Supplemental digital content is available in the text.
KEYWORDS: bilayer cellular construct, cellular and/or tissue-based products, chronic wounds, cryopreserved human skin allograft, dermal skin substitute, health economics, skin substitutes, Medicare, wound care
ABSTRACT
OBJECTIVE
To investigate differences in wound-related costs; product waste; lower-extremity amputations; and number of applications, hospitalizations, and emergency room visits among patients treated with three cellular and/or tissue-based products.
METHODS
This retrospective intent-to-treat matched-cohort study analyzed the full Medicare claims dataset from 2011 to 2014. Patients who received either a bilayer cellular construct (BLCC), dermal skin substitute (DSS), or cryopreserved human skin allograft (CHSA) were concurrently matched for Charlson Comorbidity Index, age, sex, and region, resulting in 14,546 study patients. Key variables were reported at 60, 90, and 180 days after the first product application.
RESULTS
There were no statistically significant differences in the distribution of Charlson Comorbidity Index, age, sex, and region among cohorts. Wound-related costs and product wastage were lower for CHSA patients relative to both BLCC and DSS patients at all time intervals (P < .05). Patients treated with CHSA received fewer product applications than DSS at 90 and 180 days (P < .05). Amputations were significantly higher among patients treated with DSS than either CHSA or BLCC (P < .0001).
CONCLUSIONS
The data demonstrate that wound-related costs, product waste, amputations, and frequency of applications are lower for CHSA than DSS. Wound-related costs and product waste are lower for CHSA compared with BLCC. Further claims analysis and prospective clinical trials could help develop appropriate quality measures and reimbursement models to ensure smarter spending for the growing population of patients with chronic wounds.
INTRODUCTION
Chronic wounds are an underappreciated national epidemic masked by comorbidities. More than 6.7 million patients in the US suffer from nonhealing wounds, leading to unnecessary hospitalizations and lower-extremity amputations (LEAs); these patients face significant emotional and physical pain as a result of their wound.1,2 Nussbaum and colleagues1 reported that nearly 15% of Medicare beneficiaries (8.2 million) had at least one type of wound or infection. The chronic wound epidemic is projected to grow annually as a result of an aging population with increased rates of obesity and diabetes. Today, chronic wounds in the US cost more than $50 billion annually, and amputation procedures account for $8 billion. For Medicare, the annual cost of treating chronic wounds is estimated at nearly $32 billion, with most costs accruing in various outpatient settings.1
Hospital outpatient departments (HOPDs) provide most of the advanced wound care therapies, including cellular and/or tissue-based products (CTPs), a category of advanced wound treatments reserved for patients who fail to heal with conventional care. Numerous studies have shown the clinical benefit of CTPs in terms of morbidity and mortality,3 but the relative cost-effectiveness of various products has yet to be conclusively established. This study examines the subset of US Medicare beneficiaries who received CTPs to understand if there are differences in wound-related costs, waste, amputations, number of applications, and hospitalizations/emergency room (ER) visits by CTP type.
METHODS
Cellular and/or Tissue-Based Products
For this study, investigators divided CTPs into three categories: cellular, acellular, and placental. These products can be derived from human, animal, or synthetic sources, or a combination of the three. Because the mechanism of action and patient selection vary among these three categories, the investigators focused on three cellular products: a cryopreserved human skin allograft (CHSA; TheraSkin, Solsys Medical, Virginia Beach, Virginia), a bilayer cellular construct (BLCC; Apligraf; Organogenesis, Canton, Massachusetts), and a dermal skin substitute (DSS; Dermagraft; Organogenesis).
The CHSA used in this study is a cryopreserved split-thickness bioactive human skin allograft that maintains cellular viability, growth factors, and the native architecture of human skin.4 The tissue is procured within 24 hours of death from an organ donor, while the skin tissue is still viable. The donor screening criteria for CHSA surpass those required by the American Association of Tissue Banks and the FDA; less than 2% of donated tissue meets the advanced quality standards required to become CHSA. There have been no reports of disease transmission since CHSA came to the market in 2010. After procurement, the allograft undergoes a proprietary process that includes a series of gentle antibiotic baths followed by computer-controlled cryopreservation. The CHSA is recovered, processed, distributed, and used in compliance with the FDA Human Cells, Tissues and Cellular and Tissue-Based Products (HCT/P) Section 361 regulations. As such, CHSA can be used to repair skin for all wound types where there is an adequate surrounding vascular bed.
