Abstract
fMRI relies on a localized cerebral blood flow (CBF) response to changes in cortical neuronal activity. An underappreciated aspect however is its sensitivity to contributions from autonomic physiology that may affect CBF through changes in vascular resistance and blood pressure. As is reviewed here, this is crucial to consider in fMRI studies of sleep, given the close linkage between the regulation of arousal state and autonomic physiology. Typical methods for separating these effects are based on the use of reference signals that may include physiological parameters such as heart rate and respiration; however, the use of time-invariant models may not be adequate due to the possibly changing relationship between reference and fMRI signals with arousal state. In addition, recent research indicates that additional physiological reference signals may be needed to accurately describe changes in systemic physiology, including sympathetic indicators such as finger skin vascular tone and blood pressure.
Introduction
Blood oxygen level dependent (BOLD) fMRI of sleep provides unique opportunities to investigate brain function across a range of arousal states. fMRI relies on the blood flow (hemodynamic) response to local cortical circuit activity (Iadecola 2017), involving the so-called “neurovascular” unit in which arteriolar diameter is controlled by chemical signaling secondary to synaptic activity subserving local computation. Spontaneous and evoked fMRI activity may thus inform on the function and interaction of cortical areas. However, a number of studies have shown that the fMRI signal may also be affected by widespread contributions from fluctuations in autonomic physiology (Birn et al. 2009; Shmueli et al. 2007; van Houdt et al. 2010) or activity of modulatory neurotransmitters. As reviewed in the following, these contributions may vary strongly, and potentially jointly, with arousal state, and thus -- if not modeled -- constitute a serious impediment for the interpretation of fMRI studies in general, those of sleep in particular. The main focus of this article will be on the effects of autonomic physiology on the fMRI signal.
Sources contributing to the fMRI signal
It is well recognized that various spurious factors can affect the fMRI signal, including head motion, system drift, and changes in systemic physiology such as heart rate (HR) and respiration (Liu 2016). While the effects of motion and system drift are well understood and can be adequately separated from the signals of interest, physiological effects are more difficult to deal with (Caballero-Gaudes and Reynolds 2017). The way they lead to cerebral blood flow (CBF) (and fMRI) changes, and covary with electro-cortical activity, is incompletely understood, making removal difficult. Fortunately, recent additions to a large body of prior research is starting to clarify this.
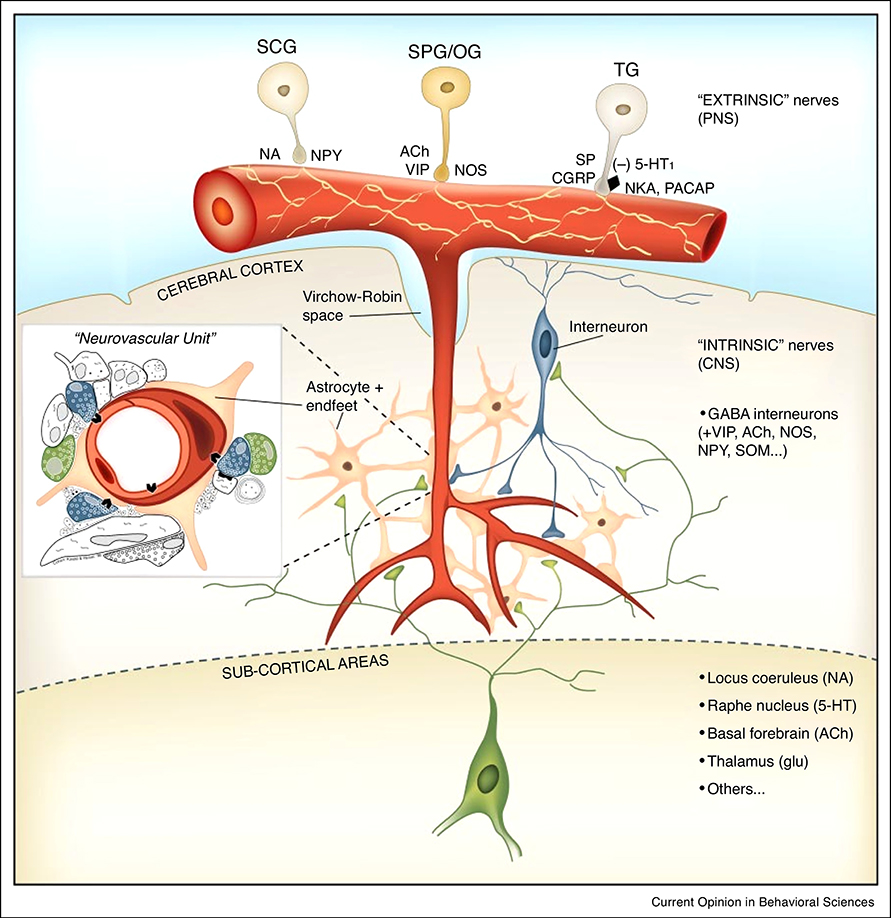
In addition to local CBF increases to local cortical activity mediated by the neurovascular unit, CBF increases may be mediated by various other mechanisms that can affect resistance of the central nervous system (CNS) vasculature (Fig. 1) (Hotta 2016; Lecrux and Hamel 2016), typically in a rather widespread manner. These include the action of circulatory vasoactive agents like CO2 and catecholamines, as well as neurogenic control of vascular tone of the extracortical arteries from vascular innervation originating from sources outside (“extrinsic”) the CNS (Hamel and Ford-Hutchinson 1985). This extrinsic innervation includes sympathetic, parasympathetic, and somatosensory nerves originating from ganglia of the autonomous nervous system (ANS), such as the superior cervical, otic, sphenopalantine, and trigeminal ganglia. Vascular tone may also be affected by the intrinsic (originating from within the CNS) innervation through fibers originating from basal forebrain (BF), raphe nuclei (RN), and locus coeruleus (LC). These (neuro-) modulatory brain regions can affect CBF in a non-local manner indirectly by modulating cortical activity, and possibly directly through direct neurotransmitter release onto endothelial cells (Lecrux and Hamel 2016). A variety of neurotransmitters and neuropeptides is involved in the neurogenic control of CBF, including noradrenaline, serotonin, glutamate, acetylcholine, and GABA. The relative contribution of these neurotransmitters to CBF regulation is not well known, is animal species and possibly arousal state dependent, and varies across brain regions.
