Abstract
A 56-year-old paramedic was admitted to hospital and treated for severe pneumonia. Shortly after initiating antibiotic treatment (including moxifloxacin), he developed bilaterally painful eyes and was diagnosed with bilateral acute anterior uveitis (AAU). Three years later, he was referred to the ophthalmology clinic with bilateral iris transillumination suggesting iris atrophy and limited pupillary dilation, indicating iris sphincter muscle paralysis. AAU typically presents unilaterally. An onset of bilateral AAU is unusual and warrants investigation for underlying systemic cause. The fluoroquinolone moxifloxacin has been reported in a limited number of cases as a causative agent of bilateral AAU and iris atrophy. This case provides additional supporting evidence that moxifloxacin may cause degradation of collagen and iris muscle in the eye, as well as elsewhere in the body, such as in blood vessels. Additionally, we present novel anterior segment ocular imaging (using optical coherence tomography) demonstrating the ability to detect iris atrophy using non-invasive imaging.
Keywords: iris, anterior chamber
Background
The recognition of ophthalmic conditions, alongside treatment and/or appropriate referral is an important, yet perhaps daunting skill required of non-ophthalmic doctors. We report a case of a patient who developed bilateral acute anterior uveitis (AAU) with iris atrophy following systemic administration of moxifloxacin.
AAU, or inflammation of the iris and ciliary body, has an incidence of 17–52 patients per 100 000 of the population each year and accounts for 50%–60% of all uveitis seen in tertiary care.1 AAU typically presents with ocular pain, red eye and photophobia. Blurring of vision and pupil irregularity can occur due to the formation of posterior synechiae, an adhesion between the iris and lens. Examination using slit-lamp biomicroscopy may additionally reveal the presence of cells (white blood cells floating in the aqueous humour) and flare (a haziness of the aqueous humour caused by protein leakage) in the anterior chamber.2 Left untreated, complications such as cystoid macular oedema, cataract and glaucoma can develop, subsequently leading to visual loss.3
Treatment consists of a tapering course of steroid eye drops and cycloplegic-mydriatics to relieve pain, while investigating and treating the underlying cause. Biological agents can also be considered in uncontrolled and recurring cases. Treatment and review should always be overseen by an ophthalmologist.4
Case presentation
A 56-year-old paramedic was referred to ophthalmology by his optometrist, who noted difficulty in pupillary dilation and irregularity of the pupil shape despite topical administration of 1% tropicamide and 2.5% phenylephrine. Also noted on examination was bilateral transillumination of the iris and pigment deposition on the anterior lens surface. A referral was made for review by the ophthalmology team due to concerns about increased risk of acute glaucoma due to aqueous outflow obstruction caused by deposition of pigment in the trabecular meshwork. The patient’s medical history included well-controlled type 2 diabetes mellitus and a splanchnic vein thrombosis 7 years previously, with haematological investigation revealing no identifiable cause. He was otherwise well. His medication history included warfarin once a day (lifelong), metformin 1 g two times per day, linagliptin 5 mg once a day, pioglitazone 45 mg once a day and atorvastatin 20 mg once a day.
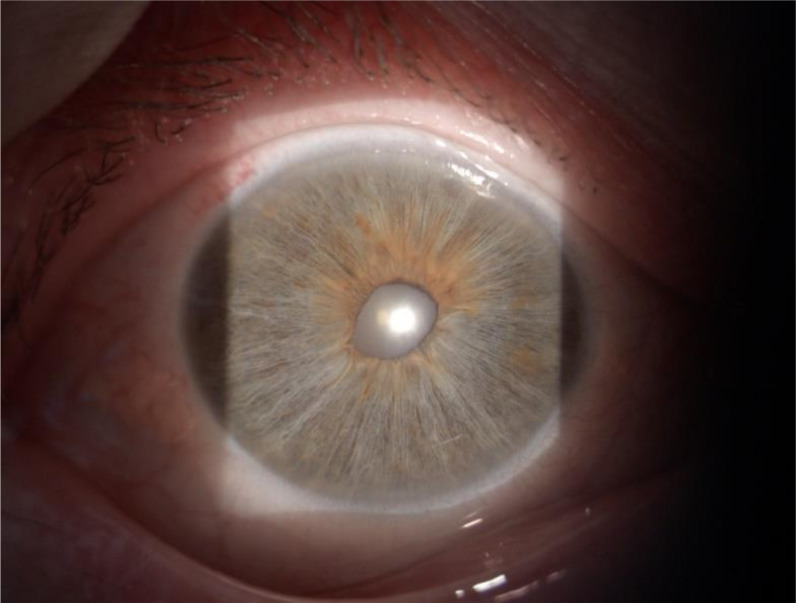
When seen in ophthalmology clinic, the patient had good visual acuity (6/6 left eye and 6/12 right eye). There was no free pigment in the anterior chamber, aside from some minimal pigment granules in the inferior iridocorneal angle visualised via gonioscopy, and no anterior chamber inflammatory cells or flare. Fundal examination was unremarkable and intraocular pressures were normal at 12 mm Hg bilaterally. However, by directing a short-slit beam through the pupil (transillumination technique), it was noted that the patient had marked diffuse bilateral thinning of the iris, poor pupillary dilation and an abnormally shaped pupil (figures 1 and 2).