Two bioengineered class III medical devices are FDA approved to treat chronic wounds. The allogeneic BLCC consists of a bovine collagen matrix with neonatal fibroblasts overlaid by a stratified epithelium containing neonatal keratinocytes. In randomized controlled trials (RCTs), BLCC was proven more effective than standard of care in the treatment of venous leg ulcers (VLUs) and diabetic foot ulcers (DFUs). The DSS consists of human fibroblasts grown in a bioabsorbable polyglactin mesh scaffold. In RCTs, both BLCC and DSS were shown to increase healing rates versus standard of care alone.2 Living cells are present in both BLCC (fibroblasts and keratinocytes) and DSS (fibroblasts) along with various signaling molecules. Unlike CHSA, both medical devices lack a native human ECM; BLCC and DSS work through cellular signaling to prompt the host to create their own scaffold.2
Retrospective and prospective analyses have suggested that CHSA is a safe and effective alternative to bioengineered skin substitutes for the treatment of VLUs, DFUs, and wounds with exposed bone and/or tendon.5–7 Two RCTs comparing CHSA to BLCC and DSS had similar findings, with CHSA having similar VLU outcomes but costing at least 40% less than BLCC per patient and significantly faster healing of DFUs than DSS.8–10
The authorized indications for use vary among the three CTPs evaluated in this study. The BLCC is indicated for both VLU and DFU, whereas the DSS is indicated for DFU only. Neither the BLCC nor DSS is FDA approved for DFUs with exposed muscle, tendon, or bone structures. The CHSA is indicated for all wound types, including those with exposed structures. Both the BLCC and DSS are available in one size: the BLCC in a 44-cm2 round disk shipped at room temperature with a shelf life of up to 10 days and the DSS in a 37.5-cm2 rectangular cryopreserved graft with a shelf life of 6 months. The CHSA has a shelf-life of 5 years and is now available in four sizes, ranging from 6 to 116 cm2. During the study period of this research, CHSA was available in two sizes, 13 and 39 cm2; the 6- and 116-cm2 size CHSA grafts were not introduced to the market until late 2016.
Database
The Medicare Standard Analytical File (SAF) spans from 2005 through 2014 and comprises the Medicare Inpatient Limited Data Set, Outpatient Limited Data Set, and Carrier file. These files include 100% of Medicare inpatient hospital billings, 100% of Medicare Outpatient hospital billings, and the 5% patient sample with all provider billings across all service locations, respectively.
A retrospective analysis of the full Medicare SAF was performed using the PearlDiver database to identify patients who received CHSA, BLCC, or DSS between January 1, 2011, and December 31, 2014. Medicare beneficiaries included in the analysis were those enrolled in Medicare Part A or B any time during the year but not enrolled in a managed Medicare plan.
Patient Eligibility
Patients with chronic wounds were deemed eligible if they were newly treated with CHSA, BLCC, or DSS. Cohorts were required to have a claim for a surgical debridement within 30 days of CTP application. This ensured debridement was performed during the treatment period, because this is standard procedure before CTP application for all wounds, and allowed for further subgroup analysis based on the size of wounds receiving the CTP. For each query, the patients had to be “active” or enrolled under Medicare for the duration of the longitudinal analysis. Patients were assigned to a cohort based on their first claim (index date).
Cohort matching was performed on patients (as opposed to records), and the matching was conducted at a proportional level based on the following criteria: age, sex, region, and Charlson Comorbidity Index (CCI; Figure 1). The exact CCI score of each patient was used in the matching criteria. Exact matches were also required for sex and region; for age, an exact matching for the age group (eg, age 65–69, 70–74 years, etc) was used. Matching was performed concurrently on all populations. Patients from each population with the same characteristics were determined. The resulting new populations were created in proportion to one another with the same makeup for each defined characteristic. The proportion was created based on the population with the lowest patient volume, which defined the proportions for all additional populations.
Figure 1.
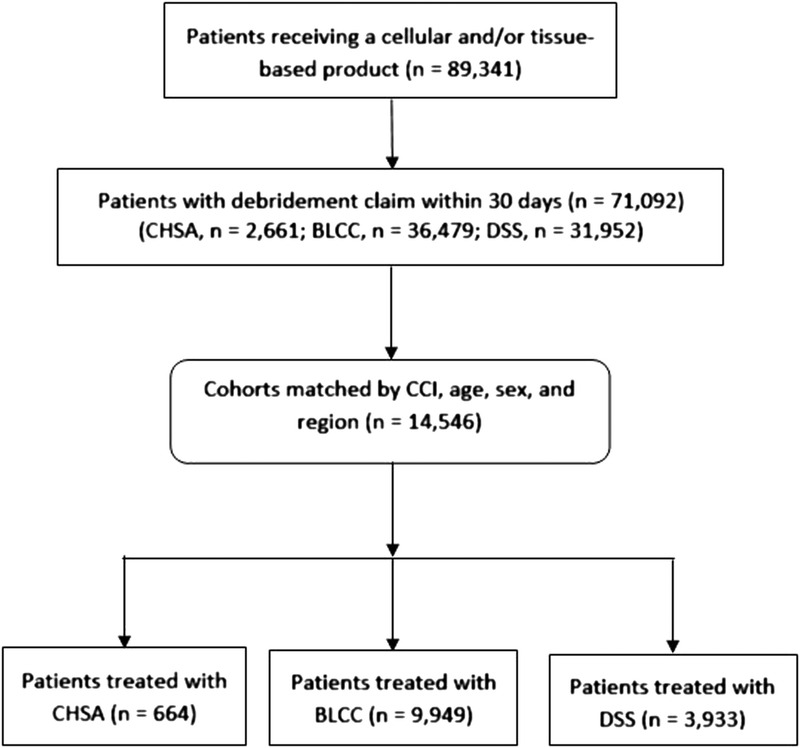
SAMPLE SELECTION
Abbreviations: BLCC, bilayer cellular construct; CCI, Charlson Comorbidity Index; CHSA, cryopreserved human skin allograft; DSS, dermal skin substitute.
Key Variables
After cohort matching, the following key variables were examined from the index date (date of first CTP claim) out to 60-, 90-, and 180-day windows: wound-related medical costs and the number of CTP applications, lower extremity amputations (LEAs), units of CTP wastage reported, wound-related hospitalizations, and wound-related ER visits. In addition, a subset analysis of smaller wounds was conducted to evaluate differences in wound-related costs, product waste, and number of applications between the matched cohorts. Physician-specific data, Medicare claims for prescription drugs, and patient-centered outcome measures were not available. There was an inadequate number of patients beyond 180 days to conduct valid statistical analysis.