Fig. 1.
Neurogenic control of CBF involving extrinsic and intrinsic innervation (reproduced from (Hamel 2006)). In addition to control of intraparenchymal vascular resistance by an intraparenchymal neurovascular unit containing a neuron-glia network, two other mechanisms of CBF control may exist that rely on vascular innervation from extra-parenchymal sources. The extrinsic innervation involves extra-parenchymal vasculature and is controlled from ganglia of the peripheral nervous system (PNS), including superior cervical, superior palantine, otic, and trigeminal ganglia. The intrinsic innervation involves the parenchymal vasculature and Is controlled from sub-cortical (neuro-) modulatory regions.
Furthermore, CBF may also be affected through changes in blood pressure effected by changes in heart rate and ejection fraction (Shivkumar and Ardell 2016), or changes in tone of the vasculature outside the CNS. Thus, a complexity of regulatory systems exist that allow for various contributions to the fMRI signal that are widespread and potentially obscure the localized response to electro-cortical activity mediated by the neurovascular unit. Because these contributions may depend on arousal state, their influence in fMRI studies of sleep may be particularly difficult to account for.
Current approaches for distinguishing contributions to fMRI signals
Due to the multitude of potential processes contributing to the fMRI signal, separation of desired and undesired contributions to the fMRI signal is a difficult problem and somewhat dependent on the goal of the study. If one is specifically interested in local neural activity, one would want to remove all contributions that do not involve the neurovascular unit, including systemic (autonomic) physiology and any direct effects on CBF from the intrinsic vascular innervation. Currently, this question is not fully resolved and still an area of active investigation.
Existing approaches for reducing systemic physiological effects are typically pragmatic and involve regressing out from the fMRI voxel time-series references signal(s) that reflect the effects one may wish to remove, including a global (brain-averaged) signal, signal from a reference region in white matter or cerebrospinal fluid (CSF), or signals that reflect fluctuations in heart rate (Shmueli et al. 2007) and/or respiratory flow rate (respiratory volume per unit time, or RVT) (Birn, Murphy, and Bandettini 2008). The rationale of these approaches is that the contribution of systemic physiology to the fMRI signal depends linearly on the reference signals with gains that are stationary (constant over the experiment). In the following, we will see that this may not be generally true. In addition, there are several other factors that may compromise the effectiveness and appropriateness of this strategy.
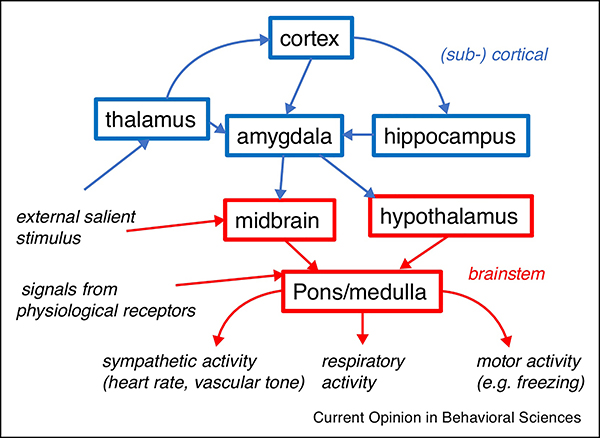
One issue is that global systemic effects may covary with neuronal effects (Scholvinck et al. 2010; Turchi et al. 2018), which then get (partially) eliminated with the removal approach. For example, joint changes in cortical excitability (and activity) and systemic physiology may result from changes in arousal state. Alternatively, changes in systemic physiology may be effectuated by neural activity in (sub-) cortical regions (Cechetto 2014; Silvani et al. 2016) (Fig. 2). Depending on the goal of the study, removal of the fMRI correlate with the aforementioned physiological regressors may not be desirable. Furthermore, variations in systemic physiology relevant for the fMRI may not be adequately described by just HR and RVT regressors (van Houdt et al. 2010).
Fig. 2.
Pathways involved in the regulation of systemic physiology (modified from (Dampney 2016)). Various sensory and physiological stimuli can affect cardiovascular and respiratory activity through pathways that can include or bypass (sub-) cortical regions. During sleep, sensory stimuli and signals from receptors sensing various physiological parameters (e.g., arterial pressure, O2 and CO2 concentration) typically affect systemic physiology without involving the (sub-) cortex.
For example, the mechanisms by which fMRI depends on HR and RVT are indirect and not well known. RVT generally assumes a close relationship with arterial CO2, which then affects BOLD through flow changes mediated by arterial and local (intracortical) vasodilatory mechanisms. The existence of this mechanism has been demonstrated in animals (Atkinson, Anderson, and Sundt 1990; Hoiland et al. 2016), and fMRI based human CO2 reactivity measurements are consistent with this (Chang and Glover 2009; Liu et al. 2017). However, as will be discussed in the next section, there are alternative ways by which CO2 (and thus RVT) can affect CBF (and thus fMRI) with potentially different spatio-temporal characteristics. Specifically, O2- and CO2-dependent brainstem mechanisms exist that can control cortical vascular tone through extrinsic vascular innervation. A successful removal strategy will need to take the various mechanisms into account as their relative contribution may vary across arousal states.
Potential contribution of vascular innervation to CBF regulation in human
While underappreciated in the fMRI literature, the notion of a neurogenic contribution to CBF regulation through extrinsic and intrinsic vascular innervation (originating from the ANS and within the CNS respectively) is supported by substantial anatomical and functional evidence. Numerous studies have reported the presence of perivascular nerves supported by various neurotransmitters, including dopamine, serotonin, acetylcholine, and GABA (for review see (Foote and Morrison 1987; Hamel and Ford-Hutchinson 1985; Larsen and Waters 2018).
