Abstract
Rapid infant weight gain predicts childhood obesity. We aimed to estimate effect size and identify critical timing for intervention-assisted smoking cessation during pregnancy to impact infant weight gain. We followed 25 mother-infant dyads in the UB Pregnancy and Smoking Cessation Study (Buffalo, NY, USA). Maternal smoking status was biochemically verified and monitored through pregnancy. Birth weight and length were extracted from birth records. Research staff measured infant weight and length at 2 weeks and monthly from 1–12 months of age. Mixed models were used to fit infant BMI-for-age z-score (ZBMI) trajectories. We found infants of quitters had lower ZBMI gain from birth to 12 months (mean ± SD, 1.13 ± 1.16) than infants of persistent smokers (2.34 ± 1.40; p=0.035), with Cohen’s d effect size being large (0.96). The infant ZBMI gain from birth to 12 months was low (<0.47) if smoking cessation was initiated between 15 and 27 weeks of pregnancy, but started to increase if quitting at 28 weeks (0.65) and accelerated with time (e.g., 3.16 if quitting at 36 weeks). We concluded maternal smoking cessation during pregnancy may reduce fetal origins of obesity through reducing infant weight gain, especially if quitting smoking by 27 weeks of pregnancy.
Keywords: Smoking, Smoking cessation, Tobacco, Pregnancy, Infant weight gain, Body mass index, Obesity
INTRODUCTION
One important contributor to fetal origins of obesity is maternal smoking during pregnancy. A recent meta-analysis concluded that the pooled adjusted odds ratio of childhood obesity related to maternal smoking during pregnancy was 1.55 (95% confidence interval, 1.36 to 1.70).[1] However, the potential protective effect of maternal smoking cessation during pregnancy against childhood overweight/obesity remained understudied. Observational research has shown preschool children (aged 3–4) of mothers who spontaneously stopped smoking before or during early pregnancy had a mean body mass index (BMI) and risk of overweight/obesity similar to children of mothers who never smoked, but lower than children of mothers who continued smoking during pregnancy.[2–4] A gap in research literature is the evaluation of the effects of intervention-assisted smoking cessation during pregnancy on childhood BMI or risk of overweight/obesity.
Previous research suggests that timing (i.e., weeks of pregnancy) of maternal smoking cessation initiation during pregnancy was associated with the magnitude of health benefits in offspring at birth such as improved birth weight, e.g., infants of mothers who quit smoking by the end of the 1st trimester of pregnancy had a mean birth weight (3,441 g) similar to infants of nonsmokers (3,452 g), whereas infants of mothers who quit smoking in the 2nd (3,268 g) or 3rd trimester (3,191 g) of pregnancy had substantially lower mean birth weight.[5] However, the critical timing of smoking cessation initiation on postnatal growth trajectory remains largely unknown, which may differ from birth outcomes.[6] Research is needed to compare the effects of smoking cessation at different time points throughout pregnancy on childhood BMI or risk of obesity.
Therefore, we had two aims in this intervention study: 1) to estimate the effect size of intervention-assisted smoking cessation during pregnancy on infant weight gain throughout the first 12 months of age, a well-established risk factor for later childhood obesity,[7] and 2) to identify the critical timing of smoking cessation initiation during pregnancy to maximize its benefits on infant weight gain.
SUBJECTS AND METHODS
Study participants
We analyzed data of 25 mother-infant dyads (8 persistent smokers and 17 quitters) in the UB Pregnancy and Smoking Cessation Study (NCT03514602), an intervention study conducted in Buffalo, NY, USA during 2015–2018. Details on its study design (i.e., non-concurrent multiple baseline) and intervention protocol can be found in our previous publication.[8] In this particular design, the intervention is introduced in a staggering order among participants recruited at different times. A total of 30 pregnant mothers (mean gestational age at enrollment, 14.8 weeks [range, 6–33 weeks]) who still smoked daily after 1 week (early intervention, n=9), 2 weeks (delayed intervention, n=14), or 3 weeks (late intervention, n=7) of multiple baseline (i.e., a waiting period with smoking monitoring only) received our 8-week multicomponent intervention. The intervention components included education and counseling, monitoring and feedback, contingent financial incentives, and family support. After post-test (i.e., 8 weeks after Quit Date), their smoking status was verified once every 2 weeks until 35 weeks, once a week between 36 weeks and delivery, and then monthly between delivery and 12 months postpartum. We were able to follow 27 participants at delivery but 2 infants were then excluded due to birth-defect-related death and very preterm delivery. Therefore, 25 infants were included in the final analysis. This study was approved by the UBIRB and all participants signed a consent form.