Determining Wound-Related Costs, Hospitalizations, and ER Visits
Only those claims that had a wound care-related diagnosis were included in the analysis (Supplemental Table 1; http://links.lww.com/NSW/A27). Because the study period (2011–2014) occurred prior to 2015, the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes were used to identify wound-related costs. A claim was considered wound related if one of the listed codes was reported in the first or second position of the claim.
Determining Product Waste
Historically, Medicare Administrative Contractors did not require providers to report the amount of product wasted at the time of application. Modifier JW is used on claims to indicate product wastage based on the amount of biologic product discarded/not administered to a patient. On January 1, 2017, the CMS revised its policy to require uniform use of the JW modifier with all discarded Part B drugs and biologics in single-use packages.11 The Medicare Administrative Contractors now require providers to report the amount of the product used on one line item and the amount wasted (using modifier JW) on another line item on the claim form. Investigators found 680 instances within the matched cohorts where providers voluntarily reported waste using the JW modifier in this way prior to the 2017 CMS policy revision (Supplemental Table 2; http://links.lww.com/NSW/A28).
Product Q codes are reported in square centimeters as “units,” which has led to confusion where providers mistakenly miscode the units used. It is worth noting that this Medicare claims analysis discovered numerous instances where only one unit of a CTP was reported, likely the result of the providers erroneously reporting that one “unit” (ie, one “graft”) was used, rather than properly reporting the square centimeter (units) of the graft used. Improper billing of the Q codes for CTPs is a serious issue beyond the scope of this article; however, the investigators accounted for billing errors by including the approximately only 680 voluntary claims that correctly reported units along with the JW modifier. In other words, only claims accurately reporting the JW modifier were deemed reliable and used in the analysis to conduct the waste analysis.
Regardless of waste, Medicare reimburses for the total product. In January 2014, the CMS implemented a bundled payment for CTPs in the HOPD where the payment for the application code included the cost of the product. Before 2014, the CTP and procedure were billed and reimbursed separately in the HOPD. Because of this, investigators focused on the number of units wasted rather than the cost associated with waste.
Determining Lower-Extremity Amputations
Amputation procedures are typically performed in the OR. For this reason, ICD-9 procedure codes related to LEAs were used to identify patients who received an amputation. Because the goal of any CTP ultimately is to achieve full wound closure, the amputation analysis included both minor and major amputations.
Identifying Wound Size
There is no reliable way to account for wound characteristics such as size (surface area) from claims data because providers are not required to report these characteristics on a claim. Large retrospective electronic medical record registry studies have found that the majority of wounds are less than 10 cm2.12,13 For this reason, the authors chose to use Current Procedural Terminology (CPT)1 codes for the reported debridement as an indirect measure of wound size. The rationale is described below, and the limitations of this method are discussed in detail in the Discussion.
On January 1, 2011, the American Medical Association issued revised and new CPT codes for debridement (97597–97598 and 11042–11047).14 These codes are billed when an extensive cleaning of a wound is needed before the application of primary dressings or CTP placed over or onto a wound that is attached with secondary dressings. When performing debridement of a single wound, providers report depth using the deepest level of tissue removed; in multiple wounds, providers sum the surface area of those wounds that are at the same depth. The appropriate debridement CPT code is billed by size in 20 cm2 increments; debridements greater than 20 cm2 are reported with add-on codes.
Real-world retrospective studies report that the average number of wounds per patient ranges from 1 to 2.2.13,15 For this analysis, wounds were considered 20 cm2 or smaller if only the 97597, 11042, 10043, or 11044 CTP code was reported (without an add-on code) within 30 days of CTP application. Alternatively, wounds were considered greater than 20 cm2 if the add-on CPT codes (97598, 11045, 11046, or 11047) were reported within 30 days of CTP application. The grouping is intended to provide directional value, and the limitations of this rationale are addressed in detail in the Discussion section.
Statistical Analysis
Differences in demographic characteristics were examined using χ2 tests. Because of the nonnormal distribution of the wound-related cost variables, nonparametric tests (Mann-Whitney U) were used. Differences in applications, wastage, ER visits, and hospital visits were tested using t tests.
RESULTS
The sample selection is described in Figure 1. From a total of 89,341 patients who received a CTP, 14,546 patients were included after concurrent cohort matching (CHSA = 644; BLCC = 9,949; DSS = 3,933). The number of patients decreased over time in each cohort because patients were required to be under active nonmanaged Medicare coverage for each of the time periods. The number of patients at 1 year and beyond was too small to conduct a valid statistical analysis.