Figure 1.
Bilateral iris transillumination. The images demonstrate slit-lamp examination of the anterior segment of the patient’s (A) right and (B) left eye. A short beam of light has been shone through the patient’s pupil reflecting off the retinal surface (transillumination technique). The atrophic iris allows penetration of light through producing the transilluminated appearance.
Figure 2.
Abnormally shaped pupil. Slit-lamp image of the anterior segment of the patient’s right eye. Abnormality of the pupil shape can be appreciated. In this patient’s case, the abnormal appearance of the pupil is likely to be due to iris muscle sphincter atrophy. More commonly, in recurrent and untreated anterior uveitis this can be caused by the formation of posterior synechiae, adherence of the lens to the iris. There is also of mottling of the iris. There was evidence of difficulty in dilation of the pupil, due to sphincter muscle paralysis.
On questioning, it transpired that 3 years prior, the patient was admitted to hospital for bilateral pneumonia. While admitted to the intensive care unit he received an array of antibiotics and antivirals, including moxifloxacin, vancomycin, rifampicin, clindamycin and oseltamivir. Three days after commencement of moxifloxacin, the patient reported severe bilateral eye pain. This was not treated during his admission, but on discharge the patient presented to eye casualty. He had evidence of cells and flare with irregularly shaped pupils. He was subsequently diagnosed with bilateral AAU and treated with a tapering course of steroids eye drops (six drops/day for 1 week, four drops/day for 2 weeks, then two drops/day for 2 weeks) and cyclopentolate 1% two times per day for 2 weeks. He was not followed up and did not represent to eye casualty, having no further episodes of eye pain. The patient did not report any lasting visual symptoms, aside from ongoing difficulty adjusting from light to dark conditions.
Investigations
Although the clinical findings were apparent on slit-lamp examination, anterior segment optical coherence tomography (AS-OCT) images were acquired and provided useful cross-sectional information to support the examination findings. We used a swept-source anterior segment (AS)-OCT device (ANTERION, Heidelberg Engineering, Heidelberg, Germany),5 which revealed marked iris thinning and light penetration through the iris which was not immediately obvious without the slit-lamp transillumination technique (figure 3). Although AS-OCT is commonly used to visualise structures such as the cornea, conjunctiva and sclera, the newer swept-source device used here employs a longer wavelength light source with improved tissue penetration.6 This allows light penetration through the iris, as demonstrated in figure 3, in cases of marked iris atrophy. Additionally, the iris thickness is visibly thinner on cross-sectional AS-OCT view compared with a normal iris (figure 3), which cannot be easily detected on slit-lamp examination.
Figure 3.
(A)–(C) Swept-source anterior segment OCT images demonstrating iris atrophy and transillumination using the ANTERION device (Heidelberg Engineering). A cross section of the patient’s (A) right and (B) left eye is shown, with the infrared en face image located in the bottom right corner of each figure. The cornea, anterior chamber and iris can be clearly visualised (labelled). A healthy eye is shown in (C). In comparison, marked iris atrophy can be visualised in the patient’s OCT scans (red triangles vs green triangles in the normal eye). Penetrating light also allows the patient’s lens to be visualised beyond the iris (A) and (B) yellow Asterix), while this cannot be appreciated on the normal eye ((C) blue Asterix). Note that the lens is normally visible through the pupil.