Functional studies have reported CBF changes in response to stimulation of the intrinsic innervation from various neuromodulatory centers including BF, RN, and LC. Vasoactive responses have been reported (Lecrux and Hamel 2016), sometimes without accompanying changes in electro-cortical activity (e.g., (Underwood et al. 1995)). Using pharmacologic manipulation of cholinergic and serotonergic activity, recent studies have shown changes in fMRI activity patterns (Hahn et al. 2012; Shah et al. 2016). Clearly, CBF changes orchestrated from a coordinating brain region, either with or without changes in cortical activity, complicate the interpretation of the fMRI signal in terms of cortico-cortical communication.
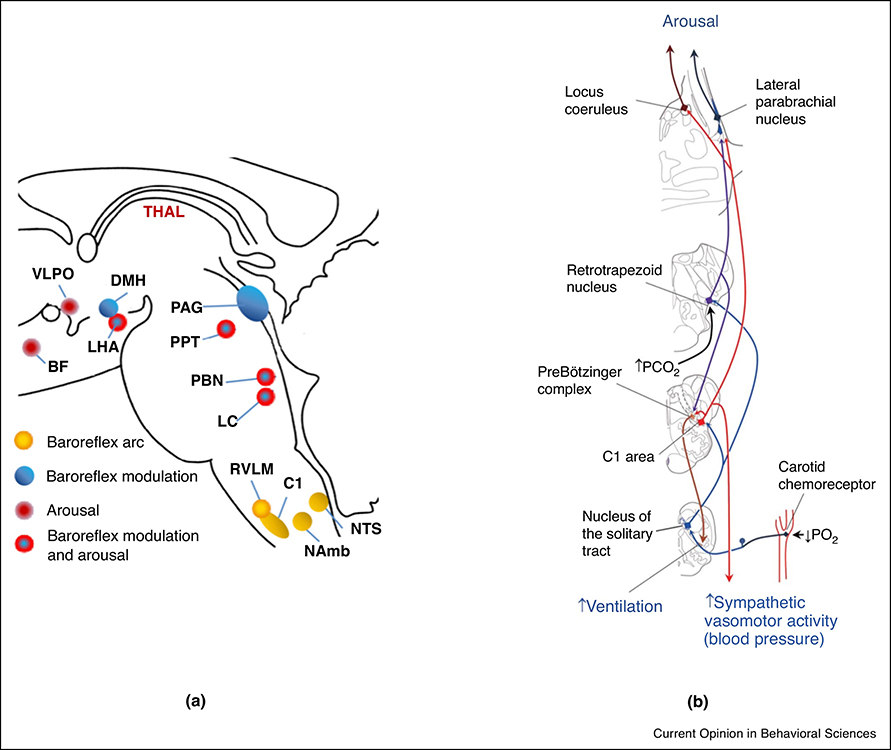
In addition to CBF changes elicited by intrinsic vascular innervation, substantial evidence exists for a contribution of extrinsic innervation originating from sympathetic and parasympathetic systems to blood-flow regulation (Hernandez-Perez, Raichle, and Stone 1975; Boysen, Dragon, and Talman 2009; Heistad, Marcus, and Gross 1978; Talman et al. 2007; Hamner et al. 2010; Zhang et al. 2002; Sandor 1999; ter Laan et al. 2013; Brassard, Tymko, and Ainslie 2017). Importantly, recent work has shown widespread correlation of fMRI with peripheral vascular tone as measured from finger skin (van Houdt et al. 2010; Özbay et al. 2018) using photoplethysmography (Shelley 2007). The fact that finger skin vascular tone is actively regulated by the sympathetic system thus would suggest a sympathetic contribution to fMRI signal. This is relevant for sleep, as sympathetic activity varies with arousal state. In fact, a recent study (Özbay et al. 2019) found joint changes peripheral vascular tone (as measured with photoplethysmography) and widespread fMRI signal to occur with EEG K-complexes during non-REM stage 2 sleep, previously associated with sympathetic activity triggered by sub-cortical arousal (Ackner and Pampiglione 1957). The general conditions under which this occurs, and the associated physiological mechanisms, are not clear due to the complex overlap of brainstem regions that mediate arousal and affect systemic physiology (Silvani et al. 2015; Benarroch 2018) (Fig. 3).
Fig. 3.
Dual role of brain areas in both arousal and autonomic physiology.
a. Joint effects on baroreflex modulation and arousal (reproduced from (Silvani et al. 2015)). In lower brain and brainstem, Locus Coeruleus (LC), Parabrachial Nucleus, Pendunculopontine Tegmental Nucleus (PPT), Raphe Ncleus (RN), and Lateral Hypothalamic Area (LHA) all have dual roles in both arousal and baroreflex modulation. Other relevant regions: Ventrolateral Preoptic Nucleus (VLPO), Basal Forebrain (BF), Dorsomedial Nucleus of the Hypothalamus (DMH), Lateral Hypothalamic Area (LHA), Periaqueductal Grey (PAG), Rostral Ventrolateral Medulla (RVLM), Cell group of adrenergic respiratory neurons in RVLM (C1), Nucleus Ambiguous (NAmb), Nucleus Tractur Solitarius (NTS).
b. Example of joint arousal and systemic (cardiovascular and respiratory) response to hypercapnic or hypoxic stimuli (reproduced from (Benarroch 2018)).