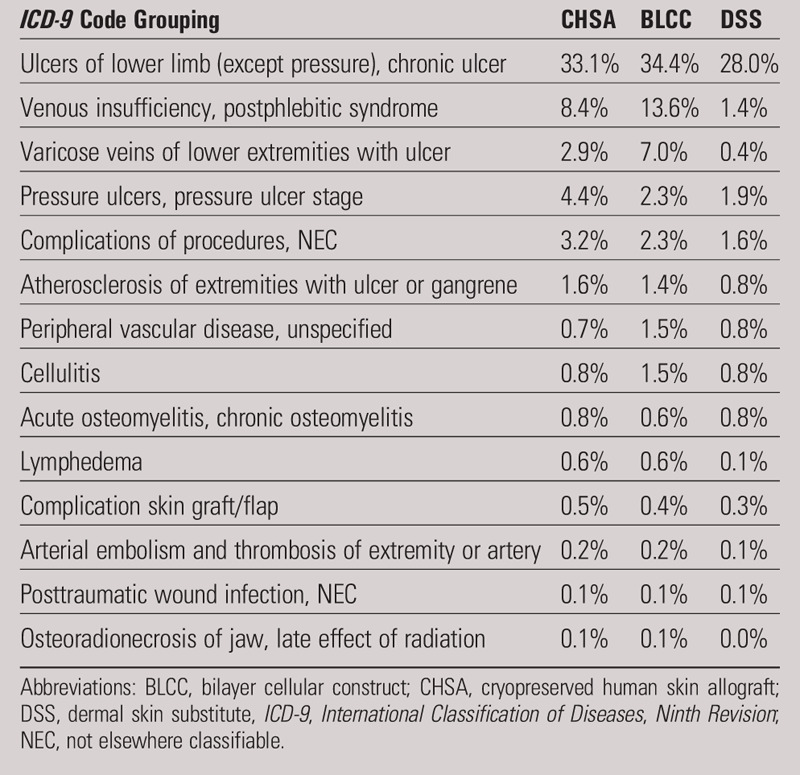
The distribution of age group, sex, region, and CCI was not statistically different across the three skin substitute cohorts (Table 1). The distribution of ICD-9 codes was analyzed and indicated that most of the wounds were lower-extremity ulcers (Table 2).
Table 1.
PATIENT DEMOGRAPHICS BY COHORT
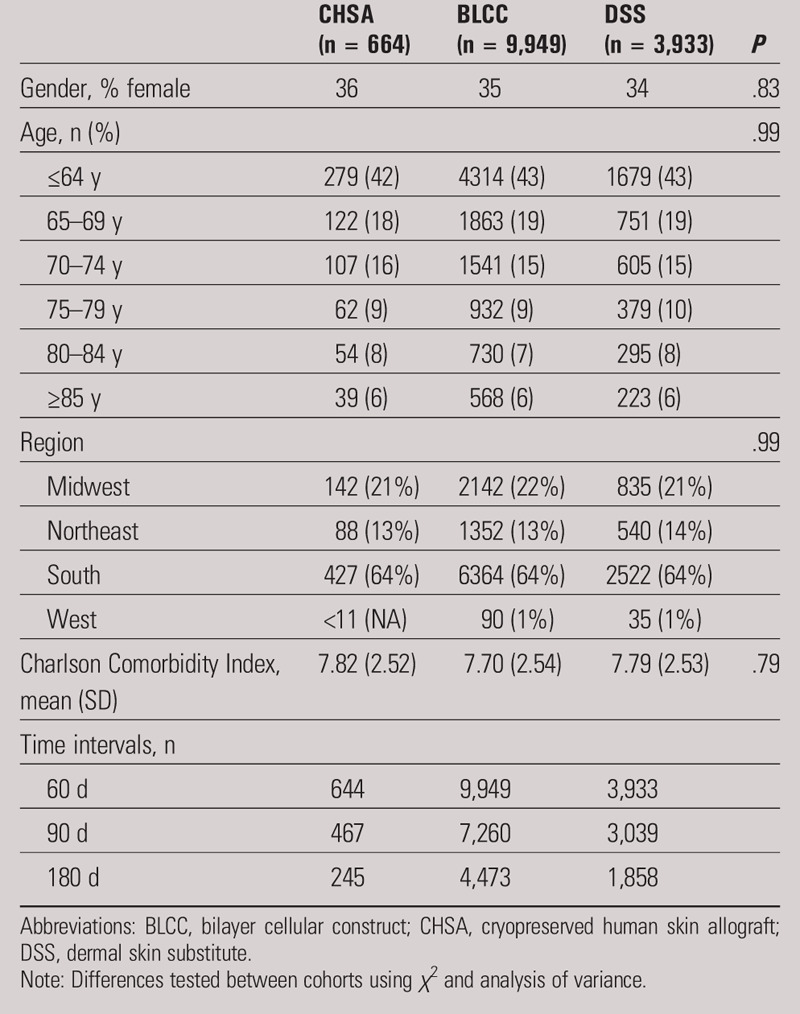
Table 2.
TOP 25 PRIMARY AND SECONDARY DIAGNOSES BY GROUP
Wound-Related Costs, Hospitalizations, and ER Visits
As illustrated in Figure 2, wound-related medical costs at 60, 90, and 180 days were significantly lower for the CHSA cohort relative to the BLCC and DSS cohorts (P < .05).
Figure 2.
