Abstract
Purpose of review
Extracellular vesicles (EVs) released by prokaryote or eukaryote cells are emerging as mechanisms of cell-to-cell communication, by either physically interacting with the surface of target cells or transferring proteins/peptides, lipids, carbohydrates, and nuclei acids to acceptor cells. Accumulating evidence indicates that EVs, among other functions, regulate innate and adaptive immune responses. We revisit here the effects that EVs of various origins have on innate immunity.
Recent findings
EVs comprise a heterogeneous group of vesicles with different biogenesis, composition and biological properties, which include exosomes, microvesicles (MVs), apoptotic cell-derived EVs, and other EVs still not well characterized. EVs released by pathogens, leukocytes, non-hematopoietic cells, tumor cells, and likely allografts, can either stimulate or suppress innate immunity via multiple mechanisms. These include transfer to target leukocytes of pro- or anti-inflammatory mediators, membrane receptors, enzymes, mRNAs and non-coding RNAs; as well as interaction of EVs with the complement and coagulation systems. As a result, EVs affect differentiation, polarization, activation, tissue recruitment, cytokine and chemokine production, cytolytic and phagocytic function, and antigen (Ag) transfer ability, of different types of innate immune cells.
Summary
The field of intercellular communication via EVs is a rapid evolving area and the effects of pathogen- and host-derived EVs on innate immunity in particular, have received increasing attention during the past decade. Future studies will be necessary to assess the full potential of the crosstalk between EVs and the innate immune system and its use for therapeutic applications to treat chronic inflammation-based diseases and cancer growth and dissemination, among the growing list of disorders in which the innate immune system plays a critical role.
Keywords: Extracellular vesicles, exosomes, microvesicles, innate immunity
INTRODUCTION
The term extracellular vesicles (EVs) encompasses a variety of vesicles of different biogenesis and composition, released by living or dying cells. The family of EVs includes exosomes, microvesicles (MVs), apoptotic cell-derived EVs and other types of EVs that have not been yet fully characterized [1,2]. Exosomes range between 30–150 nm in size. They are generated as intraluminal vesicles (ILVs) within the cellular endocytic compartment by reverse budding of the limiting membrane of multivesicular bodies (MVBs). The MVBs can fuse either with lysosomes in which the ILVs are degraded, or with the plasma membrane for release of the ILVs to the extracellular space or bodily fluids, where the ILVs are termed exosomes (Figure 1A). MVs are in general larger EVs (0.1–1 μm) that are generated by shedding of the plasma membrane (Figure 1A). Classically, apoptotic cell-derived EVs have included apoptotic blebs or MVs shed from the plasma membrane (0.1–1 μm), apoptotic bodies resulting from the apoptotic cell disassembly process (1–5 μm, although they can reach up to 10 μm), EVs from fragmentation of beaded apoptopodia (< 1μm), and apoptotic cell bodies consisting of the final rest of the apoptotic cell that does not undergo further breakup [2] (Figure 1B). However, recent evidence indicates that early apoptotic cells generate MVBs via the sphingosine-1-phosphate (S1P) pathway, which release EVs with size and protein composition similar to exosomes, so called apoptotic exosome-like vesicles, although they also contain unique marker proteins [3 *] (Figure 1B).
Figure 1: Biogenesis of EVs in living or dying eukaryotic cells.