Variation of systemic effects across arousal states
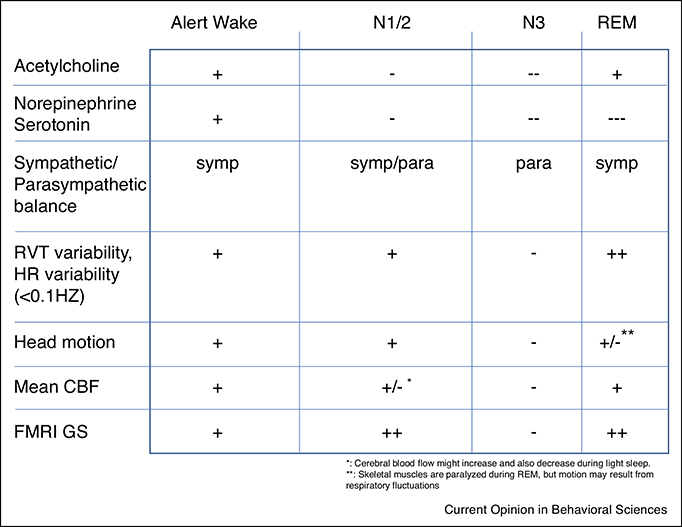
The study of sleep with fMRI is challenging as the contribution of systemic (neurogenic and non-neurogenic) sources to the fMRI signal may vary with arousal state (Fig. 4). Thus, when comparing fMRI activity patterns between arousal states, modeling of systemic contributions requires special attention.
Fig. 4.
Physiological changes across arousal states. Towards increasing sleep depth, there is a reduction in neurotransmitter activity from modulatory brain centers, an increased parasympathetic dominance, a reduction in head motion, and a reduction in baseline CBF. During N1/N2 sleep, there is an increase in fMRI global signal due to fluctuations in the balance between parasympathetic and sympathetic activity. FMRI global signal is also high during REM sleep, likely because of strong fluctuations in neural activity, as well as high respiratory variability.
As we have seen above, fMRI may be sensitive to various aspects of autonomic physiology and neurotransmitter activity and both of these are known to vary dramatically during sleep (Benarroch 2018). For example, physiological aspects such as HR, respiration rate, and respiration depth are known to undergo changes during sleep. Their variability in the 0–0.1 Hz frequency band relevant for the fMRI signal typically is relatively high during wake, light sleep, and REM sleep and relatively low during deep sleep (Bonnet and Arand 1997; Tobaldini et al. 2013; Gutierrez et al. 2016). In addition, known changes in CO2 reactivity (Meadows et al. 2003; Klingelhöfer 2012) and baroreflex sensitivity during sleep may change the level and way by which HR and respiration affect the fMRI signal during various arousal states (Benarroch 2019; Silvani et al. 2016). For example, the effect of arousal on CBF has been shown to vary with sleep state (Bangash et al. 2008).
Together with the autonomic changes, a major change with arousal state is the influence of the various neurotransmitters that modulate neural activity and affect CBF from brain regions such as BF, LC, and RN. Because of the close integration of these modulatory centers with regions affecting autonomic physiology (Fig. 3), autonomic and modulatory activity often jointly change with arousal state. Generally speaking, aminergic neurotransmitter levels reduce with increasing sleep depth, while REM sleep is associated with strong fluctuations in cholinergic activity (Saper, Scammell, and Lu 2005; Jones in press) (Fig. 4). It is unclear if this cholinergic activity affects CBF in a manner proportional to the strongly varying neuronal activity levels associated with this sleep stage. Either way, like the autonomic changes, these modulatory influences are likely to similarly confound the interpretation of fMRI correlations patterns in terms of cortico-cortical communication.
Lastly, as indicated above, the prevalence of sympathetic activity is highly dependent on arousal state (Fig. 4). During wakefulness, sympathetic activity varies with the level of alertness and attention and episodic increases can be elicited by a wide range of physiological, sensory, and cognitive stimuli. In fact, widespread fMRI signal changes have been observed with momentary increases in pupil diameter, associated with sympathetic activation during alerting stimuli (Yellin, Berkovich-Ohana, and Malach 2015; Schneider et al. 2016). Changes in sympathetic activity, indexed by skin conductance, have also been observed with arousal-inducing stimulation and tied to fMRI signal changes (Fan et al. 2012; Henderson et al. 2012). Consistent with this are the large-scale changes in fMRI signal seen with spontaneous eye closure (Chang et al. 2016; Wang et al. 2016). However, the mechanisms underlying the covariation between arousal, sympathetic activity, and fMRI signal under these conditions is not clear and may include local neurovascular control as well as involving effects from intrinsic and extrinsic vascular innervation.
During stage 1 and 2 non-REM sleep, a generally low sympathetic tone and high parasympathetic tone is punctuated by brief (seconds long) sympathetic increases associated with the occurrence of EEG K-complexes (Halász 1993; Colrain 2005). In part, these increases are thought to be part of a so-called “fight or flight” or “orienting” response (Johnson and Lubin 1967; Pampiglione and Ackner 1958). With increasing sleep depth, the rate of these episodic sympathetic surges decreases and is near zero during slow-wave sleep. During REM sleep, the typically strong variations in sympathetic activity that occur may be in part associated with both cortical activity and the strong heart rate and respiratory variations occurring in this sleep stage (Aserinsky and Kleitman 1953; Oudiette et al. 2018).
Summarized, the contributions from autonomic physiology to the fMRI signal can vary across arousal states and affect its relationship with (electro-) physiological regressors. Any strategy aimed at separating these effects from fMRI signal reflecting local cortical processing and cortico-cortical communication will need to take this into account, and as of yet have not been developed. Incomplete removal makes comparison of fMRI activity patterns across arousal state difficult and prone to misinterpretation.
Interrelationships between various brain and physiological signals
The dependence of the dynamics of the systemic physiology on arousal state can in part be explained by the strong anatomical overlap between the system components that mediate arousal, modulate neural activity, and regulate systemic physiology (Benarroch 2018; Silvani et al. 2015) (Fig. 2). For example, the various neuronal cell groups in LC have been shown to have the capability to jointly modulate breathing, arousal state, and neural activity subserving sensory processing (Yackle et al. 2017). Furthermore, many of the interactions between the various system components can vary in direction and strength, complicating causal interpretation and the understanding of their effect on fMRI.
