Abstract
Noma (canrum oris) is a mutilating necrotizing disease of uncertain etiology, but it is accepted that it is caused primarily by a polybacterial infection with secondary ischemia. The consequent necrotizing fasciitis, myonecrosis, and osteonecrosis results in destruction of facial structures with severe functional impairment and disfigurement. It most frequently affects children, particularly in sub‐Saharan Africa, who are malnourished or debilitated by systemic conditions including but not limited to malaria, measles, and tuberculosis; and less frequently debilitated HIV‐seropositive subjects. In the vast majority of cases, in susceptible subjects, noma is preceded by necrotizing stomatitis. However, it has been reported, albeit rarely, that noma can arise without any preceding oral lesions being observed. Noma is not recurrent and is not transmissible.
1. INTRODUCTION
Noma (cancrum oris) is a destructive, disfiguring, necrotizing disease affecting the orofacial tissues, predominantly of young malnourished children in central Africa (Niger, Nigeria) where HIV infection has not been reported to play a role, unlike in southern Africa (Zimbabwe, South Africa), where it appears to be an important co‐factor, although the age distribution of affected subjects is wider. Noma has a fulminating course, and if untreated is fatal in most cases. Complex dynamic interactions between dento‐gingival polybacterial plaque and host immune impairment, malnutrition, general state of debilitation, and environmental and socio‐economic factors play important roles in the pathogenesis of noma (Table 1).1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11
Table 1.
Risk factors associated with noma
| Host risk factors | Social/environmental risk factors | ||
|---|---|---|---|
| Systemic | Local | ||
| Malnutrition | Poor dento‐gingival bacterial plaque control | Young children living in impoverished circumstances | Lack of subject/parental education |
| HIV/AIDS | Subclinical oral viral infections | Living in communities with a high prevalence of HIV infection | Delay in seeking medical treatment |
| Cytokine dysregulation | |||
| Systemic bacterial or viral infections (eg malaria, measles) | Compromised oral immunity | Poor living conditions | Inadequate healthcare facilities |
| Persistent diarrhea | Lack of sanitation | Limited access to healthcare facilities | |
| Dehydration | |||
The word ‘noma’ originates from the Greek verb ‘nomein’, which means ‘to devour’, reflecting the rapidly progressive, aggressive ‘tissue eating’ nature of the disease12, 13; and the name ‘cancrum oris’, a term for noma introduced in Great Britain in the 17th century, is an incorrect use of the Latin term ‘cancer oris’, but which has gained currency despite being ‘an odd grammatical blunder’.14 Because of the context in which it almost always occurs, noma has been referred to as the ‘face of poverty’.15
Necrotizing gingivitis, the first link in the chain of clinical conditions culminating in noma, is a polybacterial infection which extends to become necrotizing periodontitis and then necrotizing stomatitis. In a small minority of cases, necrotizing stomatitis will rapidly progress to necrotizing fasciitis, myonecrosis, and osteonecrosis, resulting in a full thickness destruction of oral mucosa, muscle and skin, manifesting as noma (Figure 1).5, 11, 16, 17 However, it has been reported, albeit rarely, that noma can arise without any preceding necrotizing oral lesions.10 Noma is not recurrent and is not transmissible.1, 13, 18, 19
Figure 1.
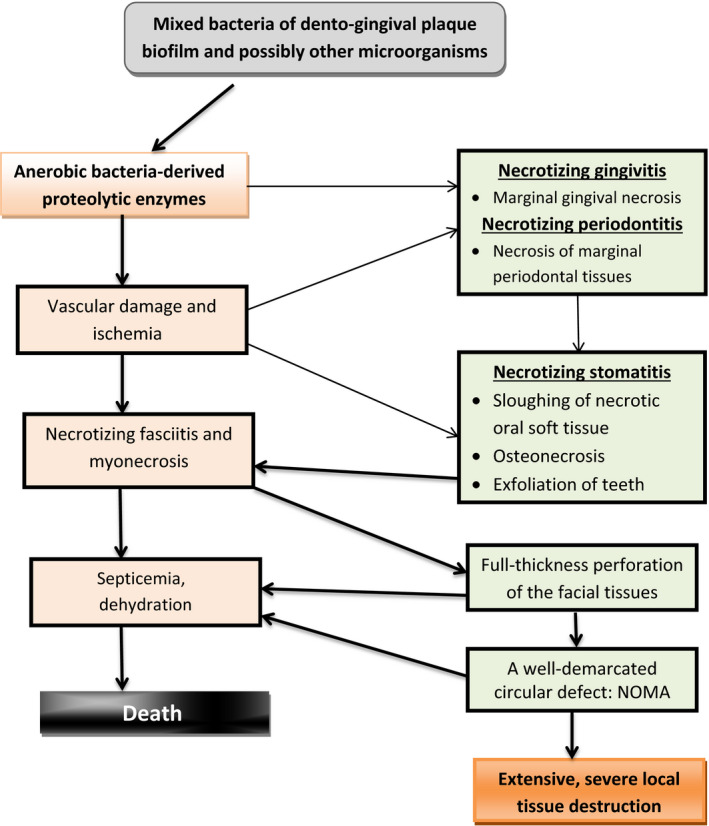
Pathogenic events in noma, and its clinical course
Early diagnosis and prompt antibiotic treatment of any of the intra‐oral necrotizing diseases which may progress to noma will prevent the development of the disease.16, 20 Subjects with necrotizing stomatitis or noma have to be urgently admitted to hospital for intravenous broad spectrum antibiotic treatment, for fluids and electrolytes, for nutritional supplementation, and for comprehensive medical care.11, 16
Noma survivors will experience severe functional impairment, facial mutilation, and tragic psycho‐social consequences. Ideally, therefore, public health measures should be put in place to enable qualified personnel to reach populations at risk and to institute effective programs for prevention, early detection, and treatment of necrotizing oral diseases or noma.9, 21, 22
In order to formulate evidenced‐based guidelines for prevention of noma, all cases of necrotizing stomatitis and noma ought to be reported and documented. This would allow the establishment of a data bank with regard to the epidemiological, clinical, bacteriological, environmental, and perhaps pathological characteristics of noma, which would enable researchers and clinicians to gain a better understanding of the disease.1, 5 However, without addressing the critical issues of poverty and lack of education, there is not much hope of reducing the incidence of noma.12
2. CLINICAL FEATURES AND COURSE OF THE DISEASE
Noma has a fulminant course. Subjects with acute noma are usually debilitated, feverish, dehydrated, anemic, and in pain.2, 4, 11, 17, 18, 23 Subjects with severe necrotizing stomatitis or acute noma require urgent hospital admission for treatment with broad spectrum antibiotics, fluid and electrolytes, and nutritional supplementation.5, 11, 17 Without urgent treatment, most subjects with severe noma will die, usually from septicemia, dehydration, or malnutrition.10, 24
The sequence of clinical events leading to noma starts as the polybacterial infection, necrotizing gingivitis (Figure 2A), characterized by bleeding, marginal gingival necrosis and pain, but also almost invariably by foetor ex ore and regional lymphadenopathy.16, 25, 26 The term ‘necrotizing gingivitis’ is preferred to the frequently used term ‘necrotizing ulcerative gingivitis’ because ulceration is merely a description of the outcome of the necrotic process.16
Figure 2.
