feasibility of 64Cu-ATSM PET/MR imaging for detecting hypoxic cells in human atherosclerotic plaque.
The cellular retention mechanism of 64Cu-ATSM is that in vitro Cu-ATSM undergoes bioreductive trapping under hypoxic conditions. Cu-ATSM Cu(II) is a lipophilic molecule, with high membrane permeability and low redox potential, and is reduced only in hypoxic cells. Upon the intracellular reduction, Cu-ATSM becomes trapped irreversibly and thus the radioactivity accumulates in these cells.3
This prospective human study was approved by the Institutional Review Board (IRB) at Washington University and all patients provided informed consent. The data that support the findings of this study are available from the corresponding author upon reasonable request. Images of sufficient diagnostic quality for further analysis were obtained in 7 neurologically asymptomatic patients (74.3 ± 6.2 years old) with carotid atherosclerosis and ≥50% diameter stenosis in at least one carotid artery. Dynamic PET imaging was done 0-60 min after IV injection of 740-925 MBq 64Cu-ATSM with simultaneously-acquired T1-, T2-, PD-, and TOF-weighted MR imaging of carotid plaque. Five patients also underwent dynamic contrast-enhanced MR imaging (DCE-MRI) afterwards (20 timepoints and 12.5-sec image interval) with 0.1 mmol/kg gadolinium-based contrast agent injected at 0.5 mL/sec. The averaged 30-60 min 64Cu-ATSM PET acquisition was reconstructed with 1.04×1.04×2.03 mm3 resolution. Volumes-of-interests were drawn on the carotid plaques on MR images to determine the maximum standard uptake values (SUVmax). As a reference, the activity of the background (SUVbkg) was calculated as the mean SUV of the blood pool within adjacent internal jugular veins. The target-to-background SUV ratios (TBRmax) defined as SUVmax corrected for SUVbkg were calculated.
Among these 7 patients there were 8 carotid arteries with stenosis of ≥50% diameter (one patient with bilateral carotid stenoses). Of these 8 arteries, 3 left arteries displayed 64Cu-ATSM focal uptake (TBRmax>>1.6; Figure 1, A–J). The TBRmax of the 3 contralateral carotid arteries and all 5 PET-negative carotid plaques as indicated by lack of detection of focal 64Cu-ATSM retention (1.48 ± 0.28 and 1.53 ± 0.17, respectively) were significantly lower (p < 0.05 and p < 0.001) than those values of the plaques with positive detection of 64Cu-ATSM hot spots (2.88 ± 0.17). Volume transfer constant (Ktrans) maps determined by describing DCE-MRI with the Patlak model was in line with literature that peak Ktrans values (0.26 ± 0.16 min−1) were identified at the highly vascularized outer rim of the plaques.4