For example, sympathetic activity is frequently generated in close association with respiratory activity (Moreira and Mulkey 2015; Guyenet and Bayliss 2015). Increases in sympathetic activity are typically associated with a biphasic heart rate change, but this is dependent on parasympathetic tone (which is in turn dependent on arousal state). As a result, the relationship between physiological parameters such as HR, RVT, end-tidal CO2, blood pressure, pupil diameter, and peripheral vascular tone is generally arousal state dependent, as is their relationship with the fMRI signal (Chang et al. 2018). This complicates the ability to isolate specifically the component of fMRI signal relating to local neurovascular coupling.
The overlap between the neural substrates controlling arousal state and systemic physiology also results in a possible covariation between the electrophysiological hallmarks of arousal and alertness on one hand, and systemic physiology one the other. For example, K-complexes, signatures of subcortical arousal, are associated with sympathetic activity and respiratory changes (Roth, Shaw, and Green 1956; Poole 1961), whereas the alertness and arousal changes associated with EEG alpha band activity may also modulate sympathetic activity. In fact, recent work reported a covariation between EEG alpha band activity, RVT, and the fMRI signal (Yuan et al. 2013). The pontine regions that mediate ponto-geniculo-occipital waves during phasic REM sleep may also be responsible for the strong respiratory variations seen during this sleep stage (Krieger et al. 1990; Sforza et al. 1990). Taken together, these possible associations between the electrophysiological signals and systemic physiology indicate that care has to be taken when interpreting EEG-fMRI correlations as reflecting local cortical processing (Jahnke et al. 2012; Laufs et al. 2006; Dang-Vu et al. 2008), as they in part may be mediated by secondary, non-neuronal mechanisms.
Future directions, outstanding questions, and current recommendations
One of the key areas needing to be addressed by future research is the mechanistic understanding of how the changes in autonomic physiology during sleep affect the fMRI signal. Current approaches for removing systemic physiological effects typically use regressors based on physiological parameters such as HR and RVT, fMRI signals from reference regions in the brain (e.g., white matter, CSF, or global brain signal), or independent components based on the spatio-temporal properties of the components (Glasser et al. 2018). However, because of the poorly understood nature of these signal contributions, and potential non-stationarity and non-linearity between physiological regressors and the fMRI signal, these approaches are unlikely to be adequate across the varying physiological states that occur during the sleep-wake cycle.
For example, a global brain signal regressor may not purely reflect systemic physiology but also contain a contribution from cortical activity, and this contribution may vary across arousal states. As a result, its removal may have varying effectivity (and accuracy) across arousal states, complicating any inference about arousal state dependent changes in neural activity patterns. Similarly, an incomplete mechanistic understanding of the effects of systemic physiology on the fMRI signal compromises the use of physiological regressors. In this regard, an important question is how vascular CO2 changes lead to changes in CBF. To what extent are CBF changes mediated by local (intracortical) CO2 changes versus central chemo-sensing and neurogenic control? What are the spatio-temporal characteristics of each of these mechanisms? How do they change across the various sleep stages? As of yet, these questions have not been fully answered.
A second area of future research concerns the general question to what extent can fMRI signal patterns be interpreted as reflecting cortico-cortical communication in so called “functional networks.” During both sleep and wake, rapid fluctuations in blood flow may occur that are mediated by intrinsic and extrinsic vascular innervation and reflect changes in systemic physiology and influence from neuromodulatory centers such as LC, RN, and BF (McGinley et al. 2015; Lecrux and Hamel 2016). These do not reflect cortico-cortical communication but can affect interpretation of the fMRI signal in terms of network function. This has special relevance for sleep studies, as these effects will strongly depend on arousal state. For example, a popular method to study the latter is to determine the fMRI signal correlation between two brain regions and take this as a measure of their “functional connectivity” (van den Heuvel and Hulshoff Pol 2010). Obviously, the presence of large scale autonomic or neuromodulatory effects will affect this measure, and because these effects vary with sleep stage, this will make comparison of functional connectivity pattern across sleep states difficult. It may be possible to use fMRI signals from neuromodulatory regions together with physiological regressors to isolate signals that specifically reflect local cortical activity or cortico-cortical interactions, but this will require additional research.
Clearly, a more mechanistic understanding is needed. Nonetheless, current recommendations are needed for the sleep neuroimager interested in local neural activity. It is recommended that sleep fMRI studies should at a minimum use nuisance regressors that model physiological noise (Horovitz et al. 2009; Shmueli et al. 2007). These regressors should include signals reflecting cardiac and respiratory cycles (Glover, Li, and Ress 2000), their rates, as well as respiratory depth (Birn, Murphy, and Bandettini 2008). In place of cardiac rate, peripheral vascular tone as measured by finger photoplethysmography may be used because both of these measures reflect sympathetic activation (Özbay et al. 2018). Alternatively, use of a whole-brain nuisance regressor may be required. The comparison of multiple preprocessing approaches may also be useful for testing for the robustness of an effect purported to be related to local neural activity.
Conclusion
FMRI has unique potential to study changes in cortical activity and cortico-cortical functional connectivity over the sleep-wake cycle. As such, it may inform about the mechanistic underpinning of sleep regulation, the nature and relevance of spontaneous activity patterns to specific sleep states and consciousness levels, and changes in neuromodulatory activity with sleep states. However, there are various overlapping signal sources whose contributions as of yet are poorly understood and difficult to eliminate. Ongoing research is starting to catalogue and reveal these additional sources and their mechanistic underpinnings, which is helping efforts to differentiate them.
Highlights.
fMRI is sensitive to contributions from variations in autonomic physiology
These affect fMRI through changes in vascular resistance and blood pressure
The neural substrates controlling autonomic physiology and arousal overlap strongly
This makes the analysis and interpretation of fMRI studies of sleep particularly challenging
Developing improved mechanistic understanding of systemic effects on fMRI is critical
Acknowledgments
This work was supported by K22ES028048 (CC) and the Intramural Research Program of the National Institute of Neurological Disorders and Stroke.