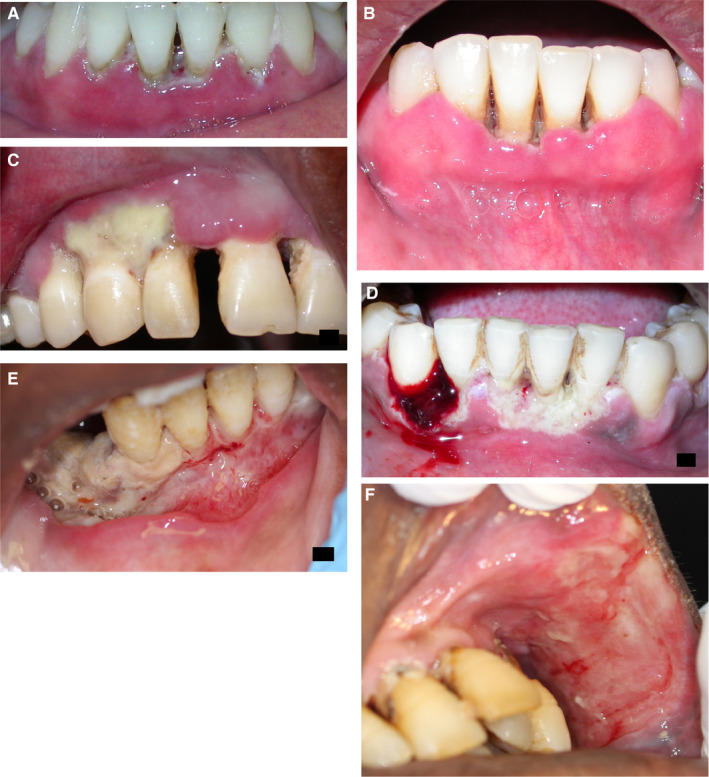
Necrotizing oral diseases in HIV‐seropositive patients. In these cases the lesions did not progress to noma. (A) Necrotizing gingivitis of the interdental papillae and the marginal gingiva (J Oral Pathol Med 2014; 43:1‐6, reproduced with permission24). (B) Necrotizing periodontitis of the marginal periodontal attachment apparatus (J Oral Pathol Med 2014; 43:1‐6, reproduced with permission24). (C‐E) Necrotizing stomatitis of the alveolar mucosa (J Oral Pathol Med 2014; 43:1‐6, reproduced with permission24). (F) Necrotizing stomatitis of the left buccal mucosa (SADJ 2014; 69:468‐470, reproduced with permission35)
Owing to local immune suppression and to bacterial virulence factors, the process of necrotizing gingivitis may spread, extending into the marginal periodontal attachment apparatus to become necrotizing periodontitis (Figure 2B). Untreated, necrotizing gingivitis/necrotizing periodontitis, particularly in an immuno‐suppressed, malnourished, debilitated subject may progress, extending beyond the mucogingival junction, when it is called necrotizing stomatitis (Figure 2C‐F). However, very rarely, necrotizing stomatitis may develop de novo.5, 16
Necrotizing stomatitis is characterized by destruction of oral soft tissues and bone, and without treatment in a minority of cases, the necrotizing process will spread rapidly to the mucosa of the cheek or lip with subsequent full thickness destruction of the mucosa, muscle, and skin (Figure 3). The affected tissues become macerated, friable, swollen, and blue‐gray in color on the skin side (Figure 4A,B). The tissues then rapidly slough leaving a full thickness circular defect, referred to as noma (Figure 5).5, 10, 11, 16 The factors that determine the extent of the orofacial defect are unknown.
Figure 3.
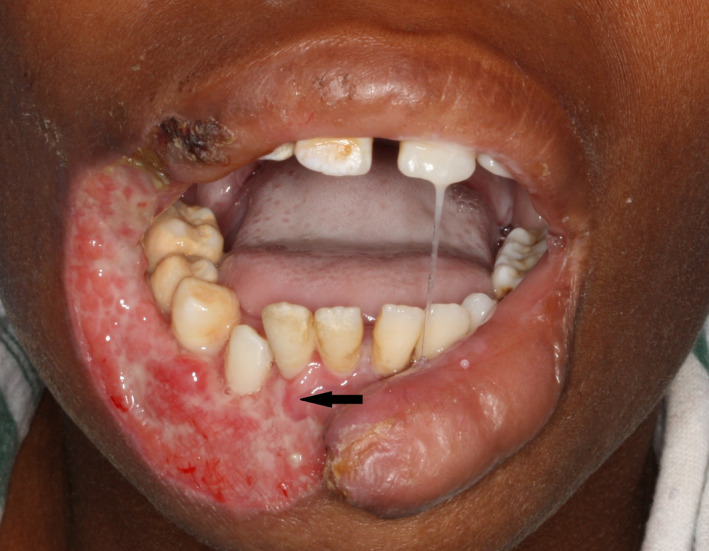
Necrosis of the right‐side of the lower lip in an HIV‐seropositive highly active antiretroviral treatment‐naïve child with a CD4 + T cell count of 13 cells/mm3. The parents reported that this lesion had developed quite rapidly (SADJ 2014; 69:468‐470, reproduced with permission35)
Figure 4.
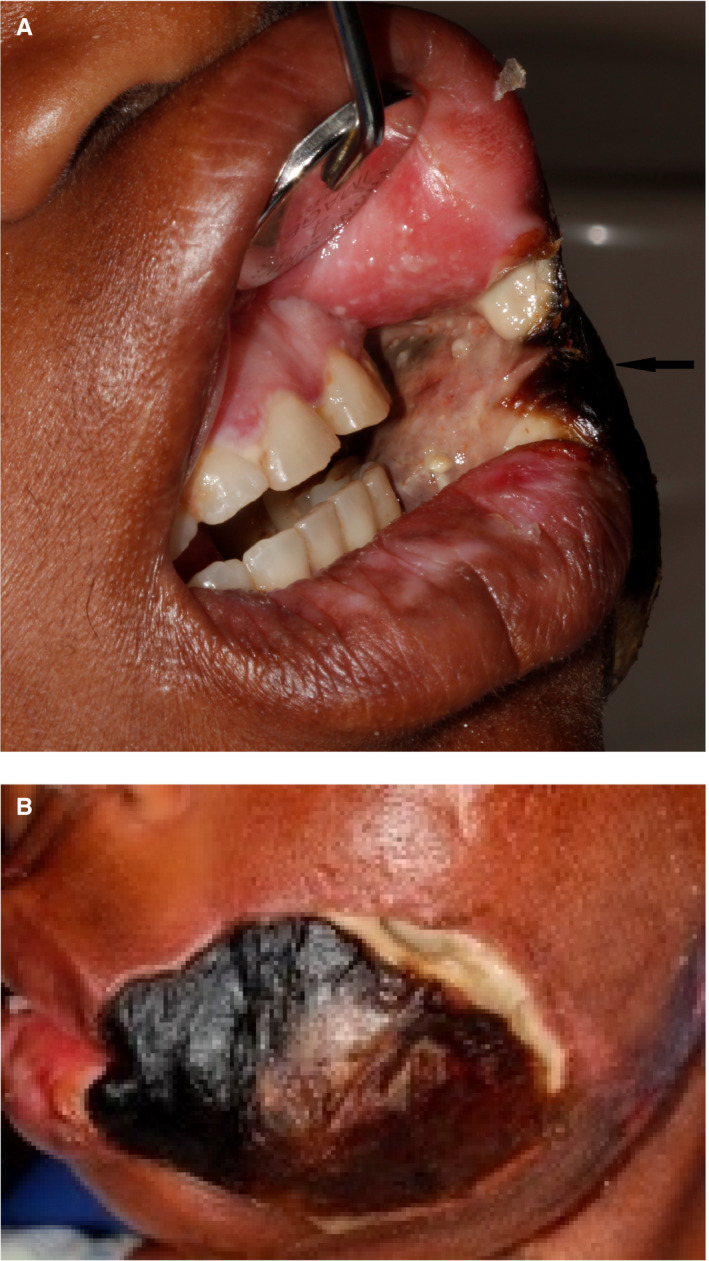
(A) Necrosis of the left buccal mucosa in a 43‐y‐old HIV‐seropositive female with a CD4 + T cell count of 4 cells/mm3, already involving the skin. According to the patient the lesion seen in (A) involving the skin of the cheek (B) developed within 3 d of the buccal mucosal lesion being noticed
Figure 5.