Measures
Participants recorded the number of smoked cigarettes every day. At each prenatal and postpartum visit, smoking abstinence was confirmed if both NicAlert urine cotinine test[9] (<100-ng/mL) and breath carbon monoxide test (CO, <8 ppm)[10] results were within the non-smoking range. Timing of smoking cessation initiation or Quit Date was defined as the first day of smoking abstinence for at least 7 consecutive days. According to the Society for Research on Nicotine and Tobacco,[11] we defined continuous abstinence as abstinence between the Quit Date and delivery without any relapse; 7-day point prevalence abstinence at delivery as the prevalence of abstinence during the 7-day window preceding delivery.
Infant gestational age, birth weight and length were obtained from birth records. Low birth weight was defined as birth weight below 2,500 grams.[12] Small-for-gestational-age (SGA) was defined as birth weight below 10th percentile within a reference population of American newborns.[13] Research staff measured infant weight with Seca 374 Digital Baby Scale and length with Seca 416 Infant Meter at approximately 2 weeks, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10,11, and 12 months of age (Supplemental Figure 1). We calculated infant BMI by dividing weight in kilograms by the square of length in meters. According to the World Health Organization Child Growth Standard,[14] we calculated infant age- and sex-specific BMI z-score (ZBMI).
Statistical analysis
Birth outcomes were compared using t-test (for continuous variables) and Chi-square test (for categorical variables). We used mixed model in SAS 9.4 software to analyze infant ZBMI trajectories. Independent variables included actual child age, smoking cessation status, their interaction term (child age ×smoking cessation), and unbalanced confounders (i.e., gestational age at enrollment and prepregnancy BMI). Other potential confounders, including socio-demographic, pregnancy, and smoking characteristics, were tested but not included in the final mixed model as they were not significantly associated with smoking cessation (p>0.1). The quadratic age term (child age × child age) was tested but not significant (p=0.635) and thus not included into the final model. Based on the fitted mixed model, we estimated group differences in infant ZBMI gain from birth to 12 months (ZBM 12m - ZBMI birth) by smoking cessation status during pregnancy. We also calculated Cohen’s d effect size as the mean difference in infant ZBMI gain between groups divided by the pooled standard deviation (SD). An exploratory analysis on timings of smoking cessation initiation were conducted among the 15 quitters with continuous abstinence. Independent variables in the mixed model included child age, timing of smoking cessation initiation, their interaction term (child age × timing of smoking cessation initiation), and confounders. We estimated infant ZBMI gain, based on the range of timings (i.e., weeks of pregnancy) of smoking cessation initiation within our sample. We recognized the potential random errors from this exploratory analysis due to small sample size and emphasized the need of validation in a larger sample.
RESULTS
Most socio-demographic, pregnancy, and smoking characteristics were comparable between persistent smokers (N=8) and quitters (N=17) (Supplemental Table 1), but quitters tended to be enrolled at earlier weeks of pregnancy (mean ± SD, 14.9 ± 6.6 vs 20.4 ± 7.9, p=0.081) and have higher prepregnancy BMI (29.6 ± 7.8 vs 24.2 ± 5.7, p=0.092), compared to persistent smokers throughout pregnancy. At birth, infants of quitters had higher mean weight (3.16 ± 0.50 vs 2.64 ± 0.35 kg; p=0.015; Cohen’s d effect size=−1.13), length (49.21 ± 2.72 vs 46.89 ± 1.94 cm; p=0.041; d=−0.93), and ZBMI (−0.39 ± 1.02 vs −1.29 ± 0.97; p=0.049; d=−0.89), compared to infants of persistent smokers (Supplemental Table 2). Maternal smoking cessation during pregnancy was significantly (p=0.017) associated with a lower risk of SGA: 11.8% among infants of quitters vs 62.5% among infants of persistent smokers. Similarly, infants of quitters tended to have a lower risk of low birth weight (5.9% vs 37.5%; p=0.081) compared to infants of persistent smokers. There was no significant difference in mean gestational age at birth between two groups.