FIGURE 1. Imaging of hypoxic cells in carotid atherosclerosis.
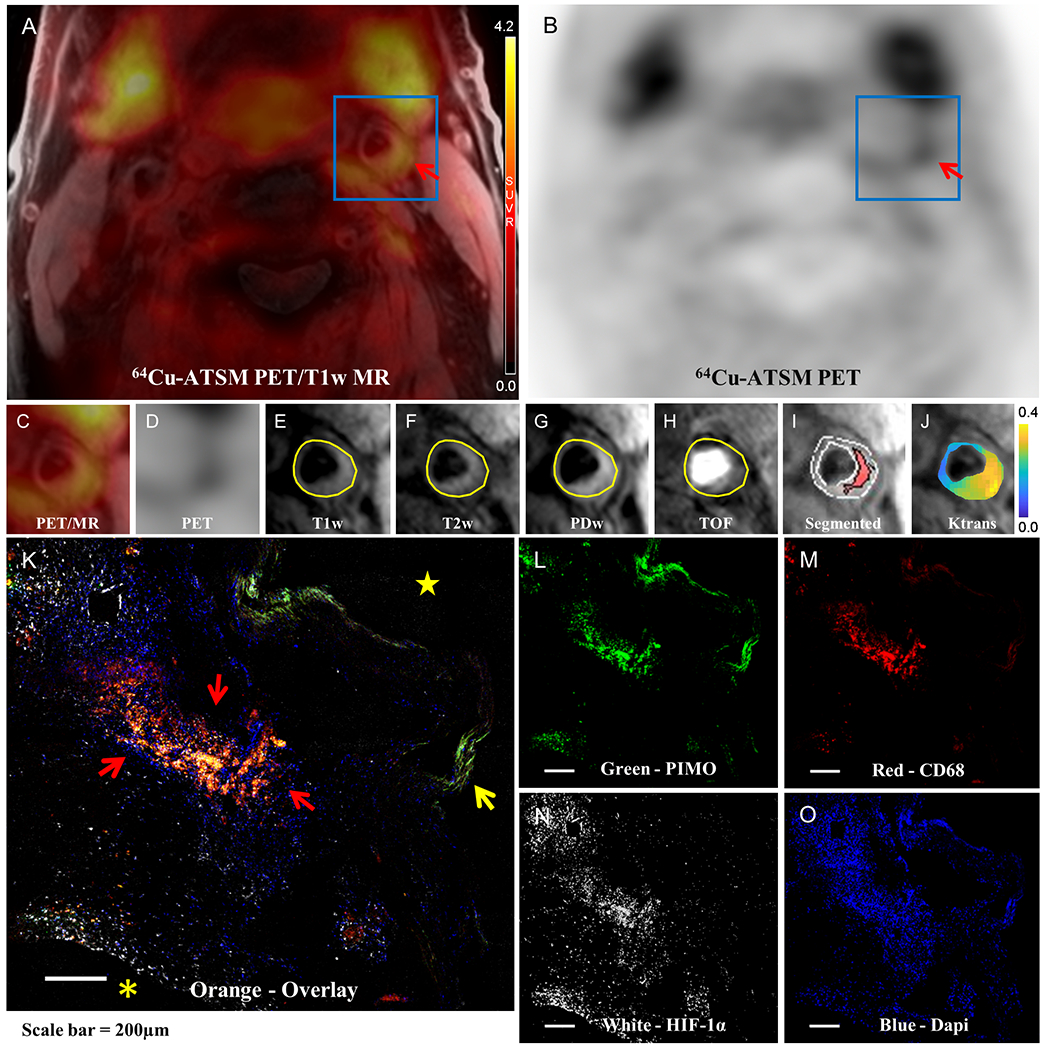
Focal 64Cu-ATSM uptake was detected in the plaque (red arrow) of the left carotid artery of a representative 69-year-old male patient as displayed in (A) 64Cu-ATSM PET/T1-weighted MR fused image and (B) 64Cu-ATSM PET only image. 64Cu-ATSM retention was also identified in the adjacent salivary glands, and a small amount of uptake is seen in muscle. (C) and (D) are the zoomed-in view of (A) and (B) with the region of interest (ROI) placed on the carotid artery (blue box). Plaque components of intraplaque hemorrhage and lipid-rich necrotic core (LRNC) are outlined in the corresponding (E) T1-weighted MRI, (F) T2-weighted MRI, (G) PD-weighted MRI, (H) TOF MRI. (I) Segmented T1-weighted MR image shows that there was one large region of hemorrhage & LRNC core comprising 25.6% of the entire plaque area. (J) Parametric map of Ktrans values color coded from 0 to 0.4 min−1 and overlaid on anatomic MR image suggest that the highly vascularized adventitia at the outer rim has the highest Ktrans values. The IHC staining of (L) PIMO for hypoxic cells, (M) CD68 for macrophages, (N) HIF-1α for hypoxia-inducible factor-1α and (O) DAPI (4′,6-diamidino-2-phenylindole) for nuclei on adjacent slices of CEA specimen slices from this patient were co-localized as shown in orange areas (red arrow) of (K) the overlaid image. Internal elastic lamina is denoted by the yellow arrow. Asterisk and star refer to the extracellular space and carotid artery lumen, respectively.
Of the 7 patients imaged, 3 went to surgery and had carotid endarterectomy (CEA) specimens available. Of the 3 CEA specimens 2 were collected from PET-positive plaques and 1 was from a PET-negative plaque. Analysis of the CEA specimens from the PET-positive plaques using IHC revealed that pimonidazole (PIMO) and HIF-1α positive cells co-localized with the macrophage marker CD68, as demonstrated by the overlay from slides of adjacent sections (Figure 1, K–O), whereas no PIMO or HIF-1α uptake was identified in the specimen of the PET-negative plaque. This suggests the presence of hypoxic macrophages in these specific carotid plaques.