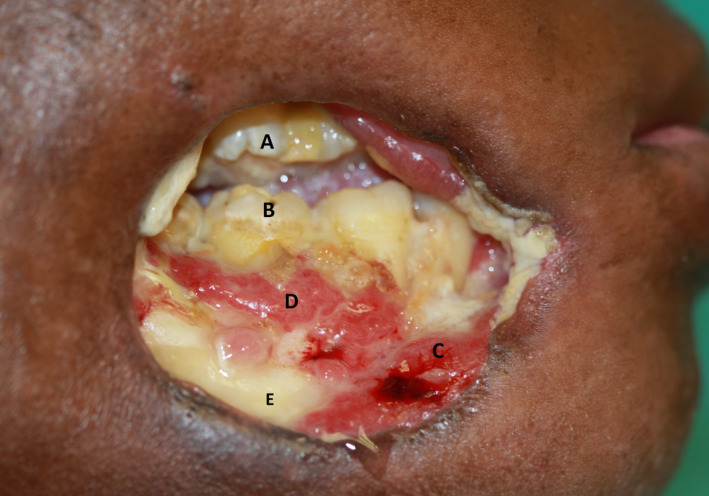
Noma of the right cheek of uncertain duration. (A) Maxillary molars; (B) mandibular molars; (C, D) necrotizing periodontitis/stomatitis; (E) mandibular bone exposed by necrotizing stomatitis. Note the remarkable, characteristic circular shape of the full‐thickness defect (J Oral Pathol Med 2014; 43:1‐6, reproduced with permission24)
If the affected subject survives, the margins of the noma defect heal with extensive fibrosis and scarring resulting in limitation of mouth opening, oral incontinence, difficulty in mastication and speech, and disfigurement.1, 5, 10, 11, 18 Not uncommonly, mouth opening is severely restricted by the formation of scar tissue between the mandible and the maxilla and zygomatic arch.15, 20
The course of the disease is very rapid with progression from necrotizing stomatitis to the full thickness destruction of noma taking just a few days (Figure 4A,B).11 Indeed, the progression is so rapid that to the patient and observing family members, necrotizing stomatitis becomes noma often before the seriousness of the condition is realized. As most cases of noma occur in subjects living in remote rural areas without adequate medical facilities, and because of the high rate of mortality in the absence of early diagnosis and prompt medical intervention, there are no evidence‐based data about the sequential clinical, microbiological, or pathological events during the progression from necrotizing stomatitis to noma.1, 5, 9, 23 However, it has been reported that in some cases the necrotizing destruction of the labial and/or buccal mucosa causes a relatively small full‐thickness perforation of the skin, which only then spreads centrifugally into a large circular defect.1, 10
Owing to a lack of evidence‐based data, any statistics about noma are at best an educated guess. Available epidemiological information on noma has been gathered from retrospective studies based on unstructured case reports, and not from well‐designed prospective studies; and owing to the stigma associated with the disfigurement, many of the children affected by noma are shunned by their communities and are isolated in remote locations. Furthermore, cases of noma in rural areas are not always recognized for what they are, and as most cases of noma are neither reported nor documented, the incidence is uncertain.1, 5, 7, 9, 11, 12, 22, 24 Nevertheless, in 1997 and 1998 it was estimated that the worldwide incidence of noma was 140,000 cases per year with a prevalence of 700,000 cases,1, 18, 27 and it has been reported that about 80%‐90% of subjects with untreated acute noma will die.28 As it is believed that fewer than 15% of acute cases present for treatment, these figures for incidence and prevalence can be regarded as an underestimation.1, 27
3. ETIOPATHOGENESIS
Although noma is clearly a polybacterial necrotizing infection, this infection is not of itself sufficient to cause noma, and other co‐factors are necessary in order for the bacteria to drive the destructive necrotizing process leading to noma (Table 1). Except for malnutrition and HIV/AIDS, which certainly appear to play roles, there is only weak scientific evidence for the direct role of each of the co‐factors listed in Table 1 in the etiopathogenesis of noma.5 Complex interactions between the various co‐factors are undoubtedly necessary for the development of noma.