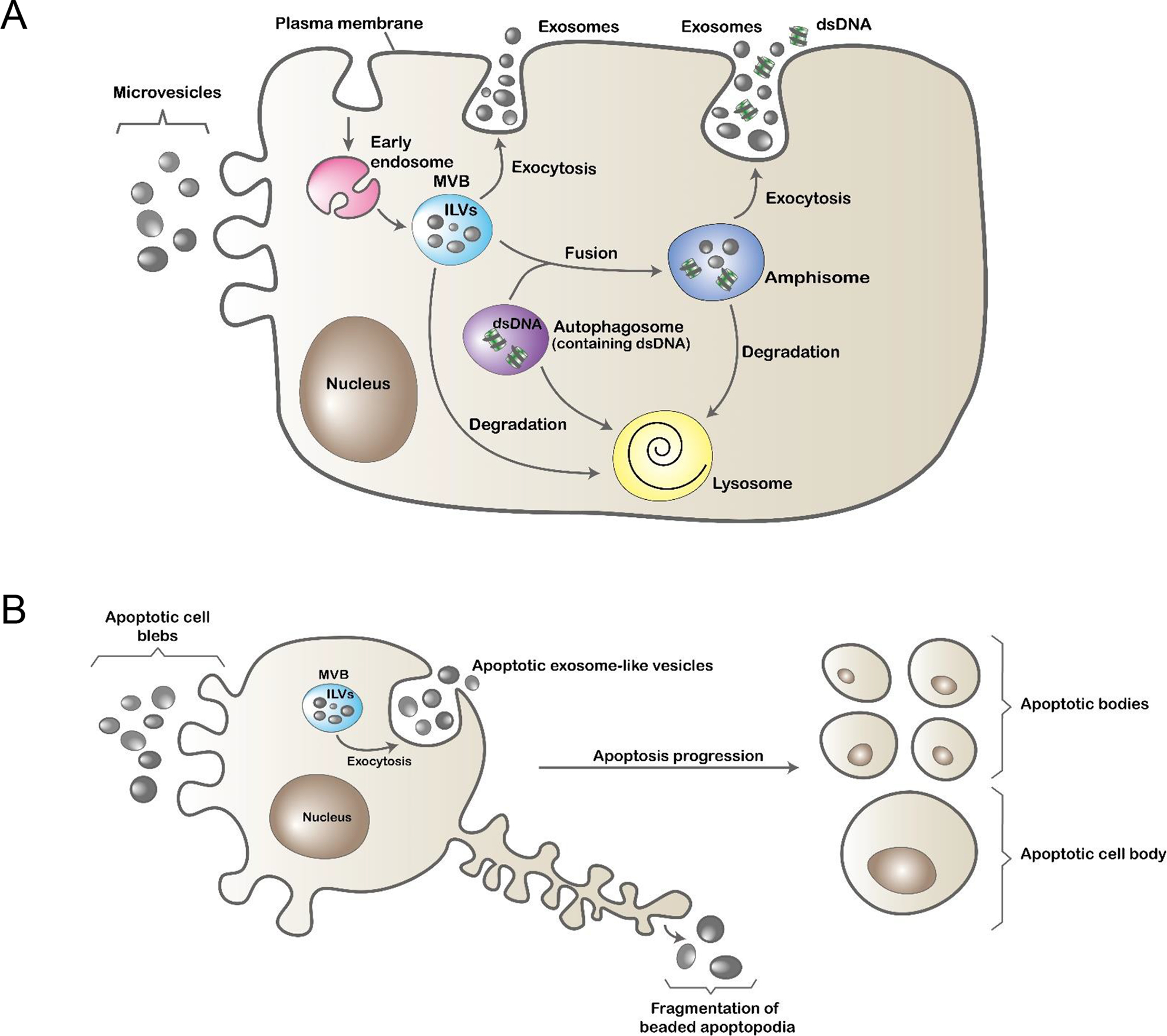
A) Living cells generate different types of EVs via multiple mechanisms. Microvesicles (MVs) are released by shedding of the plasma membrane. Exosomes are generated as intraluminal vesicles (ILVs) by reverse budding of the limiting membrane of early endosomes. Late endosomes containing ILVs are termed multivesicular bodies (MVBs). By fusing with the plasma membrane, MVBs release their cargo of ILVs to the extracellular space or bodily fluids, where the ILVs are termed exosomes. Alternatively, MVBs fuse with autophagosomes, which may contain fragments of double stranded (ds) DNA, forming intracellular vesicles termed amphisomes, which also release their content of ILVs and dsDNA to the extracellular milieu. In the latter case, the dsDNA fragments released by exocytosis are not associated physically to the exosomes secreted simultaneously from the same amphisome. MVBs, autophagosomes, and amphisomes can alternatively merge with lysosomes where their vesicular content is degraded. B) Cells undergoing early apoptosis release apoptotic cell blebs via plasma membrane shedding, apoptotic cell exosome-like vesicles through fusion of MVBs with the cell membrane, and EVs generated by fragmentation of beaded apoptopodia. At later stages of apoptosis, cells disintegrate into apoptotic bodies, some containing small nuclear fragments. The final rest(s) of the apoptotic cell that does not undergo further disintegration and still bears most of the remaining of the cell nucleus is known as apoptotic cell body. Abbreviations: dsDNA, double stranded DNA; ILVs, intraluminal vesicles; MVB, multivesicular body.
EVs carry proteins, lipids, mRNAs, and non-coding RNAs (e.g. miRNAs, long non-coding RNAs, Y RNAs, tRNAs). Some proteins are shared among determined types of EVs and therefore can be used as EV markers [4 **]. Other EV components depend on the lineage of the parent cell and its detection can be used to track the cell type(s) that release the EVs in a tissue or organ. However, within the same cell lineage, the composition of the EVs may differ depending on the stage of activation, neoplastic transformation, infection, stress, and viability of the parent cell(s).
Besides their multiples roles in adaptive B- and T-cell immunity, EVs released by leukocytes or non-hematopoietic cells regulate innate immunity due to their capacity to carry pro- and anti-inflammatory preformed mediators and nuclei acids that, at short or long distances, affect the function of acceptor leukocytes, parenchymal or stromal cells [5,6]. The EV surface also interacts with the complement and coagulation systems, and vice versa, complement activation promotes MV shedding. However, EVs express complement regulators (e.g. CD55, CD59) that protect the EVs from formation of membrane attack complexes on the vesicle surface. The cross-talk between EVs and the complement and coagulation cascades, and its effects on the immune response have been the focus of a recent review and will not be discussed here [7]. Multiple studies have analyzed the properties of EVs released by neutrophils, macrophages (MØs), mast cells (MCs), basophils, eosinophils, NK cells and dendritic cells (DCs), and their influence on leukocytes of the adaptive immune system, which have been summarized in a recent review [8]. The present review will focus on the stimulatory or suppressive effects that EVs of different origins exert on the innate immune response, under normal or pathological conditions including transplantation, the last one a field where so far there is very limited information (Figure 2).
Figure 2: Effects of EVs on innate immunity.