Footnotes
Conflict of Interest Declaration
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackner B, and Pampiglione G. 1957. ‘Some relationships between peripheral vasomotor and E.E.G. changes’, J Neurol Neurosurg Psychiatry, 20: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aserinsky E, and Kleitman N. 1953. ‘Regularly occurring periods of eye motility, and concomitant phenomena, during sleep’, Science, 118: 273–4. [DOI] [PubMed] [Google Scholar]
- Atkinson JL, Anderson RE, and Sundt TM Jr. 1990. ‘The effect of carbon dioxide on the diameter of brain capillaries’, Brain Res, 517: 333–40. [DOI] [PubMed] [Google Scholar]
- Bangash MF, Xie A, Skatrud JB, Reichmuth KJ, Barczi SR, and Morgan BJ. 2008. ‘Cerebrovascular response to arousal from NREM and REM sleep’, Sleep, 31: 321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE 2018. ‘Brainstem integration of arousal, sleep, cardiovascular, and respiratory control’, Neurology, 91: 958–66** Reviews the various brainstem regions and neurotransmitters that often have joint effects on arousal state and autonomic physiology.
- Benarroch EE 2019. ‘Control of the cardiovascular and respiratory systems during sleep, Auton Neurosci, 218: 54–63. [DOI] [PubMed] [Google Scholar]
- Birn RM, Murphy K, and Bandettini PA. 2008. ‘The effect of respiration variations on independent component analysis results of resting state functional connectivity’, Hum Brain Mapp, 29: 740–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Murphy K, Handwerker DA, and Bandettini PA. 2009. ‘fMRI in the presence of task-correlated breathing variations’, Neuroimage, 47: 1092–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet MH, and Arand DL. 1997. ‘Heart rate variability: sleep stage, time of night, and arousal influences’, Electroencephalogr Clin Neurophysiol, 102: 390–6. [DOI] [PubMed] [Google Scholar]
- Boysen NC, Dragon DN, and Talman WT. 2009. ‘Parasympathetic tonic dilatory influences on cerebral vessels’, Auton Neurosci, 147: 101–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brassard P, Tymko MM, and Ainslie PN. 2017. ‘Sympathetic control of the brain circulation: appreciating the complexities to better understand the controversy’, Auton Neurosci, 207: 37–47* Identifies the potential reasons for the often discordant reports on sympathetic control of CBF.
- Caballero-Gaudes C, and Reynolds RC. 2017. ‘Methods for cleaning the BOLD fMRI signal’, Neuroimage, 154: 128–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto DF 2014. ‘Cortical control of the autonomic nervous system’, Exp Physiol, 99: 326–31. [DOI] [PubMed] [Google Scholar]
- Chang C, Ozbay PS, de Zwart JA, Picchioni D, Chapper-Farley MG, Mandelkow H, and Duyn JH. 2018. “Covariation of pulse oximetry amplitude and BOLD fMRI across vigilance states.” In ISMRM; Paris, France. [Google Scholar]
- Chang C, and Glover GH. 2009. ‘Relationship between respiration, end-tidal CO2, and BOLD signals in resting-state fMRI’, Neuroimage, 47: 1381–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Leopold DA, Scholvinck ML, Mandelkow H, Picchioni D, Liu X, Ye FQ, Turchi JN, and Duyn JH. 2016. ‘Tracking brain arousal fluctuations with fMRI’, Proc Natl AcadSci U S A, 113: 4518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM 2005. ‘The K-complex: a 7-decade history’, Sleep, 28: 255–73. [DOI] [PubMed] [Google Scholar]
- Dampney RA 2016. ‘Central neural control of the cardiovascular system: current perspectives’, Adv Physiol Educ, 40: 283–96. [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, Schabus M, Desseilles M, Albouy G, Boly M, Darsaud A, Gais S, Rauchs G, Sterpenich V, Vandewalle G, Carrier J, Moonen G, Balteau E, Degueldre C, Luxen A, Phillips C, and Maquet P. 2008. ‘Spontaneous neural activity during human slow wave sleep’, Proc Natl Acad Sci U S A, 105: 15160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Xu P, Van Dam NT, Eilam-Stock T, Gu X, Luo YJ, and Hof PR. 2012. ‘Spontaneous brain activity relates to autonomic arousal’, J Neurosci, 32: 11176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, and Morrison JH. 1987. ‘Development of the noradrenergic, serotonergic, and dopaminergic innervation of neocortex’, Curr Top Dev Biol, 21: 391–423. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Bijsterbosch JD, Harrison SJ, Harms MP, Anticevic A, Van Essen DC, and Smith SM. 2018. ‘Using temporal ICA to selectively remove global noise while preserving global signal in functional MRI data’, Neuroimage, 181: 692–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Li TQ, and Ress D. 2000. ‘Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR’, Magn Reson Med, 44: 162–7. [DOI] [PubMed] [Google Scholar]
- Gutierrez G, Williams J, Alrehaili GA, McLean A, Pirouz R, Amdur R, Jain V, Ahari J, Bawa A, and Kimbro S. 2016. ‘Respiratory rate variability in sleeping adults without obstructive sleep apnea’, Physiol Rep, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, and Bayliss DA. 2015. ‘Neural Control of Breathing and CO2 Homeostasis’, Neuron, 87: 946–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Wadsak W, Windischberger C, Baldinger P, Hoflich AS, Losak J, Nics L, Philippe C, Kranz GS, Kraus C, Mitterhauser M, Karanikas G, Kasper S, and Lanzenberger R. 2012. ‘Differential modulation of the default mode network via serotonin-1A receptors’, Proc Natl Acad Sci U S A, 109: 2619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halász P 1993. ‘Arousals without awakening--dynamic aspect of sleep’, Physiol Behav, 54: 795–802. [DOI] [PubMed] [Google Scholar]