3.1. The role of bacteria in the etiopathogenesis of noma
It is well accepted that bacteria are essential etiological agents in the pathogenesis of noma. This is supported by the observation that numerous anerobic bacterial species including Prevotella, Spirochaetes, and Peptostreptococcus are regularly present in noma; by the fetid odour characteristic of the early active stage of noma; and by the fact that noma and its precursor diseases, necrotizing gingivitis, necrotizing periodontitis, and necrotizing stomatitis, respond well to antibiotic treatment.7, 13, 17, 18, 19
Necrotizing gingivitis is a polybacterial infection that starts at the tips of the interdental papillae and the margins of the gingiva, most probably because in these peripheral tissues the blood supply is attenuated, making them vulnerable to bacteria‐induced ischemic tissue necrosis.5, 16 The same bacteria that cause the necrotizing gingivitis may also suppress local immune responses, setting the stage for necrotizing gingivitis/necrotizing periodontitis to progress to necrotizing stomatitis in a very small number of cases of immuno‐compromised debilitated subjects (Figure 1).5
Not all the bacteria putatively implicated in the pathogenesis of noma can be cultured under laboratory conditions, and the disease cannot be induced experimentally in animals. Furthermore, some of the bacteria reportedly found in noma have been isolated only from advanced lesions and may thus represent secondary infective rather than primary causative agents. There is thus no proven causative association between any specific bacteria and noma.1, 5, 7, 13, 17
The type and density of pathogenic and potentially pathogenic bacteria may change during the progression of necrotizing gingivitis to noma owing to selective environmental pressures, with acquisition of new pathogenic bacterial species which may drive the process towards necrotizing fasciitis and myonecrosis.7, 11 On the other hand, any recruited bacteria may be merely adventitious species thriving in a microenvironment altered by tissue damage caused by the primary infection.29 However, the etiopathogenesis of infectious diseases in general can become complicated by some bacteria playing supporting rather than direct causative roles,29 while others which are not necessarily major pathogens express genes encoding virulence factors that cause much of the tissue damage.30
It is almost certain that noma is caused by a polybacterial infection in which a complex bacterial community operates as an adaptive system.5, 7, 13, 17 As the bacterial communities that can be isolated from lesions of noma and of necrotizing gingivitis are also found in the mouths of healthy subjects who do not develop these diseases,3, 7, 13, 19, 31 it is reasonable to assume that these commensals become opportunistically infective only under certain favorable circumstances. They then develop the properties of adhesion, colonization, invasion, and survival, as well as evasion of immune responses.32, 33, 34 This enables them to penetrate the epithelium into the connective tissue, and to generate immuno‐inflammatory responses and tissue damage.31
In the context of noma, systemic conditions favoring commensal microorganisms that ultimately become implicated in the etiology of the disease include malnutrition, immune suppression, and other states of debilitation; and local factors favoring infection include poor dento‐gingival bacterial plaque control, plaque‐induced inflammation, the pathogenic products of tissue breakdown, dysregulation of oral mucosal immunity, and suppression of other commensal bacterial populations.1, 5, 6, 9, 11, 16, 17 All these factors may shift the dynamic balance between the members of the complex adaptive microbial community, with the ultimate conversion of commensals into pathogens favoring opportunistic infection.13, 33
Thus, it appears that disease initiation and progression are dependent on the genes that the bacteria express rather than on the specific type of bacteria, on the complex interactions among the different members of the bacterial community, and between the bacterial community and the host.13, 32 The magnitude of tissue damage depends on the fitness and virulence of the microbial community, on the general health of the host, and on the fitness of the host's immune system.
The anerobic bacteria associated with necrotizing stomatitis and noma are highly proteolytic and invasive11 owing to the release of proteases that degrade extracellular matrix and other tissue components, and to toxins which are cytopathic to resident periodontal cells, to local endothelial cells, and to immuno‐inflammatory cells. These bacterial virulence agents cause tissue damage with disruption of local vasculature, hemorrhage, ischemia, and dysregulation of local immune responses.1, 5, 11, 16, 17
For example, Prevotella intermedia, very commonly found in acute noma, possesses virulence factors such as proteases, toxins, and other noxious agents that have the capacity not only to cause direct tissue damage but also to degrade immunoglobulins, complement components and lipopolysaccharide‐binding proteins, resulting in local immune suppression and local tissue breakdown.34
As mentioned previously, the complex nature of bacteria, not necessarily the same in all subjects with necrotizing oral diseases, by means of their virulence factors can cause necrotizing gingivitis and necrotizing periodontitis, and in a minority of cases of malnourished, immuno‐suppressed, debilitated hosts, the necrotizing gingivitis/necrotizing periodontitis rapidly progresses to necrotizing stomatitis with destruction of oral soft tissues and bone, and exfoliation of teeth (Figure 1).
From a clinical point of view, the intensity of the bacterial virulence factors together with the associated bacteria‐induced ischemic damage may cause cellulitis and rapidly spreading necrotizing fasciitis, osteonecrosis, and myonecrosis. The necrotic tissues become blue‐black in color, and within a few days the necrosis extends through the muscle layer and the overlying skin, resulting in a circular full thickness defect (Figures 4A,B and 5).5, 11, 17
During the rapid sequence of necrotizing events leading to noma, the pattern of progression of necrosis does not follow the pattern of the blood supply of the affected part and so should not be defined as gangrene, as has frequently been reported in the past. The ischemia with the accompanying necrosis in noma are in fact secondary to microbially induced vascular damage (Figure 1).17
We believe that the role in the pathogenesis of noma played by inflammatory damage to the vasculature with resultant micro‐thrombus formation and ischemic necrosis is underestimated. We cannot conceive that such extensive and rapid mucosal necrosis, myonecrosis, osteonecrosis, and cutaneous necrosis could occur without a substantive degree of centrifugally advancing ischemia, especially bearing in mind the circular shape of the tissue defect in most cases. Unfortunately, the opportunity to histologically examine surgically removed tissues does not frequently present itself, and so direct evidence in support of such a hypothesis is lacking.
3.2. Malnutrition
Many young subjects with necrotizing oral diseases or with noma have been found to be malnourished, so malnutrition has been considered to be an important co‐factor in the pathogenesis of noma. Indeed, most cases of noma are reportedly from central Africa, where it affects predominantly young children living in conditions of poverty, lack of sanitation, with very inadequate nutrition, and whose parents lack education (Table 1).1, 3, 8, 9, 10, 18, 24 Therefore, children in this population experience malnutrition, and the consequently disturbed metabolism and reduced immune fitness are thought to be major co‐factors in the initiation and progression of noma.
However, significantly, only a small minority of these malnourished children living in deprived social and economic conditions will develop necrotizing gingivitis, necrotizing periodontitis, and necrotizing stomatitis, and even when these diseases do develop, only rarely do they progress to noma.3, 5, 7
On the other hand, the malnutrition observed both in impoverished and in socio‐economically more fortunate subjects with noma may be a response to the stress of the acute critical systemic illness associated with the development of noma, and as such malnutrition may not only be a predisposing factor but also a secondary manifestation of noma. The stress response to acute noma is characterized by high levels of circulating catecholamines, cortisol, glucagon, and cytokines, which together promote gluconeogenesis and muscle proteolysis that may play important roles in the pathogenesis and progression of noma. A subject with acute noma who is secondarily malnourished will have loss of intracellular minerals, impaired cell mediated immunity and wound healing, an increased risk of other adventitious infections, and thus be more likely to die.1, 35
3.3. HIV/AIDS
HIV infection does not appear to play any significant role in the pathogenesis of noma in children in central African countries, as most of them are reportedly HIV‐seronegative.24, 36 In contrast, in South Africa5, 11, 17 and Zimbabwe19 most of the reported cases of noma have been in HIV‐seropositive subjects.5, 11, 16, 17 Thus, it is reasonable to assume that unlike in central Africa, in southern Africa HIV infection is indeed an important factor in the development of noma.