Differential diagnosis
Thirty to sixty per cent of AAU cases do not have an identifiable cause and are termed idiopathic.1 There is a strong association with Human Leukocyte Antigen B27 (HLA-B27) spondyloarthropathies, particularly ankylosing spondylitis, but also Reiter’s syndrome, reactive and psoriatic arthritis.1 2 Various infective causes should also be considered, including herpes virus, syphilis, toxoplasma, cytomegalovirus, rubella, tuberculosis (TB) and Lyme disease.1 Viral causes such as herpetic subtypes may present with increased intraocular pressure acutely and persisting iris atrophy after the acute symptoms of redness, pain and photophobia have resolved.7 Most cases of AAU affect one eye at a time (although both eyes may be sequentially involved), and simultaneous bilateral involvement should raise suspicion of an underlying systemic disease, such as sarcoidosis, Behçet's disease, diabetes mellitus, vascular disease (carotid artery stenosis)8 and renal disease (tubulointerstitial nephritis-induced uveitis (TINU) or IgA nephropathy).9
The diffuse pattern of iris atrophy, in combination with iris sphincter muscle paralysis, is most likely to be caused by AAU with an underlying viral aetiology. However, it is uncommon for viral AAU to present in a simultaneous symmetrical fashion. It is also uncommon for a single episode of AAU to result in the significant degree of iris atrophy and sphincter muscle paralysis as seen in this patient. Given the temporal relationship with moxifloxacin administration, and the known collagen-associated complications with fluoroquinolones,10 it was determined that this was likely a case of moxifloxacin-induced AAU.
Outcome and follow-up
Following the patient’s initial treatment in 2016, he had no further investigation, due to his continued oral antibiotic treatment for pneumonia having only recently been discharged from hospital. No further follow-up notes could be traced following his initial visit to the eye casualty, and our patient reports no further ophthalmology appointments.
The patient reports no further symptoms consistent with AAU. He now remains on routine ophthalmology follow-up. The patient has been able to return to work as a paramedic, although he does continue to report difficulty adjusting from light/dark environments.
Discussion
This patient was diagnosed with AAU secondary to moxifloxacin. This is a rare but recognised side effect with a small number of cases being reported in the medical literature.11–18 Bringas et al13 were the first to describe the case of a patient who developed bilateral AAU and pigment dispersion after receiving moxifloxacin. Other case studies16–18 demonstrated patients developing bilateral uveitis 7–10 days following administration of systemic moxifloxacin. Bilateral iris transillumination, mydriatic pupils and iris sphincter paralysis, all indicators of iris atrophy, were commonly identified examination findings.15–18 The ocular symptoms following administration of moxifloxacin have also been found to be persistent in follow-up.18
Patients with suspected active uveitis should be seen urgently by an ophthalmologist for slit-lamp examination and diagnosis, to avoid irreversible vision loss.4 The diagnosis of bilateral uveitis can be an indicator of underlying systemic disease. Investigations should be guided by a thorough history and findings on examination. Testing is frequently not undertaken for ‘typical’ unilateral AAU, but for bilateral anterior or posterior disease it is common practice to undertake ACE (sarcoid), syphilis/HIV serology, Quantiferon gold (TB), urine analysis (diabetes, TINU) and chest X-ray (sarcoid, TB).2 4 Additional testing will be guided by specific clinical symptoms and signs.2 A full autoimmune screen was not performed on this occasion by the ophthalmologist, as the ocular findings were clearly old and the patient had been quiescent for many years.
AAU is usually treated with a tapering course of corticosteroid eye drops to reduce inflammation as well as cycloplegic-mydriatic drugs, such as cyclopentolate 1%, to reduce ocular pain and prevent adhesions between the iris and lens. Second line immunosuppressants and biological agents can be considered in those with chronic or refractory disease.2 4 Although most patients with anterior uveitis have a good visual prognosis, sight-threatening complications may occur with secondary glaucoma being a particular concern. In this patient’s case, there was only minimal free pigment within the anterior chamber with normal intraocular pressures.