- Hamel E 2006. ‘Perivascular nerves and the regulation of cerebrovascular tone’, J Appl Physiol, 100: 1059–64. [DOI] [PubMed] [Google Scholar]
- Hamel R, and Ford-Hutchinson AW. 1985. ‘Pulmonary and cardiovascular changes in hyperreactive rats from citric acid aerosols’, J Appl Physiol, 59: 354–9. [DOI] [PubMed] [Google Scholar]
- Hamner JW, Tan CO, Lee K, Cohen MA, and Taylor JA. 2010. ‘Sympathetic control of the cerebral vasculature in humans’, Stroke, 41: 102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistad DD, Marcus ML, and Gross PM. 1978. ‘Effects of sympathetic nerves on cerebral vessels in dog, cat, and monkey’, Am J Physiol, 235: H544–52. [DOI] [PubMed] [Google Scholar]
- Henderson LA, Stathis A, James C, Brown R, McDonald S, and Macefield VG. 2012. ‘Real-time imaging of cortical areas involved in the generation of increases in skin sympathetic nerve activity when viewing emotionally charged images’, Neuroimage, 62: 30–40. [DOI] [PubMed] [Google Scholar]
- Hernandez-Perez MJ, Raichle ME, and Stone HL. 1975. ‘The role of the peripheral sympathetic nervous system in cerebral blood flow autoregulation’, Stroke, 6: 284–92. [DOI] [PubMed] [Google Scholar]
- Hoiland RL, Bain AR, Rieger MG, Bailey DM, and Ainslie PN. 2016. ‘Hypoxemia, oxygen content, and the regulation of cerebral blood flow’, Am J Physiol Regul Integr Comp Physiol, 310: R398–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, and Duyn JH. 2009. ‘Decoupling of the brain’s default mode network during deep sleep’, Proc Natl Acad Sci U S A, 106: 11376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta H 2016. ‘Neurogenic control of parenchymal arterioles in the cerebral cortex’, Prog Brain Res, 225: 3–39. [DOI] [PubMed] [Google Scholar]
- Iadecola C 2017. ‘The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease’, Neuron, 96: 17–42* Review of the current understanding of various cell types, cellular mechanisms, chemical mediators, and arterial segments involved in neurovascular coupling; also describes various roles of the neurovascular unit.
- Jahnke K, von Wegner F, Morzelewski A, Borisov S, Maischein M, Steinmetz H, and Laufs H. 2012. ‘To wake or not to wake? The two-sided nature of the human K-complex’, Neuroimage, 59: 1631–8. [DOI] [PubMed] [Google Scholar]
- Johnson LC, and Lubin A. 1967. ‘The orienting reflex during waking and sleeping’, Electroencephalogr Clin Neurophysiol, 22: 11–21. [DOI] [PubMed] [Google Scholar]
- Jones BE in press. ‘Arousal and sleep circuits’, Neuropsychopharmacology: 10.1038/s41386-019-0444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingelhöfer Jürgen. 2012. ‘Cerebral blood flow velocity in sleep’, Perspectives in Medicine, 1: 275–84. [Google Scholar]
- Krieger J, Maglasiu N, Sforza E, and Kurtz D. 1990. ‘Breathing during sleep in normal middle-aged subjects’, Sleep, 13: 143–54. [PubMed] [Google Scholar]
- Larsen RS, and Waters J. 2018. ‘Neuromodulatory Correlates of Pupil Dilation’, Front Neural Circuits, 12: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Holt JL, Elfont R, Krams M, Paul JS, Krakow K, and Kleinschmidt A. 2006. ‘Where the BOLD signal goes when alpha EEG leaves’, Neuroimage, 31: 1408–18. [DOI] [PubMed] [Google Scholar]
- Lecrux C, and Hamel E. 2016. ‘Neuronal networks and mediators of cortical neurovascular coupling responses in normal and altered brain states’, Philos Trans R Soc Lond B Biol Sci, 371:* Reviews the dependence of neurovascular coupling on arousal state, with a focus on cholinergic and adrenergic modulation. Indicates that much is still unknow about detailed mechanistic changes, including the existence of a direct pathway for modulatory neurotransmitters to affect blood flow.
- Liu P, Li Y, Pinho M, Park DC, Welch BG, and Lu H. 2017. ‘Cerebrovascular reactivity mapping without gas challenges’, Neuroimage, 146: 320–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT 2016. ‘Noise contributions to the fMRI signal: an overview’, Neuroimage, 143: 141–51. [DOI] [PubMed] [Google Scholar]
- McGinley MJ, Vinck M, Reimer J, Batista-Brito R, Zagha E, Cadwell CR, Tolias AS, Cardin JA, and McCormick DA. 2015. ‘Waking state: rapid variations modulate neural and behavioral responses’, Neuron, 87: 1143–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows GE, Dunroy HM, Morrell MJ, and Corfield DR. 2003. ‘Hypercapnic cerebral vascular reactivity is decreased, in humans, during sleep compared with wakefulness’, J Appl Physiol, 94: 2197–202. [DOI] [PubMed] [Google Scholar]
- Moreira TS, and Mulkey DK. 2015. ‘New advances in the neural control of breathing’, J Physiol, 593: 1065–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudiette D, Dodet P, Ledard N, Artru E, Rachidi I, Similowski T, and Arnulf I. 2018. ‘REM sleep respiratory behaviours mental content in narcoleptic lucid dreamers’, Sci Rep, 8: 2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özbay PS, Chang C, Picchioni D, Mandelkow H, Moehlman TM, Chappel-Farley MG, van Gelderen P, de Zwart JA, and Duyn JH. 2018. ‘Contribution of systemic vascular effects to fMRI activity in white matter’, Neuroimage, 176: 541–49* Describes fMRI evidence for joint changes in finger skin and CNS vascular tone, which is interpreted as sympathetic activity affecting CBF.
- Özbay Pinar Senay, Chang Catie, Picchioni Dante, Mandelkow Hendrik, Miranda Grace Chappel-Farley, Peter van Gelderen, Jacco Adrianus de Zwart, and Jeff Duyn. 2019. ‘Sympathetic activity contributes to the fMRI signal’, Commun Biol, 2: 421.* fMRI evidence of the episodic effect of sympathetic activity on CBF during non-REM sleep in humans.