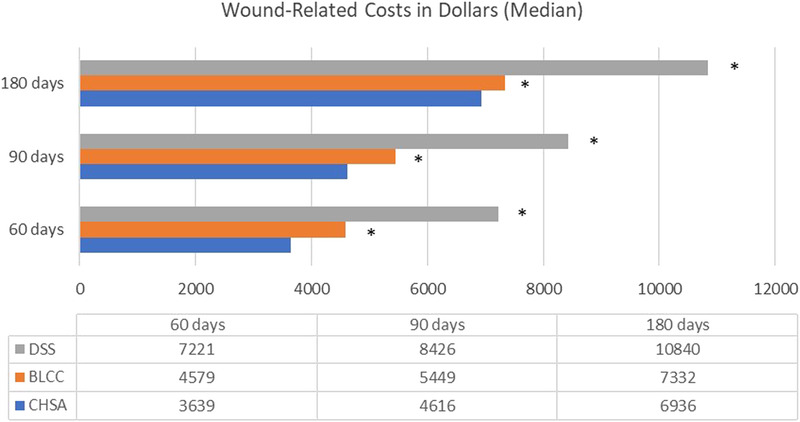
WOUND-RELATED COSTS
Abbreviations: BLCC, bilayer cellular construct; CHSA, cryopreserved human skin allograft; DSS, dermal skin substitute. aRepresents P < .05 relative to CHSA (Mann-Whitney U test was used to test for differences).
The percent of CHSA patients who experienced a hospital stay at 60, 90, and 180 days was 6.21%, 9.42%, and 13.87%, respectively. The percent of BLCC patients who experienced a hospital stay at 60, 90, and 180 days was 6.23%, 9.14%, and 15.60%, respectively. The percent of DSS patients who experienced a hospital stay at 60, 90, and 180 days was 7.39%, 10.29%, and 16.41%, respectively. There were no statistically significant differences in the number of hospitalizations across the three cohorts. The number of patients experiencing an ER visit across all three cohorts was too low to conduct reliable statistical analysis. The percent of patients experiencing at least one hospital stay or ER visit was low and similar across groups.
Number of Applications
Patients in the DSS cohort received, on average, significantly more skin substitute applications than patients in the CHSA cohort at all three time intervals (P < .05). There were no differences in the mean number of applications between the CHSA and BLCC cohorts across all three time windows (Figure 3).
Figure 3.
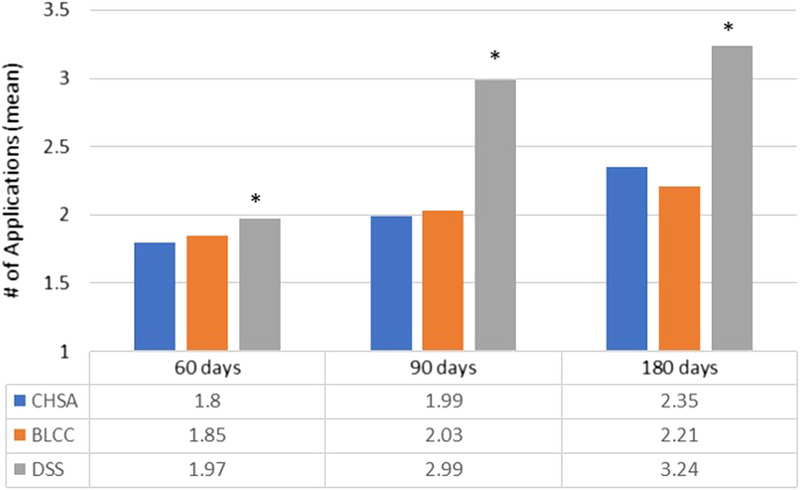
MEAN PRODUCT APPLICATIONS
Abbreviations: BLCC, bilayer cellular construct; CHSA, cryopreserved human skin allograft; DSS, dermal skin substitute. aRepresents P < .05 relative to CHSA (t test).
Lower Extremity Amputations
In the CHSA cohort, 23% (155/644) of patients underwent LEA. The BLCC cohort had 21% (2,098/9,949) LEA rates, whereas 32% (1,250/3,933) of patients in the DSS cohort underwent LEA. The difference between CHSA and BLCC was not statistically significant (P = .22). The LEA rates in the DSS cohort were statistically significant, with DSS patients experiencing higher rates of amputation than either the CHSA or BLCC group (P < .0001).
Product Wastage
The number of CHSA claims that reported the JW modifier code was too low at 180 days to allow for analysis, so a waste comparison between matched cohorts was limited to 60 and 90 days. The number of units wasted during application was significantly higher in the BLCC and DSS cohorts relative to the CHSA cohort (Figure 4).
Figure 4.
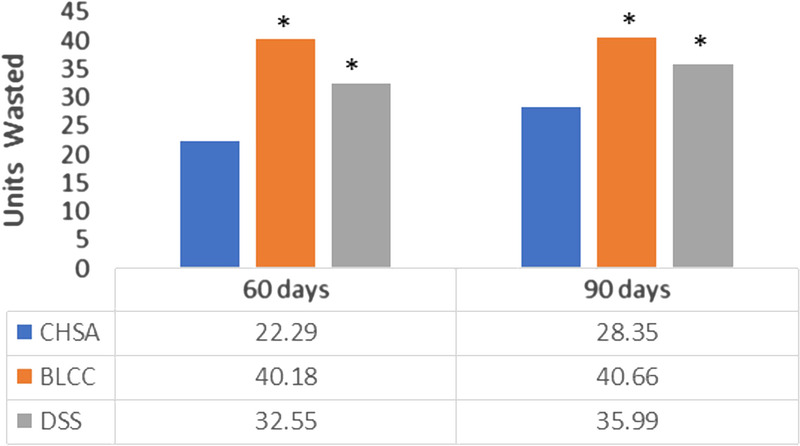
MEAN UNITS OF PRODUCT WASTED
Abbreviations: BLCC, bilayer cellular construct; CHSA, cryopreserved human skin allograft; DSS, dermal skin substitute. aRepresents P < .05 relative to CHSA (t test).