Moxifloxacin is a third generation, broad spectrum fluoroquinolone. It has been demonstrated to have a high tissue affinity and uptake, particularly in pigmented tissue19 such as the iris, compared with other fluoroquinolones, such as levofloxacin. Sampling of aqueous humour following moxifloxacin administration demonstrates high concentrations of the drug.20 Although the exact mechanisms of moxifloxacin-induced uveitis are unknown, it is hypothesised that the drug is directly toxic to iris pigment, based on data demonstrating sensitisation of skin to phototoxicity following moxifloxacin administration.19 This direct toxicity may account for the iris atrophy, mydriatic pupil and sphincter muscle paralysis which gives rise to the clinical findings reported in patients with moxifloxacin-induced uveitis.16–18 An alternative hypothesis is that moxifloxacin causes a disruption of collagen tissue in the smooth muscles of the sphincter pupillae muscles. Fluoroquinolones are known to cause collagen degradation giving rise to such conditions as achilles tendonopathy and rupture, and it has also been found that there are higher rates of aortic aneurysm and dissection, possibly due to the same mechanism.2 10 Therefore, it is possible that a similar process occurs with the collagen tissue of the iris.
The choice to use moxifloxacin should be done with due consideration for the potential adverse effects it can have across multiple systems, our patient’s case of an ocular complication highlighting one effect which may be easily overlooked.
Patient’s perspective.
I had a very difficult time in hospital being trialled on various antibiotics for the pneumonia I had developed, as well as eventually needing to go to the intensive care unit. I was tired but relieved to get better to the point of discharge, but developed very painful eyes just before leaving the hospital. I did tell the hospital doctors at the time, but I do not think it was felt to be anything serious. I went to the eye casualty shortly after getting home, and was diagnosed with uveitis. I was prescribed drops which made my symptoms better, and I did not have any similar episodes. Despite this, I found I had this lasting difficulty when moving from environments with different lighting. It was like my eyes were taking much longer to adjust than before I was admitted to hospital. This did not go away, and it was annoying, but I got used to it and did not think too much more about it. Having got better from a severe pneumonia, in comparison, this was quite minor!
I have been able to return to my work as a paramedic, and despite the continuing difficulty with light adjustment, I manage by allowing myself more time to adapt to conditions as well as wearing sunglasses. Even though my eyes have been affected by this antibiotic, I have still been able to regain normality to my life, and so feel fortunate in that respect.
Learning points.
Moxifloxacin is a rare but recognised cause of bilateral acute anterior uveitis (AAU).
Simultaneous bilateral AAU is unusual and warrants investigation for underlying systemic disease. Suspected AAU should be referred to and be managed by an ophthalmologist.
Appropriate slit examination demonstrates bilateral iris transillumination, limited dilation and abnormal shaped pupils due to iris atrophy and sphincter muscle paralysis. Examination findings can be supported with optical coherence tomography images of the anterior segment of the eye.
The mechanisms of moxifloxacin-induced AAU and iris atrophy are not clear, but potentially include direct toxicity to the pigmented iris and collagen degradation.
Acknowledgments
Many thanks for Professor Alastair K Denniston for making the diagnosis and overseeing the clinical care of this patient. We think him for his advice on this case report and assisting in the final review. We also thank Dr Aditya U Kale for his assistance in formatting of the images included in the case report.
Footnotes
Contributors: XL identified the patient case. BTKH contacted the patient and collated case details. XL and YA assisted in accessing patient details relevant to the case report. NC acquired ocular images and extraction for inclusion within case report. BTKH annotated and formatted images included in the case report. BTKH, XL and YA contributed to the literature search and concept writing of the case report. BH assembled the final case report draft. All authors contributed the review and editing of the final draft.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Tsirouki T, Dastiridou A, Symeonidis C, et al. . A focus on the epidemiology of uveitis. Ocul Immunol Inflamm 2018;26:2–16. 10.1080/09273948.2016.1196713 [DOI] [PubMed] [Google Scholar]
- 2.Guly CM, Forrester JV. Investigation and management of uveitis. BMJ 2010;341:c4976. 10.1136/bmj.c4976 [DOI] [PubMed] [Google Scholar]
- 3.Durrani OM, Tehrani NN, Marr JE, et al. . Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol 2004;88:1159–62. 10.1136/bjo.2003.037226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute for Health and Care Excellence, (NICE) Uveitis clinical knowledge summaries (Cks), 2014. Available: https://cks.nice.org.uk/uveitis [Accessed 24 Oct 2019].