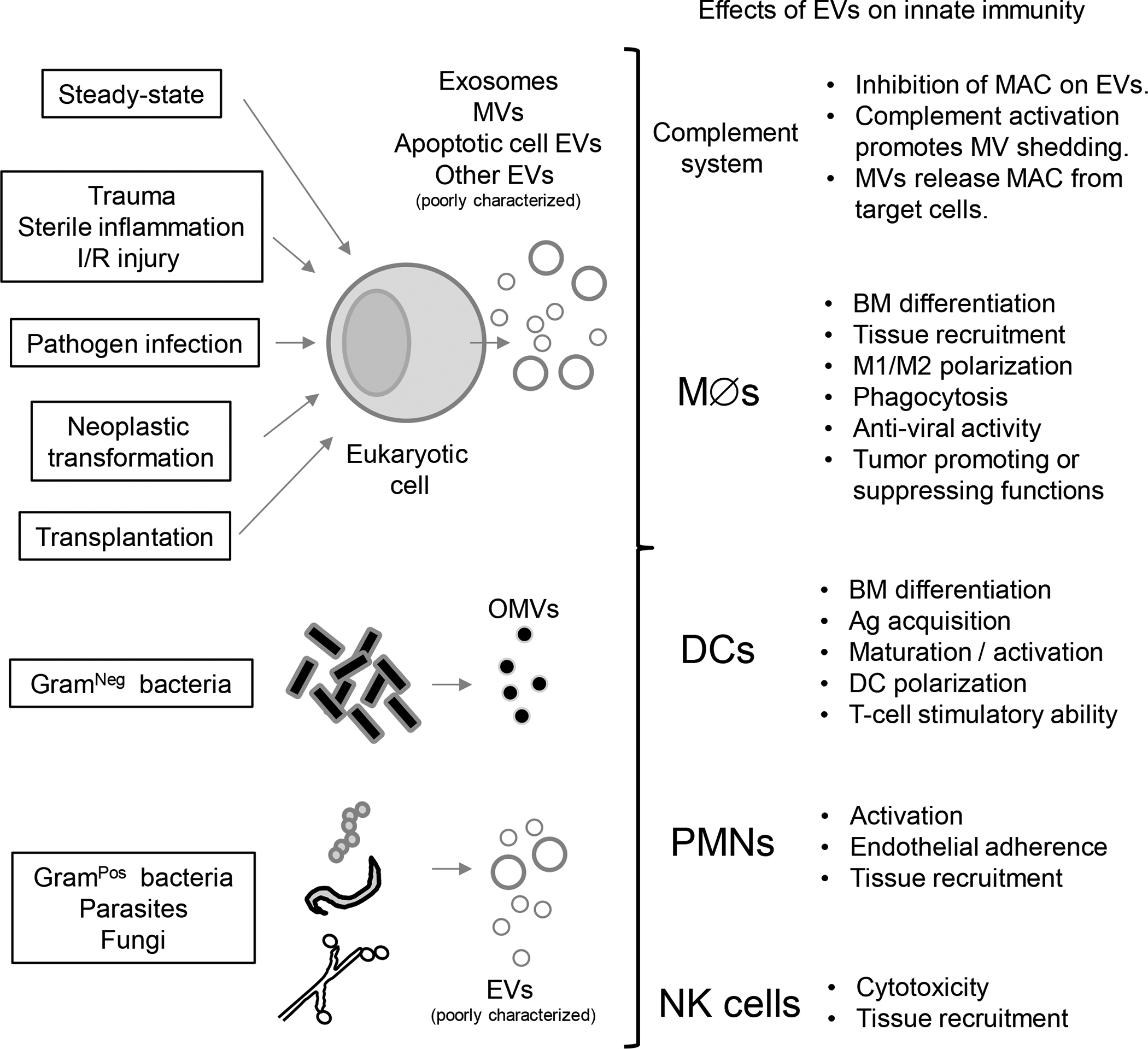
Different types of EVs released by eukaryote cells under steady-state or pathological conditions and by bacteria, parasites or fungi, interact with soluble and cellular components of the innate immune system. Abbreviations: Ag, antigen; BM, bone marrow; DCs, dendritic cells; EVs, extracellular vesicles; I/R, ischemia / reperfusion; MAC, membrane attack complex; MØs, macrophages; MVs, microvesicles; OMVs, outer membrane vesicles; PMNs, polymorphonuclear leukocytes.
STIMULATORY EFFECTS OF EVs ON INNATE IMMUNITY
Stimulatory effects of EVs on innate immunity under sterile conditions:
Under the steady-state or sterile inflammation, parenchymal and stromal cells communicate via EVs with the innate immune system. There is evidence that cells in general react to noxious stimuli by releasing EVs that promote inflammation via different mechanisms. In the lungs, after acid inhalation, epithelial cells increase production of MVs that through transfer of miR-17/221 promote local inflammation via recruitment of MØs [9].
Cells of the innate immune system, particularly MCs, secrete EVs that affect innate immunity. MCs stimulate immunity not only through secretion of preformed mediators stored in granules and de novo synthesized proteins, but also via constitutive or regulated release of EVs [10,11]. By this latter mechanism, activated MCs transfer antigen (Ag) and promote Ag-presenting cells (APC)-maturation and the T-cell stimulatory ability of acceptor DCs [12]. On the other hand, DC-derived exosomes facilitate the function of MCs during anaphylactic reactions. Anaphylaxis is a life-threatening condition triggered by allergen binding to IgE on tissue-resident MCs and possibly circulating basophils, with the consequent cross-linking of IgE, MC degranulation and systemic release of vascular and immune mediators. Allergens in circulation are captured by intravascular extensions of DCs located next to blood vessels [13 **]. The allergens are then passed on, via DC-derived MVs, to allergen-specific IgE bound to MCs [13 **].
EVs also stimulate innate immunity in the nervous system. After peripheral nerve injury, cell bodies of sensory neurons in the dorsal root ganglia (DRG) increase their content of miR-21–5p, which is released locally via exosomes [14 **]. Once secreted, the exosomes are internalized by DRG-infiltrating MØs where miR-21–5p promotes M1-biasing, which contributes to DRG inflammation and neuropathic hypersensitivity [14 **]. In the central nervous system, microglia-derived MVs are responsible for neuroinflammation following traumatic brain injury [15].
Depending on the stimulus that triggers apoptosis, the tissue micro-environment where it occurs, and the efficiency of apoptotic cell clearance, different types of apoptotic cell-derived EVs either promote or down-regulate innate immunity. A recent study has demonstrated that the more recently described apoptotic exosome-like vesicles promote synthesis of interleukin (IL)-1β in MØs [3 *]. Following fusion of the apoptotic exosome-like vesicles with acceptor MØs, the active Gα12/13 proteins coupled to the S1P receptors transferred by the EVs provide the platform to the MØs for NF-κβ activation, subsequent IL-1β mRNA transcription and IL-1β processing by the inflammasome [3 *]. In other models, apoptotic exosome-like vesicles have been shown to inhibit innate immunity by promoting transforming growth factor (TGF) β secretion in MØs [16].