In summary, we confirmed the feasibility of 64Cu-ATSM PET/MR imaging for identifying hypoxic cells within human carotid atherosclerosis. A nearly 3-fold TBRmax was observed in the plaques having 64Cu-ATSM retention, which is close to the published results using animal models with atherosclerotic-like lesions.2
The uptake of 64Cu-ATSM was found centrally within lipid-rich cells of the plaque on MRI that would correspond to regions of macrophage-rich necrotic core. This conclusion was further validated by IHC staining showing that the macrophages were the hypoxic cells that were the targets of the 64Cu-ATSM tracer. The demonstration that 64Cu-ATSM is retained in hypoxic macrophages in advanced atherosclerosis was shown previously through ex vivo co-localization of PIMO and CD68 IHC staining with 64Cu-ATSM autoradiography in preclinical studies.2
Although 18F-fluorodeoxyglucose (FDG) is the most widely validated PET tracer for evaluation of atherosclerotic inflammation based on the glucose metabolism of macrophages,2 64Cu-ATSM which targets hypoxic cells has the potential to provide complementary information for detection of advanced atherosclerosis in humans. 18F-FMISO has also been used to detect plaque hypoxia.5 Compared to 18F-FMISO, 64Cu-ATSM has two major advantages: higher cellular uptake and faster washout from normoxic tissue, resulting in earlier imaging after tracer injection and higher TBRmax (0-60 vs 120-180 min and 2.9 vs ~1.1).5
Although the results are preliminary, utilizing 64Cu-ATSM PET imaging for identification of hypoxic cells, in association with MRI may be useful as a method of stratifying asymptomatic patients, determining which patients have plaques that may rupture and benefit from the surgical intervention of CEA, and which patients may be treated more conservatively by optimal medical therapy.
ACKNOWLEDGEMENTS
We thank the staff of the Center for Clinical Imaging and Research (CCIR) at the Washington University School of Medicine (WUSM) for their technical support in PET/MR image acquisition and the cyclotron facility of the Mallinckrodt Institute of Radiology (MIR) at WUSM for supplying 64Cu.
SOURCE OF FUNDING
This research received funding from the Diabetic Cardiovascular Disease Center (DCDC), Washington University School of Medicine, the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448 from the National Center for Advancing Translational Sciences (NCATS) of NIH, Department of Energy grant DESC0002032, and NIH National Research Service Award (5-T32-HL07081-38) from the National Heart, Lung and Blood Institute. The vascular surgery biobank samples are in part supported by K08 HL132060 the NHLBI.
Footnotes
DISCLOSURES
There is no conflict of interest to disclose.
REFERENCES
- 1.Sluimer JC, Gasc JM, van Wanroij JL, Kisters N, Groeneweg M, Sollewijn Gelpke MD, Cleutjens JP, van den Akker LH, Corvol P, Wouters BG, et al. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol. 2008;51:1258–65. [DOI] [PubMed] [Google Scholar]
- 2.Nie X, Laforest R, Elvington A, Randolph GJ, Zheng J, Voller T, Abendschein DR, Lapi SE and Woodard PK. PET/MRI of Hypoxic Atherosclerosis Using 64Cu-ATSM in a Rabbit Model. J Nucl Med. 2016;57:2006–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujibayashi Y, Taniuchi H, Yonekura Y, Ohtani H, Konishi J and Yokoyama A. Copper-62-ATSM: a new hypoxia imaging agent with high membrane permeability and low redox potential. J Nucl Med. 1997;38:1155–60. [PubMed] [Google Scholar]
- 4.Gaens ME, Backes WH, Rozel S, Lipperts M, Sanders SN, Jaspers K, Cleutjens JP, Sluimer JC, Heeneman S, Daemen MJ, et al. Dynamic contrast-enhanced MR imaging of carotid atherosclerotic plaque: model selection, reproducibility, and validation. Radiology. 2013;266:271–9. [DOI] [PubMed] [Google Scholar]
- 5.Joshi FR, Manavaki R, Fryer TD, Figg NL, Sluimer JC, Aigbirhio FI, Davenport AP, Kirkpatrick PJ, Warburton EA and Rudd JH. Vascular Imaging With (18)F-Fluorodeoxyglucose Positron Emission Tomography Is Influenced by Hypoxia. J Am Coll Cardiol. 2017;69:1873–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]