- 5.Asam JS, Polzer M, Tafreshi A. Anterior segment OCT. high resolution imaging in microscopy and ophthalmology. Springer, 2019: 285–99. [PubMed] [Google Scholar]
- 6.Venkateswaran N, Galor A, Wang J, et al. . Optical coherence tomography for ocular surface and corneal diseases: a review. Eye Vis 2018;5:13. 10.1186/s40662-018-0107-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Lelij A, Ooijman FM, Kijlstra A, et al. . Anterior uveitis with sectoral iris atrophy in the absence of keratitis. Ophthalmology 2000;107:1164–70. 10.1016/S0161-6420(00)00115-9 [DOI] [PubMed] [Google Scholar]
- 8.Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome - a systematic review. Med Sci Monit 2012;18:RA138–44. 10.12659/MSM.883260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okafor LO, Hewins P, Murray PI, et al. . Tubulointerstitial nephritis and uveitis (TINU) syndrome: a systematic review of its epidemiology, demographics and risk factors. Orphanet J Rare Dis 2017;12:1–9. 10.1186/s13023-017-0677-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasternak B, Inghammar M, Svanström H. Fluoroquinolone use and risk of aortic aneurysm and dissection: nationwide cohort study. BMJ 2018;360:k678. 10.1136/bmj.k678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncombe A, Gueudry J, Massy N, et al. . [Severe pseudouveitis associated with moxifloxacin therapy]. J Fr Ophtalmol 2013;36:146–50. 10.1016/j.jfo.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 12.Cano Parra J, Díaz-Llopis M. [Drug induced uveitis]. Arch Soc Esp Oftalmol 2005;80:137–49. 10.4321/s0365-66912005000300004 [DOI] [PubMed] [Google Scholar]
- 13.Bringas Calvo R, Iglesias Cortiñas D, Bringas RC. [Acute and bilateral uveitis secondary to moxifloxacin]. Arch Soc Esp Oftalmol 2004;79:357–9. 10.4321/s0365-66912004000700011 [DOI] [PubMed] [Google Scholar]
- 14.Broens A, Collignon N. [Moxifloxacin and iris transillumination]. Rev Med Liege 2015;70:606–8. [PubMed] [Google Scholar]
- 15.Morshedi RG, Bettis DI, Moshirfar M, et al. . Bilateral acute iris transillumination following systemic moxifloxacin for respiratory illness: report of two cases and review of the literature. Ocul Immunol Inflamm 2012;20:266–72. 10.3109/09273948.2012.670359 [DOI] [PubMed] [Google Scholar]
- 16.Tranos P, Lokovitis E, Masselos S, et al. . Bilateral acute iris transillumination following systemic administration of antibiotics. Eye 2018;32:1190–6. 10.1038/s41433-018-0054-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rangel CM, Parra MM, Frederick G, et al. . An unusual case of bilateral anterior uveitis related to moxifloxacin: the first report in Latin America. GMS Ophthalmol Cases 2017;7:7. 10.3205/oc000069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wefers Bettink-Remeijer M, Brouwers K, van Langenhove L, et al. . Uveitis-like syndrome and iris transillumination after the use of oral moxifloxacin. Eye 2009;23:2260–2. 10.1038/eye.2009.234 [DOI] [PubMed] [Google Scholar]
- 19.Sandhu HS, Brucker AJ, Ma L, et al. . Oral fluoroquinolones and the risk of uveitis. JAMA Ophthalmol 2016;134:38–43. 10.1001/jamaophthalmol.2015.4092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinkle DM, Kruh-Garcia NA, Kruh JN, et al. . Moxifloxacin concentration and proteomic analysis of aqueous humor in human uveitis associated with oral moxifloxacin therapy. Open Ophthalmol J 2017;11:107–16. 10.2174/1874364101711010107 [DOI] [PMC free article] [PubMed] [Google Scholar]