- Pampiglione G, and Ackner B. 1958. ‘The effects of repeated stimuli upon EEG and vasomotor activity during sleep in man’, Brain, 81: 64–74. [DOI] [PubMed] [Google Scholar]
- Poole EW 1961. ‘Nervous activity in relation to the respiratory cycle’, Nature, 189: 579–81. [DOI] [PubMed] [Google Scholar]
- Roth M, Shaw J, and Green J. 1956. ‘The form voltage distribution and physiologicalsignificance of the K-complex’, Electroencephalogr Clin Neurophysiol, 8: 385–402. [DOI] [PubMed] [Google Scholar]
- Sandor P 1999. ‘Nervous control of the cerebrovascular system: doubts and facts’, Neurochem Int, 35: 237–59. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, and Lu J. 2005. ‘Hypothalamic regulation of sleep and circadian rhythms’, Nature, 437: 1257–63. [DOI] [PubMed] [Google Scholar]
- Schneider M, Hathway P, Leuchs L, Samann PG, Czisch M, and Spoormaker VI. 2016. ‘Spontaneous pupil dilations during the resting state are associated with activation of the salience network’, Neuroimage, 139: 189–201. [DOI] [PubMed] [Google Scholar]
- Scholvinck ML, Maier A, Ye FQ, Dduyn j. H., and Leopold DA. 2010. ‘Neural basis of global resting-state fMRI activity’. Proc Natl Acad Sci U S A, 107: 10238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sforza E, Krieger J, Weitzenblum E, Apprill M, Lampert E, and Ratamaharo J. 1990. ‘Long-term effects of treatment with nasal continuous positive airway pressure on daytime lung function and pulmonary hemodynamics in patients with obstructive sleep apnea’, Am Rev Respir Dis, 141: 866–70. [DOI] [PubMed] [Google Scholar]
- Shah D, Blockx I, Keliris GA, Kara F, Jonckers E, Verhoye M, and Van der Linden A. 2016. ‘Cholinergic and serotonergic modulations differentially affect large-scale functional networks in the mouse brain’, Brain Struct Funct, 221: 3067–79. [DOI] [PubMed] [Google Scholar]
- Shelley KH 2007. ‘Photoplethysmography: beyond the calculation of arterial oxygen saturation and heart rate’, Anesth Analg, 105: S31–6, tables of contents. [DOI] [PubMed] [Google Scholar]
- Shivkumar K, and Ardell JL. 2016. ‘Cardiac autonomic control in health and disease’, J Physiol, 594: 3851–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, Jansma JM, and Duyn JH. 2007. ‘Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal’, Neuroimage, 38: 306–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvani A, Calandra-Buonaura G, Benarroch EE, Dampney RA, and Cortelli P. 2015. ‘Bidirectional interactions between the baroreceptor reflex and arousal: an update’, Sleep Med, 16: 210–6** Reviews the various brainstem regions and their interactions to jointly change arousal and autonomic physiology.
- Silvani A, Calandra-Buonaura G, Dampney RA, and Cortelli P. 2016. ‘Brain-heart interactions: physiology and clinical implications’, Philos Trans A Math Phys Eng Sci, 374. [DOI] [PubMed] [Google Scholar]
- Talman WT, Corr J, Nitschke Dragon D, and Wang D. 2007. ‘Parasympathetic stimulation elicits cerebral vasodilatation in rat’, Auton Neurosci, 133: 153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Laan M, van Dijk JM, Elting JW, Staal MJ, and Absalom AR. 2013. ‘Sympathetic regulation of cerebral blood flow in humans: a review’, Br J Anaesth, 111: 361–7. [DOI] [PubMed] [Google Scholar]
- Tobaldini E, Nobili L, Strada S, Casali KR, Braghiroli A, and Montano N. 2013. ‘Heart rate variability in normal and pathological sleep’, Front Physiol, 4: 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchi J, Chang C, Ye FQ, Russ BE, Yu DK, Cortes CR, Monosov IE, Duyn JH, and Leopold DA. 2018. ‘The basal forebrain regulates global resting-state fMRI fluctuations’, Neuron, 97: 940–52 e4* Reports evidence from chemical inhibition experiment in macaque for a role of BF in widespread fMRI signal changes.
- Underwood MD, Bakalian MJ, Arango V, and Mann JJ. 1995. ‘Effect of chemical stimulation of the dorsal raphe nucleus on cerebral blood flow in rat’, Neurosci Lett, 199: 228–30. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, and Hulshoff Pol HE. 2010. ‘Exploring the brain network: a review on resting-state fMRI functional connectivity’, Eur Neuropsychopharmacol, 20: 519–34. [DOI] [PubMed] [Google Scholar]
- van Houdt PJ, Ossenblok PP, Boon PA, Leijten FS, Velis DN, Stam CJ, and de Munck JC. 2010. ‘Correction for pulse height variability reduces physiological noise in functional MRI when studying spontaneous brain activity’, Hum Brain Mapp, 31: 311–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Ong JL, Patanaik A, Zhou J, and Chee MW. 2016. ‘Spontaneous eyelid closures link vigilance fluctuation with fMRI dynamic connectivity states’, Proc Natl Acad Sci U S A, 113: 9653–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yackle K, Schwarz LA, Kam K, Sorokin JM, Huguenard JR, Feldman JL, Luo L, and Krasnow MA. 2017. ‘Breathing control center neurons that promote arousal in mice’, Science, 355: 1411–15* Suggests a role of brainstem respiratory neurons on arousal state from cellular ablation experiments mice.
- Yellin D, Berkovich-Ohana A, and Malach R. 2015. ‘Coupling between pupil fluctuations and resting-state fMRI uncovers a slow build-up of antagonistic responses in the human cortex’, Neuroimage, 106: 414–27. [DOI] [PubMed] [Google Scholar]
- Yuan H, Zotev V, Phillips R, and Bodurka J. 2013. ‘Correlated slow fluctuations in respiration, EEG, and BOLD fMRI’, Neuroimage, 79: 81–93. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, and Levine BD. 2002. ‘Autonomic neural control of dynamic cerebral autoregulation in humans’, Circulation, 106: 1814–20. [DOI] [PubMed] [Google Scholar]