Stimulatory effects of EVs on microbe-induced innate immunity:
Pathogenic and commensal Gram-negative bacteria release EVs (10–300 nm in size) generated by budding of the outer membrane, so termed outer membrane vesicles (OMVs), which carry components of the periplasmic space and the outer bacterial membrane, including lipopolysaccharide (LPS) and lipoproteins. More recently, it has been shown that Gram-positive bacteria, which lack an outer membrane and in which the cell membrane is covered by a peptidoglycan wall, also release EVs. Bacteria-derived EVs contribute to pathogenesis of bacterial infections by delivering resistance to anti-microbials and the complement system, and by transferring virulence factors [17–20]. Bacteria-derived EVs are a vehicle for spreading of pathogen-associated molecular patterns (PAMPs) that trigger pattern recognition receptors (PRRs) signaling, inflammasome activation, stimulate the Stimulator of Interferon (IFN) Genes (STING) pathway, and activate the host’s innate immune cells to control microbial infection and/or amplify tissue damage [17–20]. In some instances, bacteria-derived EVs can also poise the innate immune system for facilitation or amplification of Th2-biased adoptive immune responses. Indeed, during the course of atopic dermatitis, EVs released by Staphylococcus aureus increase secretion the pro-inflammatory and T helper (Th) 2-biasing mediators thymic stromal lymphopoietin, MØ inflammatory protein-1 α (MIP-1α) and eotaxin, which increase the number of eosinophils and worsen the manifestations of the disease [21].
EVs released by bacteria can be pathogenic to the host even when the bacteria are transported by a carrier organism. OMVs from Gram-negative bacteria that colonize house dust mites trigger airway inflammation in mice by stimulating alveolar MØs [22]. The ability of OMVs to co-deliver bacterial Ags plus pro-inflammatory mediators make them good candidates as potential antibacterial vaccines. Bacteria are not the only microbes that release pathogenic EVs that trigger the host’s innate immune response. EVs produced by certain fungi also activate innate immunity by promoting M1 MØ-polarization [23].
Cells infected by pathogens or exposed to PAMPs or IFNs, release EVs carrying PAMPs, pro-inflammatory mediators and anti-viral molecules that enhance the anti-microbial defenses of innate immune leukocytes and parenchymal cells targeted by the EVs [24,25]. In mice, LPS-stimulated MØs release exosomes carrying endoplasmic reticulum aminopeptidase 1, tumor necrosis factor (TNF)-α, IFN-γ and CCL3 that via complementary mechanisms stimulate phagocytosis and nitric oxide production in neighboring MØs [26]. Exosomes released by MØs and liver nonparenchymal cells pre-incubated with type I and II IFNs, exert anti-viral activity on hepatitis B or C virus–replicating hepatocytes [27–29]. Kaposi’s Sarcoma-associated herpesvirus-infected human endothelial cells release EVs that induce IFN-stimulated genes in bystander endothelial cells via activation of the cGAS-STING pathway triggered by mitochondrial DNA carried on the EVs [30]. Herpes simplex virus (HSV-1)-infected cells release STING and STING-related factors packaged in EVs that suppress viral gene expression and replication in acceptor cells [31].
In some cases, the inflammatory response against pathogens requires crosstalk via EVs between different components of the innate immune system. Platelets bound to neutrophils reprocess neutrophil-derived EVs containing arachidonic acid into thromboxane A2, which promotes neutrophil adherence to endothelium and extravasation [32].
Stimulatory effects of tumor-derived EVs on innate immunity:
Monocytes and MØs play either tumor-suppressive or -promoting functions depending on their activation and polarization, which are both affected by tumor-derived EVs. Exosomes from non-metastatic melanomas, unlike those from metastatic melanomas, stimulate innate immunity by expanding Ly6Clow patrolling monocytes in bone marrow (BM) and promoting their differentiation into phagocytic M1 MØs, which remove metastatic cells in the lung [33].
Stimulatory effects of EVs on innate immunity during ischemia / reperfusion (I/R) injury:
Through their effects on the innate immune system, EVs may exacerbate I/R injury, a major complication in transplantation, surgical resection, myocardial infarction and stroke. Liver I/R injury triggers hepatocyte upregulation of IFN Regulatory Factor (IRF)-1, a transcription factor that activates Rab27a, a small GTPase that facilitates EV exocytosis [34 *]. Liver I/R injury not only increases release of hepatic EVs, but also augments the EV content of oxidized phospholipids, which activate neutrophils via Toll-like receptor (TLR)-4 [34 *].
Stimulatory effects of donor EVs on innate immunity after transplantation:
Recent studies have shown that following transplantation, donor MHC cross-dressing of recipient’s APCs is mediated via transfer of clusters of donor-derived EVs that are released directly by the grafts or by passenger leukocytes homed in graft-draining secondary lymphoid tissues [35–37]. A percentage of the transferred EVs remains attached to the recipient’s APC surface within a localized region of the plasma membrane and for enough time, so the donor intact MHC molecules carried by the EVs can be detected by directly allo-reactive T cells via the semi-direct pathway [35]. Recipient’s B cells also recognize donor- and self-derived Ags carried, intact or partially degraded, on the surface of donor EVs [38–40]. Donor EVs are also internalized by recipient’s APCs for Ag-processing or degradation, and possibly for delivery of the intravesicular content into the cytosol of the recipient’s APCs [41]. Thus, it is likely that donor-derived EVs function as a platform for delivery not only of donor Ags but also pro-inflammatory mediators (e.g. DAMPs, RNAs) that activate the recipient’s immune system [35,38]. Indeed, immature DCs that acquire exosomes released by fully-mature allogeneic DCs, up-regulate their surface expression of MHC class-II, CD40 and CD86, and increase their T-cell stimulatory ability [35].