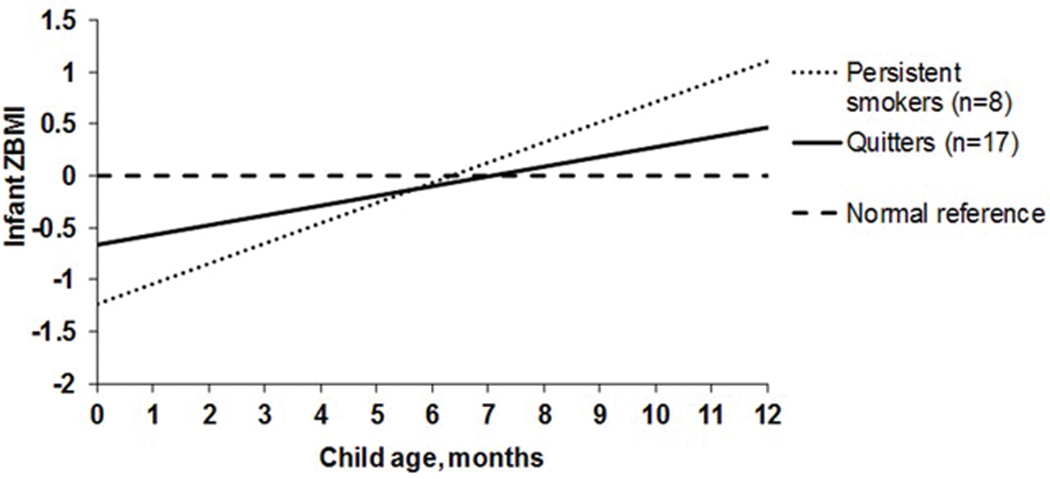
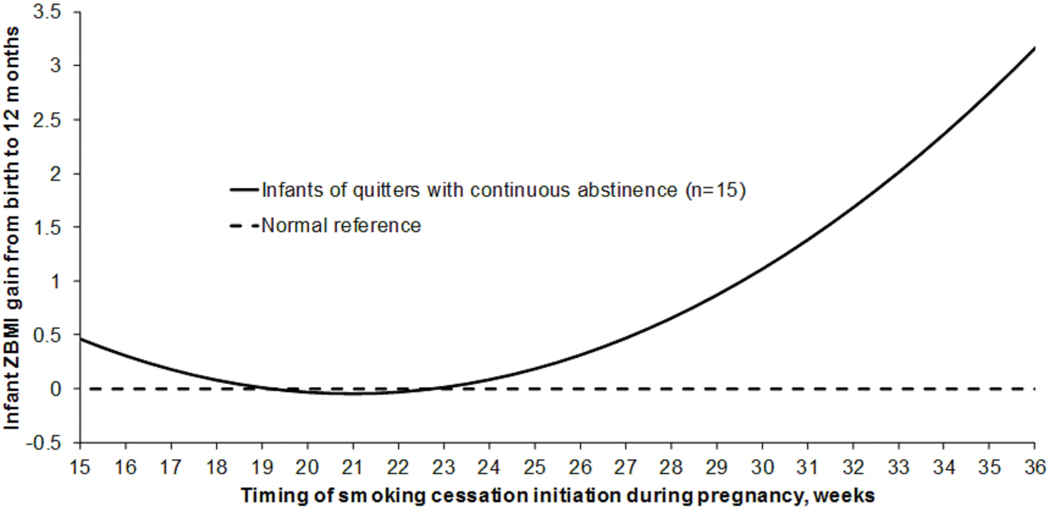
In the final mixed model, the regression coefficient for the interaction term of “child age × smoking cessation” was 0.101 (standard error, 0.047) and significant (p=0.035), adjusting for gestational age at enrollment and prepregnancy BMI (Supplemental Table 3). Infants of quitters had a lower mean ZBMI gain from birth to 12 months (mean ± SD, 1.13 ± 1.16) than infants of persistent smokers (2.34 ± 1.40; p=0.035 (Figure 1); and their estimated mean difference was −1.21 (95% confidence interval, −2.34 to −0.09). The estimated Cohen’s d effect size was 0.96. The estimated ZBMI at 12 months of age among infants of quitters (0.46) was closer to normal reference (0.00), whereas was high among infants of persistent smokers (1.11). Among the 15 quitters with continuous abstinence, timings of smoking cessation initiation ranged from 14.7 to 36.3 weeks (mean ± SD, 21.9 ± 5.8) (Supplemental Figure 2). The infant ZBMI gain from birth to 12 months of age was low (<0.47) if smoking cessation was initiated between 15 and 27 weeks of pregnancy, but started to increase if quitting at 28 weeks (0.65) and accelerated with time (e.g., 3.16 if quitting at 36 weeks) (Figure 2).
Figure 1. Infant ZBMI gain from birth to 12 months by maternal smoking cessation status during pregnancy.