Outcomes for Wounds 20 cm2 or Less
The originally matched cohorts from Table 1 were separated into two groups using the rationale described in the Methods section. The resulting number of patients in each size category is described in Table 3. The proportion of patients in the group with wound sizes 20 cm2 or less was significantly different in the three cohorts (P < .05) and largest in the DSS cohort (Z test). For all three cohorts, the results show that most patients had a debridement of 20-cm2 wound surface area or less within 30 days of CTP application. Because of this, the investigators conducted further analysis on this population of patients. Directionally, the wound-related costs for wounds 20 cm2 or less were lower than the general matched cohort population (Figures 2 and Table 4).
Table 3.
WOUNDS BY SIZE

Table 4.
WOUND-RELATED COSTS, PRODUCT WASTE, AND NUMBER OF APPLICATIONS
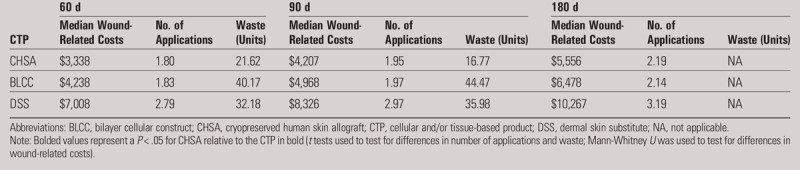
The wound-related costs, product waste, and the number of applications for wounds 20 cm2 or less are summarized in Table 4. Wound-related costs for wounds 20 cm2 or less were lower for CHSA compared with BLCC (statistically significant at 60 and 90 days; P < .05) and DSS (statistically significant at all time intervals; P < .05). In wounds 20 cm2 or less, both BLCC and DSS had significantly more waste than CHSA at 60 and 90 days (P < .05). The number of CHSA claims that reported the JW modifier code was too low at 180 days to allow for analysis, so waste comparison between matched cohorts was limited to 60 and 90 days. The results mirror those in the general matched cohort analysis. Similarly, DSS had a significantly higher number of applications than CHSA at all time windows (P < .05). There was no statistically significant difference in the number of applications between CHSA and BLCC in wounds 20 cm2 or less.
DISCUSSION
This is the first research addressing the clinical outcomes and health economic impact of a specific class of CTPs by analyzing Medicare claims data. Previous research has shown that Medicare spending for beneficiaries with wounds is higher than the general Medicare population.1
In 2015, Rice and colleagues2 published an analysis of Medicare claims data to demonstrate the benefits of BLCC and DSS over conventional care in the treatment of DFUs. Both BLCC and DSS have components of human skin: BLCC contains two living cell types (fibroblasts and keratinocytes), and DSS has one living cell type (fibroblasts). Both bioengineered grafts have demonstrated that they are more effective than standard of care, but no RCT to date has proven that they are more effective or safer than a human skin allograft such as CHSA. This is an important point because the intent of bioengineered tissue is to emulate the properties of human skin and act as a skin replacement.
A retrospective study by Treadwell and colleagues16 comparing CHSA with BLCC in the treatment of VLU reported superiority of BLCC to CHSA. The discrepancy between that study and the present study could have been a result of lack of controls (treating clinicians selected the treatment option) and lack of a matched-pairs methodology in the Treadwell article.16
This large matched-cohort study aimed to validate the results observed in smaller head-to-head RCTs comparing CHSA to BLCC and DSS in the Medicare population. Interestingly, three head-to-head RCTs comparing CHSA to BLCC or DSS have shown either equivalency or improved outcomes with CHSA over others.8–10 Of importance to this analysis, however, is that all three of these RCTs also reported either fewer applications or overall cost savings with CHSA compared with BLCC or DSS. Further, in this Medicare claims analysis, all wound types were included, whereas the RCTs focused on only DFU and VLU; this potentially could have impacted the results, although the investigators did not see a significant difference in diagnosis codes among the three cohorts (Table 2).
The observed reduction in wound-related costs with CHSA at 60, 90, and 180 days (Figure 2) could be attributed to numerous factors. The frequency of hospitalizations and ER visits were low among all cohorts, and no statically significant differences were noted. The low number of hospitalizations and ER visits in all three cohorts potentially validates previously published research on the value of CTPs in this population.2,3
The main driver of cost seems to be in the outpatient setting, which is consistent with Nussbaum and colleagues’1 findings. Because CTPs are most commonly applied in the HOPD setting, these data are not surprising. In the HOPD setting, the wound-related cost reduction observed with CHSA could be attributable to fewer visits (as a result of faster healing), fewer LEAs, fewer applications, or lower use of other advanced modalities such as hyperbaric oxygen therapy and negative-pressure wound therapy. Patients with complex wounds often require multiple advanced modalities, which are sometimes used concomitantly with CTPs or provided sequentially in a stepwise fashion in the continuum of care. The investigators also saw an increase in costs across all three cohorts with each progressive time period, likely a reflection of harder to heal wounds that required more resources. Further analysis in the future would help identify the source of the wound-related cost savings.