INHIBITORY EFFECTS OF EVs ON INNATE IMMUNITY
Inhibitory effects of EVs on innate immunity under sterile conditions:
Neutrophil-derived MVs have been shown to promote the pro-resolution and wound healing properties of MØs, in part due to EV expression of the pro-resolution protein annexin-1 and EV ability to prevent classical activation of M1 MØs, MØ differentiation into DCs and DC maturation, and to promote TGF-β secretion [42–44]. Indeed, neutrophil-derived MVs exert anti-inflammatory effects when injected intraarticularly in mouse models of rheumatoid arthritis [44,45]. At systemic level, increased numbers of EVs have been detected in circulation of patients undergoing severe tissue trauma, major burns, or sepsis, which negatively correlated with patient survival [46–48]. In such situations, shedding of neutrophil-derived MVs carrying the complement receptor C5aR1 has been associated to reduced C5aR1 expression on neutrophils, which leads to neutrophil dysfunction [7,49].
EVs also control the innate immune response against bioengineered materials. The ability of mammalian extracellular matrix bioscaffolds to promote anti-inflammatory MØs is mediated to some extent to its cargo of matrix-bound EVs and its miRNA content [50].
Adipose tissue MØs control adipose tissue function in healthy conditions and chronic obesity. Adipocytes release exosome-like, lipid-filled EVs that are produced at a higher rate in obese mice [51 **]. The adipocyte-derived EVs promote differentiation of BM progenitors into adipose tissue MØs that hydrolyze the triacyl-glycerides delivered by the EVs creating a local metabolic loop within the adipose tissue [51 **]. Exosomes released by white adipose tissue-derived stem cells from lean mice promote M2 MØ biasing, improve insulin sensitivity, and reduce obesity and hepatic steatosis in diet-induced obese mice [52 *]. Whether adipocyte EVs exert pro-inflammatory effects during onset of obesity remains unknown.
Inhibitory effects of EVs on pathogen-induced innate immunity:
Pathogen-derived EVs may suppress the host’s innate immune response or increase pathogen adsorption / attachment to the host’s cells, both to the microbe’s benefit. This phenomenon has been well documented in parasites. Leishmania-derived EVs decrease TNF-α secretion and augment IL-10 release in infected monocytes, and reduce the Th1-driving ability of DCs, thus priming the host for parasite infection [53]. Heligmosomoides polygyrus, a nematode that infest the mouse intestine, secretes EVs that suppress type 2 innate responses, eosinophilia and IL-33 synthesis. [54]. EVs released by highly-adherent strains of the sexually transmitted parasite Trichomonas vaginalis augment attachment to human cervical epithelial cells of poorly adherent strains of the same parasite [55].
Inhibitory effects on innate immunity of tumor-derived EVs:
Tumor-derived EVs affect tumor growth, invasion and dissemination through their effects on neutrophils, monocytes, MØs and NK cells. This interaction may occur within the tumor microenvironment, draining secondary lymphoid tissues, BM, or non-lymphoid metastatic niches. In certain neoplasias, tumor growth and dissemination have been shown to be facilitated indirectly through the effects of tumor-derived exosomes on tumor-infiltrating neutrophils [56,57].
Tumor-derived EVs also exert pro-tumorigenic effects via M2-biasing of tumor-associated MØs (TAMs). Indeed, EVs released by highly metastatic pancreatic adenocarcinoma cell lines promote differentiation of MØs into pro-tumorigenic M2 cells more efficiently than EVs secreted by less aggressive pancreatic tumors [58]. M2-polarization of TAMs induced by colorectal cancer cells correlates with delivery of mir-145 through EVs, which silences the histone deacetylase HDAC11 that indirectly promotes IL-10 production [59]. Tumor-derived EVs also interact with MØs at longer distances. Subcapsular sinus MØs located in tumor-draining lymph nodes restrain dissemination of melanoma-derived EVs mobilized via lymphatics, preventing interaction of the EVs with B cells and anti-tumor humoral immunity [60]. Exosomes released by metastatic melanomas facilitate tumor dissemination by reprogramming BM progenitors that migrate to future sites of metastasis [61]. Tumor-derived exosomes also suppress differentiation of BM precursors into DCs [62].
EVs also reduce the cytotoxicity against tumors of leukocytes of the innate immune system. Exosomes from plasma of patients with head and neck cancer decrease NKG2D expression on NK cells and suppress their cytotoxic activity against tumor cells [63].
Tumor-derived exosomes can also compromise the anti-viral innate immunity of the host by passing on activated Epidermal Growth Factor receptor (EGFR) from tumor cells to MØs. The transferred EGFR activates MEKK2 that phosphorylates the Ser173 of IFN regulatory factor (IRF) 3, a transcription factor key for production of type I IFNs. Ser173 phosphorylation of IRF3 blocks its dimerization and consequent nuclear translocation, and triggers poly-ubiquitination and degradation of the transcription factor [64 **].
Inhibitory effects of EVs on innate immunity during I/R injury:
Some of the beneficial effects of cell-based therapies on I/R injury, initially assumed to be caused by the immuno-regulatory, pro-resolution or tissue regenerative properties of the injected cells, are indeed mediated by their EVs through regulatory effects on innate immunity. In a pig model of acute myocardial infarction, intramyocardial administration of exosomes released by cardiosphere-derived cells recapitulates the cardioprotective effects of the parent cells, by inhibiting accumulation of MØs at the infarct border and shifting MØ differentiation away from M1 polarization [65]. Similarly in mice, local administration of mesenchymal stromal cell-derived exosomes attenuates myocardial I/R injury by promoting M2 polarization and reducing TLR-4 activity in infiltrating MØs, a phenomena mediated likely by delivery of miR-182 through EVs [66]. Interestingly, a non-coding Y RNA fragment highly enriched in human cardiosphere-derived EVs promotes IL-10 synthesis in MØs and confers cardioprotection in a rat model of I/R injury [67].
EVs released by mesenchymal stromal or stem cells administered systemically exert protective effects against I/R injury in kidney, liver and lung [68–70]. In rat models, these beneficial effects were associated to reduction of renal NK cell infiltration and amelioration of hepatic neutrophil inflammation and oxidative stress, the latter via delivery of the antioxidant enzyme manganese superoxide dismutase through the injected EVs [68,69 *].