Infant ZBMI estimated from the mixed model, adjusting for maternal gestational age at enrollment and prepregnancy BMI.
Figure 2. Infant ZBMI gain from birth to 12 months by timing of maternal smoking cessation initiation during pregnancy among 15 quitters with continuous abstinence.
Infant ZBMI gain estimated from the mixed model, adjusting for maternal gestational age at enrollment and prepregnancy BMI.
DISCUSSION
Consistent with previous studies,[5,15,16] we found that intervention-assisted smoking cessation during pregnancy was associated with improved birth outcomes including normalized birth weight and length as well as lower risk of SGA (a risk factor for abdominal or central obesity in later life). In our sample, infants of quitters had mean birth weight (3.15 kg) and risk of low birth weight (5.9%) similar to the U.S. national levels (e.g., 3.27 kg and 8.1%, respectively in 2016).[17] Therefore, smoking-induced fetal growth restriction can be largely reduced by smoking cessation.
Maternal smoking cessation during pregnancy is an opportunity neglected in existing childhood obesity interventions.[18] Our finding that infants of intervention-assisted quitters had slower ZBMI gain compared to infants of persistent smokers extended and supported previous observational research among spontaneous quitters.[19,20] Rapid infant weight gain is a strong risk factor for later obesity, e.g., each +1 unit increment in infant weight SD score from birth to 1 year is associated with a twofold higher risk of childhood obesity, and a 23% higher risk of adult obesity, according to a meta-analysis of 10 cohort studies.[7] Therefore, we expect that infants of quitters in our study (mean ZBMI gain, 1.13) will have a substantially reduced risk (~50%) of obesity in later childhood than infants of persistent smokers (mean ZBMI gain, 2.34). The estimated effect size was relatively large (Cohen’s d=0.96). This supports our hypothesis that maternal smoking cessation during pregnancy may reduce fetal origins of childhood obesity through reducing infant weight gain.
Our study provided the unique evidence that 27 weeks of pregnancy might be the latest critical timing to obtain maximal benefits of smoking cessation during pregnancy on reducing infant ZBMI gain, after which the effect size was attenuated. Possible reasons are related to trajectories of fetal development of energy-balance-related organs (e.g., hypothalamus, central and sympathetic norepinephrine systems, fat cells, liver, and pancreas) and/or hormone profiles (e.g., leptin, ghrelin, and thyroid hormones). Alternatively, mothers who quit smoking during pregnancy might also change their other health behaviors that could alter infant ZBMI gain, such as diet, physical activity, alcohol, marijuana and other substance use. Furthermore, these mothers might be more likely to adhere to infant feeding guidelines including breastfeeding; they might also invest more time on infant care, which could serve as an alternative reinforcement to infant eating.
Our study had several strengths. First, smoking status was biochemically verified and monitored throughout pregnancy, which allowed us to identify critical timing of smoking cessation initiation. Second, monthly infant growth data facilitated trajectory analyses on infant ZBMI gain. However, our study was limited by 1) relatively small sample size, especially for the exploratory analysis on timing of smoking cessation initiation; 2) regional generalizability due to single-city recruitment from Buffalo, NY that is characterized with high proportions of African Americans and of populations living in poverty; 3) inclusion of a partial period of pregnancy (15–36 weeks) in analyzing timing of smoking cessation initiation; 4) relative short follow-up (12 months) that prevented us from examining risk of childhood overweight/obesity; and 5) residual confounding by other factors such as partner smoking.
In conclusion, our intervention study suggested that maternal smoking cessation during pregnancy was associated with improved birth outcomes, slower infant ZBMI gain, and closer-to-normal ZBMI at 12 months of age, especially if the mother quit smoking by 27 weeks of pregnancy. A larger randomized controlled trial is needed to confirm our novel findings.
Supplementary Material
Acknowledgements:
We appreciate the administrative support from Teresa Quattrin, MD, Former Chair, Department of Pediatrics, State University of New York at Buffalo; Vanessa M. Barnabei, MD, Chair, Department of Obstetrics and Gynecology, State University of New York at Buffalo; and Aimée C. Gomlak, FACHE, MBA, BS, Vice President of Women’s Services of Catholic Health System, as well as the assistance on recruitment by the staff in the Kaleida Health OB/GYN Centers, the Sisters of Charity Hospital of Buffalo, the Buffalo Prenatal-Perinatal Network, and other local OB/GYN clinics. The authors also thank the study participants for their time and efforts, and Drs. Katelyn A. Carr and Tinuke Oluyomi Daniel for their assistance on training counselors.