Because there was no statistically significant difference in the number of applications between CHSA and BLCC (Figure 3), the researchers assume that the number of applications was not responsible for the higher wound-related costs in the BLCC cohort but rather the other possible causes described above. The fewer number of applications of CHSA relative to DSS (Figure 3) is consistent with findings from a head-to-head RCT.10 One explanation is that CHSA is typically applied every other week, whereas DSS is applied weekly. In addition, it is possible that more cell types or the presence of human collagen are important in healing, but there are little data available today to understand the implications for real-world practice. In general, the number of applications for all three cohorts seems to reflect other studies conducted on these products to date.2,5
In 2015, Rice and colleagues2 performed an analysis of the Medicare 5% dataset from 2006 to 2012 and found that 27.6% of BLCC patients experienced an LEA, compared with 22.2% in the DSS group. The results from the full Medicare dataset from 2011 to 2014 presented here found the inverse, in that amputation rates were 21% for BLCC and 32% for the DSS cohort. This analysis found that 23% of CHSA patients experienced an amputation. This is possibly because of the initial matching of the cohorts for medical complexity (CCI), age, sex, and region to account for a more complex patient population.
Although the difference in BLCC and CHSA amputations was not statistically significant, the investigators did see a statistically lower percentage of amputations for both BLCC and CHSA compared with DSS. This also may account for the more significant difference between CHSA and DSS in wound-related costs observed in this study (Figure 2).
In addition, CHSA is approved for wounds with exposed structures, whereas DSS is not; clinically, the ability to treat these wounds may have impacted the LEA rate because diabetic wounds with exposed structures (Wagner 2 and greater) have higher amputation rates.17,18 In a retrospective cohort study of 312,744 wound from a large electronic medical record database, the majority of DFUs at the time of presentation were Wagner grade 2 and greater.19 The amputation rates for all three cohorts are lower than the recently published rate of 36.2%; in that study, 45.7% of patients healed, 9.5% of patients died before ulcer healing, and 8.5% were lost to follow-up.17 The lower amputation rates reported from this analysis potentially validate the reduction in amputations reflected in both RCTs and real-world evidence of CHSA, BLCC, and DSS.
Finally, real-world retrospective studies report that the number of wounds per patient ranges from 1 to 2.2 on average; that is, a patient may have multiple chronic wounds for which they are undergoing concurrent treatment.13,15 The investigators assumed that amputations were a result of the treated wound, but it is possible that the amputation was a result of a separate wound.
There was a marked decrease in the amount of waste with CHSA compared with DSS and BLCC at 60 and 90 days (Figure 4). Before 2017, when Medicare required the reporting of product wastage, reporting waste with the JW modifier was voluntary. As a result, not only could there be differences in coding, but there was also no consistent Medicare requirement on how the providers should report the wastage. Large retrospective registry studies have found that most wounds are less than 10 cm2.5,13,19 Whereas DSS and BLCC are still provided in one size (37.5 and 44 cm2, respectively), the smaller size of CHSA (6 cm2) became available in late 2016 to reduce product wastage. The CHSA is premeshed at 1:1.5 ratio, allowing each graft to “stretch” approximately 30% of its original size; this property of CHSA has the potential to provide further cost savings and reduced waste. Further research into product waste with more current Medicare claims data may show a more significant reduction in product waste with CHSA after 2016 when the smaller CHSA 6 cm2 became available. These data could help the CMS identify opportunities to further minimize unnecessary CTP waste and help manufacturers develop more flexible product sizing.
Wounds measuring less than 20 cm2 had directionally lower wound-related costs compared with the full matched cohort (Figure 2 and Table 4). It is possible that wounds larger than 20 cm2 are more challenging to heal and require more medical resources. The amount of product waste and the number of applications in this subset mirrored those of the general matched cohort analysis (Figures 2–4 and Table 4). The analysis of wounds less than 20 cm2 requires further discussion because the methodology and rationale do have limitations. Because the method used identifies these patients using debridement claims after implementation of the new billing policy in 2011, it is difficult to discern whether the size of the wound debridement was for one or multiple wounds. However, because the CTP codes reflect the total surface area debrided of all wounds, one can assume that the wound being treated with the CTP had to be less than 20 cm2 if no add-on code was reported. The results in Table 3 validate previous research, indicating that most wounds are small. This is consistent with a large retrospective study of CHSA for the treatment of VLU and DFU where it was found that the average CHSA-treated VLU (n = 134) was 11.8 cm2 in size, and the average wound size in the DFU (n = 54) was 6.2 cm2.5
Although the cohorts were matched for CCI, a limitation of claims analysis in wound care studies is that there is no reliable and consistent way to measure wound severity. In addition, chronic wounds often require other surgical interventions such as venous ablation or arterial intervention to achieve healing or prevent recidivism. After cohort matching, this dataset was not large enough to conduct this type of subanalysis; future real-world matched-cohort studies looking at timely surgical intervention and recidivism are needed.
Study Limitations
The limitations of the Medicare SAF include:
Conditions must be diagnosed in order to appear in the utilization files; however, some diseases such as hypertension, depression, and diabetes are often underdiagnosed. In addition, although the files provide a reliable record of the care received by the beneficiary, they do not provide information on the care needed. It is difficult to study disease recurrence in detail because the data may indicate the start of a new treatment only.
Another important point is that services that providers know in advance will be denied (eg, the application of noncovered dressings) may be inconsistently submitted as bills and therefore inconsistently recorded in the files.
Diagnosis information may not be comprehensive enough in some cases to allow for detailed analysis. For example, a cancer diagnosis is included in the data (eg, lung cancer is 162.xx), but no information on stage or histology is included in the Medicare claims data.
The data contain information on chronic diseases; however, knowing that someone has a chronic disease does not reveal how long they have had the condition or the severity of their condition.