Inhibitory effects of EV-based therapies on innate immunity during graft rejection:
Systemic injection of exosomes produced by donor-derived immature DCs, alone or in combination with suboptimal doses of immunosuppressants or regulatory T cells, have been shown to decrease the anti-donor T-cell response and prolong heart, liver and intestine allograft survival in murine models [71–73]. However, whether such beneficial effects are mediated via interaction of the donor-derived EVs with leukocytes of the innate or adaptive immune system remains unknown.
CONCLUSION
The influence that microbe- and host-derived EVs exert on subsets of leukocytes of the innate immune system is beginning to be elucidated. Comparisons between different studies, generalizations on the effects of EVs on innate immunity, and attribution of specific effects of a particular type of EVs to a given function of a subset of leukocytes, have to be all done cautiously, since laboratories have employed different methods of EV isolation and distinct criteria for naming the vesicles. In absence of reliable discriminatory markers for EVs, the best criteria to identify individual subtypes of EVs is its biogenesis, which can’t be tracked back in EVs harvested from bodily fluids or cell culture supernatants. Besides, most of the previous studies have explored the effects of EVs on innate immunity using in vitro or ex vivo systems. There are ongoing efforts to develop new animal models and improve the few ones in existence, to follow the fate and biology of endogenous EVs in vivo, by using high resolution intravital multiphoton microscopy, and the inclusion of lack-of-function or gain-of-function models, which together will provide the final evidence of the biological impact of EVs on innate immunity in vivo. The rapidly accumulating information on the crosstalk between EVs and the immune system will likely have an impact on future development of biomarkers, vaccines and therapeutic approaches.
KEY POINTS.
Extracellular vesicles (EVs) include a heterogenous family of vesicles released by prokaryote or eukaryote cells, alive or dying, with different biogenesis, composition and function.
EVs target leukocytes of the innate and adaptive immune systems via interaction with cell surface receptors or intra-cellular delivery of inflammatory mediators, receptors, enzymes, mRNAs and non-coding RNAs.
EVs from microorganisms and host’s cells may stimulate or inhibit the innate immune response through multiple mechanisms.
EVs regulate the extent of ischemia / reperfusion (I/R) injury and allo-sensitization in murine models of transplantation via their effects on the recipient’s innate and adaptive immune responses.
The ability of EVs to carry antigen, plus their stimulatory or suppressive effects on the innate and adaptive immune responses, make EVs good candidates as platforms for positive or negative vaccination.
Financial support and sponsorship
This work was supported by grants from the NIH (R01-HL130191 and R01-AI148690 to A.E. Morelli, and R01AR068249 and R01AR071277 to A.T. Larregina).
Footnotes
Conflict of Interest
The authors of this manuscript have no conflict of interest to disclose.
References and recommended reading
Papers of particular interest, published within the annual period review, have been highlighted as:
(*) of special interest
(**) of outstanding interest
- 1.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014, 30:255–289. [DOI] [PubMed] [Google Scholar]
- 2.Poon IKH, Parkes MAF, Jiang L, et al. Moving beyond size and phosphatidylserine exposure: evidence for a diversity of apoptotic cell-derived extracellular vesicles in vitro. J Extracell Vesicles 2019, 8:1608786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park SJ, Kim JM, Kim J, et al. Molecular mechanisms of biogenesis of apoptotic exosome-like vesicles and their roles as damage-associated molecular patterns. Proc Natl Acad Sci U S A 2018, 115:E11721–E11730. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Characterization of the recently described apoptotic exosome-like vesicles and its biogenesis via the shipngosine-1 phosphate (S1P) – S1P receptor pathway. Analysis of the mechanism by which such EVs promote IL-1β secretion in target MØs.
- 4.Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of Exosome Composition. Cell 2019, 177:428–445 e418. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** A thorough analysis by high resolution density gradient, of protein, RNA and DNA composition of small EVs and the non-vesicular extracellular matter that usually contaminates EV samples. Demonstration that small EVs are not carriers of DNA, which can be released together with the EVs -but not physically associated to the EVs- via an autophagy- and MVB-dependent mechanism.
- 5.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009, 9:581–593. [DOI] [PubMed] [Google Scholar]
- 6.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 2014, 14:195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karasu E, Eisenhardt SU, Harant J, et al. Extracellular Vesicles: Packages Sent With Complement. Front Immunol 2018, 9:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groot Kormelink T, Mol S, de Jong EC, et al. The role of extracellular vesicles when innate meets adaptive. Semin Immunopathol 2018, 40:439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H, Zhang D, Wu J, et al. Lung Epithelial Cell-Derived Microvesicles Regulate Macrophage Migration via MicroRNA-17/221-Induced Integrin beta1 Recycling. J Immunol 2017, 199:1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll-Portillo A, Surviladze Z, Cambi A, et al. Mast cell synapses and exosomes: membrane contacts for information exchange. Front Immunol 2012, 3:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moon TC, Befus AD, Kulka M. Mast cell mediators: their differential release and the secretory pathways involved. Front Immunol 2014, 5:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skokos D, Botros HG, Demeure C, et al. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol 2003, 170:3037–3045. [DOI] [PubMed] [Google Scholar]
- 13.Choi HW, Suwanpradid J, Kim IH, et al. Perivascular dendritic cells elicit anaphylaxis by relaying allergens to mast cells via microvesicles. Science 2018, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** During anaphylaxis, blood-borne Ags are sampled by intravascular prolongations of perivascular DCs. The DCs then transfer the captured Ags via MVs to perivascular MCs, a phenomenon that triggers MC degranulation and amplification of the inflammatory response.
- 14.Simeoli R, Montague K, Jones HR, et al. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat Commun 2017, 8:1778. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Upon nerve injury, DRG neuron cell bodies release EVs that through delivery of miR-21–5p promote M1-polarization of target MØs. Deletion of miR-21–5p decreases neuropathic hypersensitivity and inflammatory MØ recruitment in the DRG.