Neutropenia, low CD4 + T cell count, diminution in the number of Langerhans cells in the oral epithelium, and cytokine dysregulation, are some of the factors associated with HIV infection which favor bacterial adhesion and colonization of the mouth, thus promoting the development of necrotizing oral diseases that are the precursors of noma.5, 16 In addition, HIV‐seropositive subjects have an increased burden of subclinical and clinical fungal and viral oral infections which can further dysregulate local immunity with consequent risk of bacterial colonization and invasion.16
In our experience, in South Africa, most of the HIV‐seropositive subjects with noma have either not yet started highly active antiretroviral treatment or they have defaulted on their antiretroviral medication in the period just before developing noma. Most of them have AIDS and are physically wasted. In the context of AIDS, HIV‐seropositive subjects may develop nutrient deficiencies, not only because of insufficient dietary intake, but also owing to increased metabolic requirements as a stress response to their illness, or possibly owing to abnormal metabolism.35
HIV‐seropositive subjects with necrotizing oral diseases who are highly active antiretroviral therapy‐naïve should immediately receive antiretroviral medication as any improvement in the immune status consequent to a decrease in the HIV load may help contain the necrotizing process.5, 11, 16, 17
4. TREATMENT
Noma is preceded by necrotizing gingivitis, necrotizing periodontitis, and necrotizing stomatitis (Figure 1), so early diagnosis and treatment of any stage in this sequence of progression will prevent the development of noma. The treatment of necrotizing gingivitis and early necrotizing periodontitis in both HIV‐seropositive and ‐seronegative subjects is simple and effective, and is done on an outpatient basis. This treatment comprises appropriate orally administered antibiotic, frequent use of chlorhexidine mouthwash, toothbrushing to remove dento‐gingival bacterial plaque, and analgesic. Once the acute phase of the necrotizing disease has been somewhat controlled, debridement of roots, necrotic soft tissue and necrotic bone, and removal of hopelessly mobile teeth should follow. If assiduously carried out, this treatment regimen not only will prevent the progression of the necrotizing process to necrotizing stomatitis, but will also control the necrotizing periodontal disease.16, 28, 37
Debilitated patients with established necrotizing stomatitis should immediately be admitted to hospital for intravenous broad spectrum antibiotics, fluid and electrolytes, for nutritional supplementation, and for supportive medical care. Again, superficial necrotic tissue and mobile teeth should be removed, followed by frequent irrigation. This treatment protocol will usually prevent the progression of necrotizing stomatitis to necrotizing fasciitis and myonecrosis (Figure 6A‐C).5, 11, 16, 17
Figure 6.
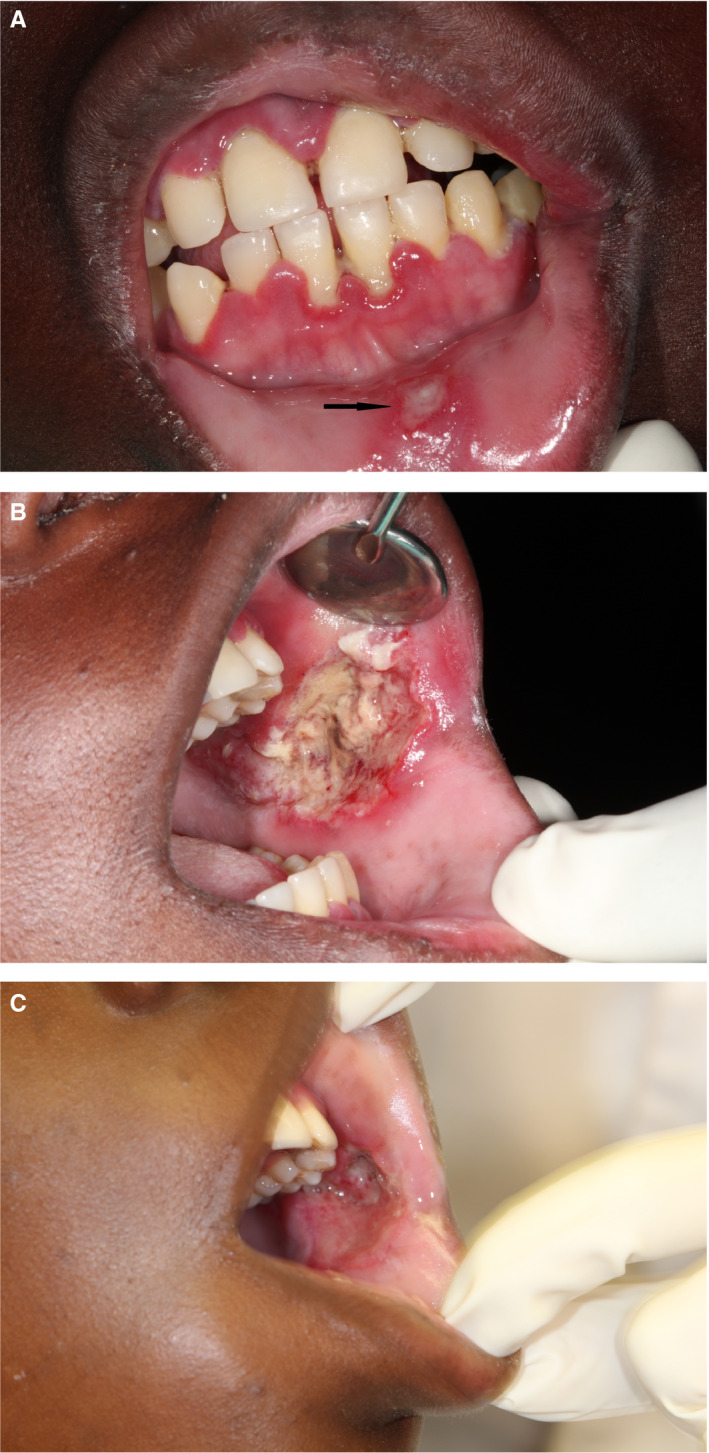
(A) Mandibular and maxillary necrotizing gingivitis, with early necrotizing stomatitis of the labial mucosa (arrow) apparently from contact with the gingival lesions, in a 16‐y‐old HIV‐seropositive adolescent with a CD4 + T cell count of 17 cells/mm3, who had been on highly active antiretroviral treatment for the previous 4 y. (B) Severe necrotizing stomatitis of the left buccal mucosa in the same patient. The gingival, labial, and buccal lesions developed rapidly. The patient was admitted to hospital and penicillin, metronidazole, and gentamycin were administered with fluid replacement and daily irrigation of the intra‐oral lesions with saline. A semi‐fluid high‐protein diet was given. The lesions were debrided, and dressed daily with bismuth‐iodoform‐paraffin paste. The patient was referred to the local HIV clinic to optimize the highly active antiretroviral treatment regimen. (C) 5 d after admission to hospital, the left buccal mucosa was healing satisfactorily and the necrotizing gingivitis had resolved. The patient was discharged from hospital, but was lost to follow‐up (SADJ 2014; 69:468‐470, reproduced with permission35)
Once the necrotizing process has already progressed to noma, a treatment protocol similar to that for necrotizing stomatitis should immediately be introduced, except for tissue debridement and tooth extraction, which will be performed only after the general condition of the patient has been stabilized (Figure 7A‐E).36 There seems to be no particular antibiotic regimen that is superior in preventing noma or in minimizing its morbidity or complications.38 Broad spectrum antibiotics should be administered. A combination of penicillin/clavulanic acid, an aminoglycoside and metronidazole will usually arrest the disease, and reduce the morbidity and the likelihood of mortality.1, 6, 11, 17, 23, 38, 39
Figure 7.