There is no reliable and consistent way to measure wound severity.
Some services are not covered by Medicare and are therefore not included.
CONCLUSIONS
Medicare beneficiaries receiving CHSA experienced fewer wound-related costs and waste compared with BLCC and DSS. The CHSA patients also had fewer applications and lower LEAs compared with the DSS cohort. Future prospective research that combines cost, clinical outcomes, and patient-centered outcomes could provide insight into the use of advanced modalities in this challenging patient population and enhance the development of more appropriate quality measures and reimbursement models to ensure smarter spending for this growing and costly population.
CPT is a registered trademark of the American Medical Association, Chicago, Illinois.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website.
REFERENCES
- 1.Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and Medicare policy implications of chronic nonhealing wounds. Value Health 2018;21(1):27–32. [DOI] [PubMed] [Google Scholar]
- 2.Rice J, Desai U, Ristovska L, et al. Economic outcomes among Medicare patients receiving bioengineered cellular technologies for treatment of diabetic foot ulcers. J Med Econ 2015;18(8):586–95. [DOI] [PubMed] [Google Scholar]
- 3.Santema TB, Poyck PPC, Ubbink DT. Systematic review and meta-analysis of skin substitutes in the treatment of diabetic foot ulcers: highlights of a Cochrane systematic review. Wound Repair Regen 2016;24(4):737–44. [DOI] [PubMed] [Google Scholar]
- 4.Landsman A, Rosines E, Houck A, et al. Characterization of a cryopreserved split-thickness human skin allograft—TheraSkin. Adv Skin Wound Care 2016;29:399–406. [DOI] [PubMed] [Google Scholar]
- 5.Landsman AS, Cook J, Cook E, et al. A retrospective clinical study of 188 consecutive patients to examine the effectiveness of a biologically active cryopreserved human skin allograft (TheraSkin) on the treatment of diabetic foot ulcers and venous leg ulcers. Foot Ankle Spec 2011;4:29–41. [DOI] [PubMed] [Google Scholar]
- 6.Wilson TC, Wilson JA, Crim B, Lowery NJ. The use of cryopreserved human skin allograft for the treatment of wounds with exposed muscle, tendon, and bone. Wounds 2016;28:119–25. [PubMed] [Google Scholar]
- 7.Budny AM, Ley A. Cryopreserved allograft as an alternative option for closure of diabetic foot ulcers. Podiatry Manage 2013;131–6. [Google Scholar]
- 8.DiDomenico L, Landsman AR, Emch KJ, et al. A prospective comparison of diabetic foot ulcers treated with either a cryopreserved skin allograft or a bioengineered skin substitute. Wounds 2011;23:184–9. [PubMed] [Google Scholar]
- 9.Sanders L, Landsman AS, Landsman A, et al. A prospective, multicenter, randomized, controlled clinical trial comparing a bioengineered skin substitute to a human skin allograft. Ostomy Wound Manage 2014;60:26–38. [PubMed] [Google Scholar]
- 10.Towler MA, Rush EW, Richardson MK, Williams CL. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatric Med Surg 2018;35(3):357–65. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Medicare & Medicaid Services. Medicare Program. JW Modifier: Drug/Biological Amount Discarded/Not Administered to Any Patient: Frequently Asked Questions. 2016. www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Downloads/JW-Modifier-FAQs.pdf. Last accessed August 20, 2019.
- 12.Margolis D, Allen-Taylor L, Hoffstad O, Berlin J. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Repair Regen 2004;12:163–8. [DOI] [PubMed] [Google Scholar]
- 13.Fife C, Eckert K. Harnessing electronic healthcare data for wound care research: standards for reporting observational registry data obtained directly from electronic health records. Wound Repair Regen 2017;25(2):192–209. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Medicare & Medicaid Services. Billing and Coding Guidelines for Wound Care. 2017. https://downloads.cms.gov/medicare-coverage-database/lcd_attachments/34587_21/L34587_GSURG051_BCG.pdf. Last accessed August 20, 2019.
- 15.Kirsner R, Sabolinksi M, Parson N, Skornicki M, Marston W. Comparative effectiveness of a bioengineered living cellular construct vs. a dehydrated human amniotic membrane allograft for the treatment of diabetic foot ulcers in a real world setting. Wound Repair Regen 2015;23(5):737–44. [DOI] [PubMed] [Google Scholar]
- 16.Treadwell T, Sabolinski M, Skornicki M, Parsons N. Retrospective medical records review comparing patients treated with Apligraf or TheraSkin. Adv Wound Care 2017;7(3):1–8. [Google Scholar]
- 17.Zhan LX, Branco BC, Armstrong DG, Mills JL., Sr The Society for Vascular Surgery lower extremity threatened limb classification system based on wound, ischemia, and foot infection (WIfI) correlates with risk of major amputation and time to wound healing. J Vasc Surg 2015;61(4):939–44. [DOI] [PubMed] [Google Scholar]
- 18.Smith-Strøm H, Iversen MM, Igland J, et al. Severity and duration of diabetic foot ulcer (DFU) before seeking care as predictors of healing time: a retrospective cohort study. PLoS One 2017;12(5):e0177176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilcox J, Carter M, Covington S. Frequency of debridements and time to heal- a retrospective cohort study of 312,744 wounds. JAMA Dermatol 2013;149(9):1050–8. [DOI] [PubMed] [Google Scholar]