- 15.Kumar A, Stoica BA, Loane DJ, et al. Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J Neuroinflammation 2017, 14:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H, Kasagi S, Chia C, et al. Extracellular Vesicles from Apoptotic Cells Promote TGFbeta Production in Macrophages and Suppress Experimental Colitis. Sci Rep 2019, 9:5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos JC, Dick MS, Lagrange B, et al. LPS targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation. EMBO J 2018, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Codemo M, Muschiol S, Iovino F, et al. Immunomodulatory Effects of Pneumococcal Extracellular Vesicles on Cellular and Humoral Host Defenses. MBio 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Yoon YJ, Kim JH, et al. Outer Membrane Vesicles Derived From Escherichia coli Regulate Neutrophil Migration by Induction of Endothelial IL-8. Front Microbiol 2018, 9:2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nandakumar R, Tschismarov R, Meissner F, et al. Intracellular bacteria engage a STING-TBK1-MVB12b pathway to enable paracrine cGAS-STING signalling. Nat Microbiol 2019, 4:701–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong SW, Kim MR, Lee EY, et al. Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation. Allergy 2011, 66:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi JP, Jeon SG, Kim YK, et al. Role of house dust mite-derived extracellular vesicles in a murine model of airway inflammation. Clin Exp Allergy 2019, 49:227–238. [DOI] [PubMed] [Google Scholar]
- 23.Bitencourt TA, Rezende CP, Quaresemin NR, et al. Extracellular Vesicles From the Dermatophyte Trichophyton interdigitale Modulate Macrophage and Keratinocyte Functions. Front Immunol 2018, 9:2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatnagar S, Shinagawa K, Castellino FJ, et al. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 2007, 110:3234–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatnagar S, Schorey JS. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J Biol Chem 2007, 282:25779–25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goto Y, Ogawa Y, Tsumoto H, et al. Contribution of the exosome-associated form of secreted endoplasmic reticulum aminopeptidase 1 to exosome-mediated macrophage activation. Biochim Biophys Acta Mol Cell Res 2018, 1865:874–888. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Liu K, Liu Y, Xu Y, et al. Exosomes mediate the cell-to-cell transmission of IFN-alpha-induced antiviral activity. Nat Immunol 2013, 14:793–803. [DOI] [PubMed] [Google Scholar]
- 28.Cai C, Koch B, Morikawa K, et al. Macrophage-Derived Extracellular Vesicles Induce Long-Lasting Immunity Against Hepatitis C Virus Which Is Blunted by Polyunsaturated Fatty Acids. Front Immunol 2018, 9:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao Z, Jia X, Megger DA, et al. Label-Free Proteomic Analysis of Exosomes Secreted from THP-1-Derived Macrophages Treated with IFN-alpha Identifies Antiviral Proteins Enriched in Exosomes. J Proteome Res 2019, 18:855–864. [DOI] [PubMed] [Google Scholar]
- 30.Jeon H, Lee J, Lee S, et al. Extracellular Vesicles From KSHV-Infected Cells Stimulate Antiviral Immune Response Through Mitochondrial DNA. Front Immunol 2019, 10:876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deschamps T, Kalamvoki M. Extracellular Vesicles Released by Herpes Simplex Virus 1-Infected Cells Block Virus Replication in Recipient Cells in a STING-Dependent Manner. J Virol 2018, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossaint J, Kuhne K, Skupski J, et al. Directed transport of neutrophil-derived extracellular vesicles enables platelet-mediated innate immune response. Nat Commun 2016, 7:13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plebanek MP, Angeloni NL, Vinokour E, et al. Pre-metastatic cancer exosomes induce immune surveillance by patrolling monocytes at the metastatic niche. Nat Commun 2017, 8:1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang MQ, Du Q, Goswami J, et al. Interferon regulatory factor 1-Rab27a regulated extracellular vesicles promote liver ischemia/reperfusion injury. Hepatology 2018, 67:1056–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]; * By using murine in vivo models and human hepatocyte cell lines, the study demonstrates that Rab27a -a GTPase that controls exosome exocytosis- is upregulated in hypoxic hepatocytes, in livers undergoing warm I/R, and in hepatic isografts. The Rab27a increase leads to increase secretion of hepatocyte-derived EVs with higher content of oxidized phospholipids on their surface, which activate TLR-4 on neutrophils.