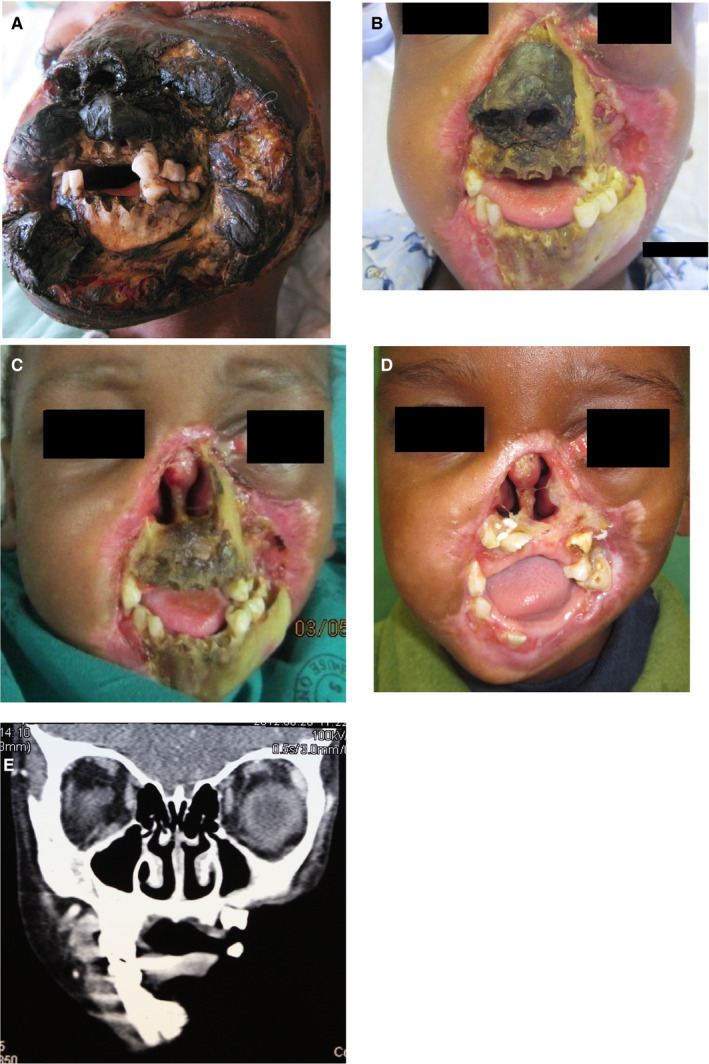
(A) Severe necrotic destruction of the nose, lips, and cheeks extending to the infra‐orbital margin, and denudation and necrosis of the anterior mandible and anterior maxilla of a 6‐y‐old HIV‐seropositive highly active antiretroviral treatment‐naïve child with a CD4 + T cell count of 6 cells/mm3. (B) The appearance 2 wk after hospital admission: much of the necrotic soft tissue has been removed. (C) All the necrotic soft tissue removed. (D) The appearance 3 mo after resection of all the necrotic soft tissue and bone with extensive scarring around the defect. (E) Computed tomography image showing the extent of the damage to the mandible and maxilla (Head Neck Pathol 2013; 7:188‐192, reproduced with permission8)
Some subjects with noma of limited extent may benefit from early reconstructive surgery as soon as the active phase of the disease is under control (Figure 8A‐F)2; but usually surgical reconstruction and functional rehabilitation should await healing and fibrosis, which may take up to 1 year.12, 20
Figure 8.
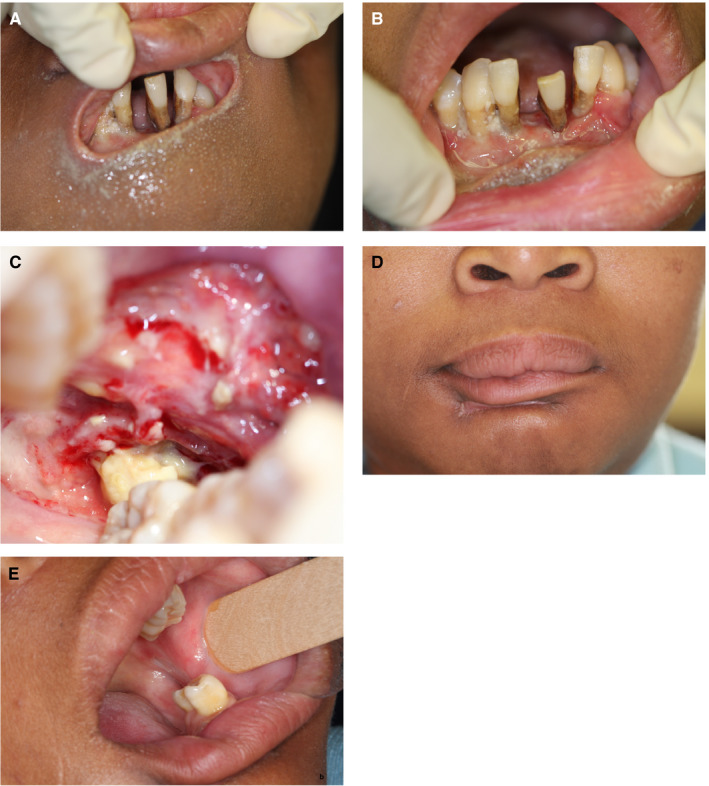
A 32‐y‐old malnourished HIV‐seropositive female with noma and a CD4 + T cell count of 118 cells/mm3, who had been on highly active antiretroviral treatment for 6 y. (A) A well‐defined, oval, full thickness defect of the lip below the vermilion border. (B) Necrotizing periodontitis affecting the anterior mandibular teeth with necrotizing stomatitis of the labial mucosa. (C) Posteriorly extending necrotizing stomatitis of the left buccal mucosa. (D) The sublabial scar 8 wk after admission to hospital and 6 wk after suturing. The patient was satisfied despite the aesthetically imperfect result. (E) Healed left posterior buccal mucosa 8 wk after treatment (AIDS Res Hum Retroviruses 2014; 30: 213‐216, reproduced with permission34)
Even under ideal conditions, the outcome of the complex surgical and functional rehabilitation of a subject with severe mutilating noma is at best a compromise. In underdeveloped countries in sub‐Saharan Africa, central Asia and Latin America, where most cases of noma occur,1 and where the medical facilities, if available at all, are far from optimal, then outcome of treatment may leave the victims of the disease physically disfigured and emotionally traumatized for the rest of their lives.36
5. PUBLIC HEALTH
Noma is a truly enigmatic disease, and very little global data are available about its incidence, mortality rate, or other epidemiological factors; or about the reasons for the marked variations in the incidence and prevalence among comparable population groups of different but equally impoverished underdeveloped countries.5, 9
The nature of the link between HIV/AIDS and noma, and the potential host‐related and environmental factors that either confer protection against or favor the development of noma are obscure, as are the reasons why only a small minority of cases of necrotizing gingivitis, necrotizing periodontitis, and necrotizing stomatitis progress to noma.5, 11 Adequate information on these issues could be of value in formulating and implementing public health policies and programs for prevention and early detection and treatment of this terrible disease.