Funding: This work was supported in part through Clinical and Translational Science Award (CTSA) Pilot Study support from National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) grant UL1TR001412, and seed funding from the Department of Pediatrics, State University of New York at Buffalo (both awarded to Xiaozhong Wen). STH received salary support from R01HD075669, R01HD078332, and P20GM103644 while assisting with this study. This work is solely the responsibility of the authors and does not represent official views of the sponsors. The funders had no role in writing the manuscript or the decision to submit it for publication.
Footnotes
Conflicts of interest: No financial disclosures were reported by the authors of this paper.
Ethical approval: All study procedures were conducted in accordance with the ethical standards of the responsible committee on social and behavioral science research and with the Helsinki Declaration of 1975, as revised in 2000. All participants signed consent form and this study was approved by University at Buffalo Institutional Review Board.
Supplementary information is available at the International Journal of Obesity’s website.
REFERENCES
- 1.Rayfield S, Plugge E. Systematic review and meta-analysis of the association between maternal smoking in pregnancy and childhood overweight and obesity. J Epidemiol Community Health. 2017;71:162–173. [DOI] [PubMed] [Google Scholar]
- 2.Oken E, Huh SY, Taveras EM, Rich-Edwards JW, Gillman MW. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res 2005;13:2021–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fasting MH, Oien T, Storro O, Nilsen TI, Johnsen R, Vik T. Maternal smoking cessation in early pregnancy and offspring weight status at four years of age. A prospective birth cohort study. Early human development. 2009;85:19–24. [DOI] [PubMed] [Google Scholar]
- 4.Durmus B, Kruithof CJ, Gillman MH, et al. Parental smoking during pregnancy, early growth, and risk of obesity in preschool children: the Generation R Study. Am J Clin Nutr 2011;94:164–171. [DOI] [PubMed] [Google Scholar]
- 5.Yan J, Groothuis PA. Timing of prenatal smoking cessation or reduction and infant birth weight: evidence from the United Kingdom Millennium Cohort Study. Matern Child Health J. 2015;19:447–458. [DOI] [PubMed] [Google Scholar]
- 6.Hebel JR, Fox NL, Sexton M. Dose-response of birth weight to various measures of maternal smoking during pregnancy. Journal of clinical epidemiology. 1988;41:483–489. [DOI] [PubMed] [Google Scholar]
- 7.Druet C, Stettler N, Sharp S, et al. Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatr Perinat Epidemiol. 2012;26:19–26. [DOI] [PubMed] [Google Scholar]
- 8.Wen X, Eiden RD, Justicia-Linde FE, et al. A multicomponent behavioral intervention for smoking cessation during pregnancy: a non-concurrent multiple baseline design. Translational behavioral medicine. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nymox Pharmaceutical Corporation. NicAlert®: Product Insert (Urine Samples). 2017; http://www.nymox.com/default.action?itemid=47 Accessed 12/13, 2017.
- 10.Gaalema DE, Higgins ST, Bradstreet MP, Heil SH, Bernstein IM. Using NicAlert strips to verify smoking status among pregnant cigarette smokers. Drug and alcohol dependence. 2011;119:130–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res 2003;5:13–25. [PubMed] [Google Scholar]
- 12.World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. Vol 2 2010 Edition ed. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 13.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl 2006;450:76–85. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman E, Gremy I, Lang JM, Cohen AP. Low birthweight at term and the timing of fetal exposure to maternal smoking. Am J Public Health. 1994;84:1127–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCowan LM, Dekker GA, Chan E, et al. Spontaneous preterm birth and small for gestational age infants in women who stop smoking early in pregnancy: prospective cohort study. BMJ. 2009;338:b1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.United States Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Vital Statistics. Natality public-use data 2007–2016. 2018; http://wonder.cdc.gov/natality-current.html Accessed 3/22, 2018.
- 18.Reilly JJ, Martin A, Hughes AR. Early-Life Obesity Prevention: Critique of Intervention Trials During the First One Thousand Days. Current obesity reports. 2017;6:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mine T, Tanaka T, Nakasone T, Itokazu T, Yamagata Z, Nishiwaki Y. Maternal smoking during pregnancy and rapid weight gain from birth to early infancy. J Epidemiol. 2017;27:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Layte R, Bennett A, McCrory C, Kearney J. Social class variation in the predictors of rapid growth in infancy and obesity at age 3 years. Int J Obes (Lond). 2014;38:82–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.