- 35.Liu Q, Rojas-Canales DM, Divito SJ, et al. Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J Clin Invest 2016, 126:2805–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marino J, Babiker-Mohamed MH, Crosby-Bertorini P, et al. Donor exosomes rather than passenger leukocytes initiate alloreactive T cell responses after transplantation. Sci Immunol 2016, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng F, Morelli AE. Extracellular vesicle-mediated MHC cross-dressing in immune homeostasis, transplantation, infectious diseases, and cancer. Semin Immunopathol 2018, 40:477–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dieude M, Bell C, Turgeon J, et al. The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci Transl Med 2015, 7:318ra200. [DOI] [PubMed] [Google Scholar]
- 39.Gunasekaran M, Xu Z, Nayak DK, et al. Donor-Derived Exosomes With Lung Self-Antigens in Human Lung Allograft Rejection. Am J Transplant 2017, 17:474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma M, Liu W, Perincheri S, et al. Exosomes expressing the self-antigens myosin and vimentin play an important role in syngeneic cardiac transplant rejection induced by antibodies to cardiac myosin. Am J Transplant 2018, 18:1626–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montecalvo A, Larregina AT, Shufesky WJ, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 2012, 119:756–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eken C, Gasser O, Zenhaeusern G, et al. Polymorphonuclear neutrophil-derived ectosomes interfere with the maturation of monocyte-derived dendritic cells. J Immunol 2008, 180:817–824. [DOI] [PubMed] [Google Scholar]
- 43.Dalli J, Norling LV, Renshaw D, et al. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood 2008, 112:2512–2519. [DOI] [PubMed] [Google Scholar]
- 44.Rhys HI, Dell’Accio F, Pitzalis C, et al. Neutrophil Microvesicles from Healthy Control and Rheumatoid Arthritis Patients Prevent the Inflammatory Activation of Macrophages. EBioMedicine 2018, 29:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Headland SE, Jones HR, Norling LV, et al. : Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci Transl Med 2015, 7:315ra190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogura H, Kawasaki T, Tanaka H, et al. Activated platelets enhance microparticle formation and platelet-leukocyte interaction in severe trauma and sepsis. J Trauma 2001, 50:801–809. [DOI] [PubMed] [Google Scholar]
- 47.O’Dea KP, Porter JR, Tirlapur N, et al. Circulating Microvesicles Are Elevated Acutely following Major Burns Injury and Associated with Clinical Severity. PLoS One 2016, 11:e0167801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lehner GF, Harler U, Haller VM, et al. Characterization of Microvesicles in Septic Shock Using High-Sensitivity Flow Cytometry. Shock 2016, 46:373–381. [DOI] [PubMed] [Google Scholar]
- 49.Unnewehr H, Rittirsch D, Sarma JV, et al. Changes and regulation of the C5a receptor on neutrophils during septic shock in humans. J Immunol 2013, 190:4215–4225. [DOI] [PubMed] [Google Scholar]
- 50.Huleihel L, Bartolacci JG, Dziki JL, et al. Matrix-Bound Nanovesicles Recapitulate Extracellular Matrix Effects on Macrophage Phenotype. Tissue Eng Part A 2017, 23:1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flaherty SE 3rd, Grijalva A, Xu X, et al. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science 2019, 363:989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Adipocytes release lipid-filled vesicles that are a source of lipids for neighboring MØs and promote differentiation of BM precursors into adipose tissue MØs.
- 52.Zhao H, Shang Q, Pan Z, et al. Exosomes From Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes 2018, 67:235–247. [DOI] [PubMed] [Google Scholar]; * Exosomes from adipose-derived stem cells promote M2-polarization of MØs in vitro and when administered systemically in obese mice, they improve insulin sensitivity, reduce hepatic steatosis, and decrease obesity.
- 53.Silverman JM, Clos J, Horakova E, et al. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J Immunol 2010, 185:5011–5022. [DOI] [PubMed] [Google Scholar]
- 54.Buck AH, Coakley G, Simbari F, et al. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat Commun 2014, 5:5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Twu O, de Miguel N, Lustig G, et al. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate hostratioparasite interactions. PLoS Pathog 2013, 9:e1003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bobrie A, Krumeich S, Reyal F, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res 2012, 72:4920–4930. [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, Shi H, Yuan X, et al. Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Mol Cancer 2018, 17:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Linton SS, Abraham T, Liao J, et al. Tumor-promoting effects of pancreatic cancer cell exosomes on THP-1-derived macrophages. PLoS One 2018, 13:e0206759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shinohara H, Kuranaga Y, Kumazaki M, et al. Regulated Polarization of Tumor-Associated Macrophages by miR-145 via Colorectal Cancer-Derived Extracellular Vesicles. J Immunol 2017, 199:1505–1515. [DOI] [PubMed] [Google Scholar]
- 60.Pucci F, Garris C, Lai CP, et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science 2016, 352:242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peinado H, Aleckovic M, Lavotshkin S, et al. : Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012, 18:883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu S, Liu C, Su K, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol 2007, 178:6867–6875. [DOI] [PubMed] [Google Scholar]
- 63.Ludwig S, Floros T, Theodoraki MN, et al. Suppression of Lymphocyte Functions by Plasma Exosomes Correlates with Disease Activity in Patients with Head and Neck Cancer. Clin Cancer Res 2017, 23:4843–4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao L, Wang L, Dai T, et al. Tumor-derived exosomes antagonize innate antiviral immunity. Nat Immunol 2018, 19:233–245. [DOI] [PubMed] [Google Scholar]; ** Tumors can suppress the patient’s antiviral innate immunity through release of tumor-derived exosomes that promote degradation of a transcription factor key for production of type I IFNs in target MØs.
- 65.de Couto G, Gallet R, Cambier L, et al. Exosomal MicroRNA Transfer Into Macrophages Mediates Cellular Postconditioning. Circulation 2017, 136:200–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao J, Li X, Hu J, et al. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res 2019, 115:1205–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cambier L, de Couto G, Ibrahim A, et al. Y RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL-10 expression and secretion. EMBO Mol Med 2017, 9:337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zou X, Gu D, Zhang G, et al. NK Cell Regulatory Property is Involved in the Protective Role of MSC-Derived Extracellular Vesicles in Renal Ischemic Reperfusion Injury. Hum Gene Ther 2016, 27:926–935. [DOI] [PubMed] [Google Scholar]
- 69.Yao J, Zheng J, Cai J, et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J 2019, 33:1695–1710. [DOI] [PubMed] [Google Scholar]; * Systemic administration of EVs released by mesenchymal stem cells protects the liver against warm I/R injury by decreasing infiltration of neutrophils and tissue oxidative stress, a phenomenon associated to passage via EVs of the antioxidant enzyme manganese superoxidase dismutase.
- 70.Stone ML, Zhao Y, Robert Smith J, et al. Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia-reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir Res 2017, 18:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peche H, Heslan M, Usal C, et al. Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection. Transplantation 2003, 76:1503–1510. [DOI] [PubMed] [Google Scholar]
- 72.Yang X, Meng S, Jiang H, et al. Exosomes derived from immature bone marrow dendritic cells induce tolerogenicity of intestinal transplantation in rats. J Surg Res 2011, 171:826–832. [DOI] [PubMed] [Google Scholar]
- 73.Li X, Li JJ, Yang JY, et al. Tolerance induction by exosomes from immature dendritic cells and rapamycin in a mouse cardiac allograft model. PLoS One 2012, 7:e44045. [DOI] [PMC free article] [PubMed] [Google Scholar]