Most subjects at risk of noma live in impoverished rural areas, lack education and financial resources, and do not have ready access to healthcare services. Furthermore, owing to cultural beliefs and practices such as consulting traditional healers rather than conventional medical practitioners, subjects with necrotizing gingivitis and necrotizing periodontitis, or more often their parents, delay seeking medical treatment, or neglect to seek medical treatment at all. As a result, the disease progresses, so by the time of diagnosis it has already advanced to necrotizing stomatitis and noma. Those who do develop noma are doomed to suffer the tragic consequences of disfigurement and functional impairment, or death.5, 11, 17
Survivors of acute noma with severe mid‐facial destruction have to undergo lengthy, daunting, and complex surgical reconstruction and functional rehabilitation, the outcome of which is, at best, a compromise.5, 10, 11, 17 In underdeveloped countries lacking the medical expertise for such advanced surgical procedures, many victims of noma with facial mutilation have little hope of living normal lives again, and are often subjected to social discrimination because of superstitious beliefs, and the stigma associated with gross facial defects. Therefore, it is not uncommon for them to be shunned by their communities and to have to live in remote areas, almost in isolation.5, 17, 20 The social consequences and the psychological impact can be devastating.
In formulating a public health policy on noma, one should not lose sight of the fact that necrotizing gingivitis/necrotizing periodontitis are the precursors of necrotizing stomatitis and noma, and since the principle cause is a polybacterial, primarily anerobic infection, prevention of noma could certainly be as simple as treatment of the precursor conditions by education and instruction in dento‐gingival bacterial plaque control, and the use of antibiotics.28, 37 Unfortunately, the logistical difficulties of introducing even these simple preventive measures for noma in remote, impoverished, widely separated and poorly educated populations appear all but insuperable, because even these preventative programs, which to the developed world may seem simple, may be beyond the financial resources, and perhaps the political will, of many, if not most, underdeveloped countries. In the meantime, allocation of adequate funding by developed countries and by private philanthropic organizations is required for research into all aspects of noma, and for making available experienced surgeons to treat those affected by noma wherever it may be required.36
It should be incumbent upon any healthcare practitioner encountering necrotizing stomatitis and noma to report cases to the appropriate health authorities, although in the foreseeable future there will always be many cases that, for the reasons outlined above, will remain undiagnosed and untreated. The reporting of known cases will at least add significantly to the present national and international pools of data on noma.
However, without addressing poverty, sanitation, education, and not least personal dento‐gingival bacterial plaque control, and without the political will to allocate the necessary financial resources to address these issues, the likelihood of reducing the incidence of noma and the number of those affected by noma remains small.
In this regard, at the turn of the 20th century the World Health Organization launched an initiative to reduce the incidence of this disease. The steps in the World Health Organization oral health program to reduce the incidence of noma consisted of: initiating training and heightening awareness with regard to early diagnosis and treatment among healthcare practitioners; raising awareness among at‐risk populations; promoting epidemiological and etiological research; and setting up regional treatment centers.40 Little is known concerning the progress of this initiative, but noma remains an unresolved global concern.
6. CONCLUSION AND FUTURE DIRECTIONS
Noma is a mutilating disease of unclear etiopathogenesis, which frequently affects children, particularly those in sub‐Saharan Africa. Those affected by noma may experience disfigurement, functional impairment, social discrimination, and/or death.
Further research is necessary to identify the risk factors in the pathogenesis of noma, and the reasons for the marked variations in incidence and prevalence among comparable populations in different but equally impoverished countries. More information is needed regarding the molecular virulence factors that confer fitness advantage to the putative pathogenic bacteria, increasing the probability of them causing noma. Identifying the virulence genes of the bacteria, facilitating their attachment, invasion, and immune evasion, and mediating the biosynthesis of toxins that cause tissue damage, will be an important step in understanding the pathogenesis of noma.32
In this context, it is important to know whether herpesviruses, which can persist in a variety of cells in the periodontium,41 upon reactivation contribute to the development of noma, either by a direct cytopathic effect, by dysregulation of local immune responses, or by upregulation of local inflammatory reactions.42, 43
It is evident from the fulminating clinical course of noma that the immune system is in some manner dysregulated in subjects with the disease. It is imperative to identify the elements of the immune system which are impaired in subjects with precursor lesions or early noma, whether these be the cytokine profile, the functional activity of systemic or local immuno‐inflammatory cells such as neutrophils, macrophages or lymphocytes, or the antibody‐mediated humoral reactions. Taken together this information would give a much needed insight into the pathogenesis of noma and should facilitate the formulation and implementation of appropriate public health policies and programs for prevention, and for developing new treatment modalities.
Meanwhile, funding from developed countries and private philanthropic organizations is needed to provide experienced surgeons to treat those affected by noma, to promote education regarding personal and particularly oral hygiene, and to make basic healthcare services accessible to impoverished, poorly educated populations.
Feller L, Khammissa RAG, Altini M, Lemmer J. Noma (cancrum oris): An unresolved global challenge. Periodontol 2000. 2019;80:189–199. 10.1111/prd.12275
REFERENCES
- 1. Baratti‐Mayer D, Pittet B, Montandon D, et al. Noma: an “infectious” disease of unknown aetiology. Lancet Infect Dis. 2003;3:419‐431. [DOI] [PubMed] [Google Scholar]
- 2. Chidzonga MM, Mahomva L. Noma (cancrum oris) in human immunodeficiency virus infection and acquired immunodeficiency syndrome (HIV and AIDS): clinical experience in Zimbabwe. J Oral Maxillofac Surg. 2008;66:475‐485. [DOI] [PubMed] [Google Scholar]
- 3. Enwonwu CO, Falkler WA, Idigbe EO. Oro‐facial gangrene (noma/cancrum oris): pathogenetic mechanisms. Crit Rev Oral Biol Med. 2000;11:159‐171. [DOI] [PubMed] [Google Scholar]
- 4. Enwonwu CO, Falkler WA Jr, Phillips RS. Noma (cancrum oris). Lancet. 2006;368:147‐156. [DOI] [PubMed] [Google Scholar]
- 5. Feller L, Altini M, Chandran R, et al. Noma (cancrum oris) in the South African context. J Oral Pathol Med. 2014;43:1‐6. [DOI] [PubMed] [Google Scholar]
- 6. Hatcher J, Williamson L. Noma in a patient with HIV. Lancet Infect Dis. 2017;17:672. [DOI] [PubMed] [Google Scholar]
- 7. Huyghe A, Francois P, Mombelli A, et al. Microarray analysis of microbiota of gingival lesions in noma patients. PLoS Negl Trop Dis. 2013;7:e2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Idigbe EO, Enwonwu CO, Falkler WA, et al. Living conditions of children at risk for noma: Nigerian experience. Oral Dis. 1999;5:156‐162. [DOI] [PubMed] [Google Scholar]
- 9. Srour ML, Marck K, Baratti‐Mayer D. Noma: overview of a neglected disease and human rights violation. Am J Trop Med Hyg. 2017;96:268‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tempest MN. Cancrum oris. Br J Surg. 1966;53:949‐969. [DOI] [PubMed] [Google Scholar]
- 11. van Niekerk C, Khammissa RA, Altini M, Lemmer J, Feller L. Noma and cervicofacial necrotizing fasciitis: clinicopathological differentiation and an illustrative case report of noma. AIDS Res Hum Retroviruses. 2014;30:213‐216. [DOI] [PubMed] [Google Scholar]
- 12. Ogbureke KU, Ogbureke EI. NOMA: a preventable, “scourge” of African children. Open Dent J. 2010;4:201‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Whiteson KL, Lazarevic V, Tangomo‐Bento M, et al. Noma affected children from niger have distinct oral microbial communities based on high‐throughput sequencing of 16S rRNA gene fragments. PLoS Negl Trop Dis. 2014;8:e3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marck KW. Cancrum oris and noma: some etymological and historical remarks. Br J Plast Surg. 2003;56:524‐527. [DOI] [PubMed] [Google Scholar]
- 15. Coupe MH, Johnson D, Seigne P, Hamlin B. Special article: airway management in reconstructive surgery for noma (cancrum oris). Anesth Analg. 2013;117:211‐218. [DOI] [PubMed] [Google Scholar]
- 16. Khammissa RAG, Ciya R, Munzhelele TI, et al. Oral medicine case book 65: necrotising stomatitis. SADJ. 2014;69:464‐467. [PubMed] [Google Scholar]
- 17. Masipa JN, Baloyi AM, Khammissa RA, Altini M, Lemmer J, Feller L. Noma (cancrum oris): a report of a case in a young AIDS patient with a review of the pathogenesis. Head Neck Pathol. 2013;7:188‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baratti‐Mayer D, Gayet‐Ageron A, Hugonnet S, et al. Risk factors for noma disease: a 6‐year, prospective, matched case‐control study in Niger. Lancet Glob Health. 2013;1:e87‐e96. [DOI] [PubMed] [Google Scholar]
- 19. Bolivar I, Whiteson K, Stadelmann B, et al. Bacterial diversity in oral samples of children in niger with acute noma, acute necrotizing gingivitis, and healthy controls. PLoS Negl Trop Dis. 2012;6:e1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simon F, Wolfe SA, Guichard B, Bertolus C, Khonsari RH. Paul Tessier facial reconstruction in 1970 Iran, a series of post‐noma defects. J Craniomaxillofac Surg. 2015;43:503‐509. [DOI] [PubMed] [Google Scholar]
- 21. Ahlgren M, Funk T, Marimo C, Ndiaye C, Alfven T. Management of noma: practice competence and knowledge among healthcare workers in a rural district of Zambia. Glob Health Action. 2017;10:1340253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ravinetto R. Noma: time to address a collective moral failure. Am J Trop Med Hyg. 2017;96:263‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ashok N, Tarakji B, Darwish S, Rodrigues JC, Altamimi MA. A review on noma: a recent update. Glob J Health Sci. 2015;8:53‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Enwonwu CO, Falkler WA Jr, Idigbe EO, et al. Pathogenesis of cancrum oris (noma): confounding interactions of malnutrition with infection. Am J Trop Med Hyg. 1999;60:223‐232. [DOI] [PubMed] [Google Scholar]
- 25. Feller L, Wood NH, Raubenheimer E. Complex oral manifestations of an HIV‐seropositive patient. J Int Acad Periodontol. 2006;8:10‐16. [PubMed] [Google Scholar]
- 26. Periodontology Aao . International Workshop for a Classification of Periodontal Diseases and Conditions. Consensus report. Ann Periodontol. 1999;4:1‐112. [DOI] [PubMed] [Google Scholar]
- 27. Tonna JE, Lewin MR, Mensh B. A case and review of noma. PLoS Negl Trop Dis. 2010;4:e869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shangase L, Feller L, Blignaut E. Necrotising ulcerative gingivitis/periodontitis as indicators of HIV‐infection. SADJ. 2004;59:105‐108. [PubMed] [Google Scholar]
- 29. Friedrich MJ. Microbiome project seeks to understand human body's microscopic residents. JAMA. 2008;300:777‐778. [DOI] [PubMed] [Google Scholar]
- 30. Duran‐Pinedo AE, Chen T, Teles R, et al. Community‐wide transcriptome of the oral microbiome in subjects with and without periodontitis. ISME J. 2014;8:1659‐1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feller L, Altini M, Khammissa RA, Chandran R, Bouckaert M, Lemmer J. Oral mucosal immunity. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:576‐583. [DOI] [PubMed] [Google Scholar]
- 32. Ang MY, Heydari H, Jakubovics NS, et al. FusoBase: an online Fusobacterium comparative genomic analysis platform. Database (Oxford). 2014;1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feller L, Khammissa RA, Chandran R, Altini M, Lemmer J. Oral candidosis in relation to oral immunity. J Oral Pathol Med. 2014;43:563‐569. [DOI] [PubMed] [Google Scholar]
- 34. Ruan Y, Shen L, Zou Y, et al. Comparative genome analysis of Prevotella intermedia strain isolated from infected root canal reveals features related to pathogenicity and adaptation. BMC Genom. 2015;16:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Halsted CH. Malnutrition and nutritional assessment In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, eds. Harrisons Principles of Internal Medicine. New York, NY: McGraw Hill Medical; 2005:411‐422. [Google Scholar]
- 36. Marck KW, de Bruijn HP. Surgical treatment of noma. Oral Dis. 1999;5:167‐171. [DOI] [PubMed] [Google Scholar]
- 37. Phiri R, Feller L, Blignaut E. The severity, extent and recurrence of necrotizing periodontal disease in relation to HIV status and CD4 + T cell count. J Int Acad Periodontol. 2010;12:98‐103. [PubMed] [Google Scholar]
- 38. Elliott D, Kufera JA, Myers RA. The microbiology of necrotizing soft tissue infections. Am J Surg. 2000;179:361‐366. [DOI] [PubMed] [Google Scholar]
- 39. Weledji EP, Njong S. Cancrum oris (noma): the role of nutrition in management. J Am Coll Clin Wound Spec. 2015;7:50‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bourgeois DM, Leclercq MH. The World Health Organization initiative on noma. Oral Dis. 1999;5:172‐174. [DOI] [PubMed] [Google Scholar]
- 41. Slots J. Herpesviruses, the missing link between gingivitis and periodontitis? J Int Acad Periodontol. 2004;6:113‐119. [PubMed] [Google Scholar]
- 42. Feller L, Lemmer J. Necrotizing periodontal diseases in HIV‐seropositive subjects: pathogenic mechanisms. J Int Acad Periodontol. 2008;10:10‐15. [PubMed] [Google Scholar]
- 43. Slots J. Herpesviruses in periodontal diseases. Periodontol 2000. 2005;38:33‐62. [DOI] [PubMed] [Google Scholar]


