ABSTRACT
OBJECTIVE
To describe venous ulcer care and wound care practices in Gauteng, a province of South Africa, according to the Donabedian structure-process-outcome quality improvement model.
METHODS
Forty-eight facilities were selected randomly from public and private wound care practices in Gauteng. Structured interviews were conducted with care providers via questionnaire to assess the structural aspects of the Donabedian model. Within these facilities, investigators randomly selected 160 patient files and extracted data using a checklist to assess processes implemented and outcomes reached for patients who had previously presented with lower-leg venous ulcers.
RESULTS
Facilities lack the necessary equipment to perform vital assessments. Handheld Dopplers were available in 66% (n = 48) of the facilities. Sixty-one percent (n = 48) of the personnel at the facilities indicated that they had no formal wound care training. Although the majority of files (92%, n = 147) indicated that an assessment tool was used, many elements were not evaluated comprehensively according to the best available evidence. Aspects such as smoking, body mass index, and anemia were assessed in fewer than 30% of the patients. Distinguishing between superficial and deep infection and the accompanying overuse of antimicrobials and antibiotics were among the challenges identified. Further, 71% of patients received compression therapy, although the ankle-brachial pressure index of only 30% of patients was known. In 27 cases (17%), the outcome was amputation.
CONCLUSIONS
From this survey, it is evident that not all clinicians providing wound care in Gauteng are adequately trained or fully implementing best practice guidelines, and the consequences are detrimental to patients, particularly in terms of amputation. This article highlights the need for improved legislation and regulation for practitioners who deliver wound care services.
KEYWORDS: ankle-brachial pressure index, compression therapy, Donabedian model, handheld Doppler, lower-leg ulcer, venous insufficiency, venous ulcer
INTRODUCTION
A lack of evidence-based care not only contributes to extended healing trajectories and increased costs, but also may have detrimental effects for the patient.1 Chronic wounds, specifically lower-leg venous ulcers, are often complex and challenging to heal. In addition, they place a significant socioeconomic burden on the patient, the healthcare system, and the community.1 Chronic venous disease is the underlying cause of between 40% and 80% of leg ulcers and is listed as the seventh most common chronic disease worldwide.2 Inappropriate care contributes to unfavorable outcomes and could be ascribed to the lack of knowledge and skills of the practitioner delivering the care.3 Clinical guidelines are one of the most effective ways to apply evidence to practice and improve quality of care.4 Access to high-quality, effective care contributes to the timely healing of venous leg ulcers.5
Chronic venous leg ulcers are defined as full-thickness skin lesions around the gaiter area and are a result of chronic venous insufficiency.6 Chronic wounds fail to progress through a normal or timely sequence of tissue repair, resulting in protracted healing trajectories.7 Inaccurate diagnosis and not managing the underlying cause contribute to wound chronicity and recurrence.5
Several best practice guidelines advocate a holistic assessment of the patient with a lower-leg ulcer to identify and treat underlying causes.8–10 Assessment includes diagnostic tests, such as an ankle-brachial pressure index (ABPI) to exclude peripheral arterial disease, and developing a patient-centered care plan, which could include the application of compression.8 The implementation and application of standardized protocols could improve outcomes for patients with lower-leg venous ulcers.11
Andrews and Langley12 point out that there are no standards for wound care in South Africa; therefore, quality of care cannot be measured. There is a need for standardization of care within the South African context. South Africa currently spends 8.8% of gross domestic product on healthcare. This is relatively high by international standards. However, there are clear inequalities in the extent to which different sections of the South African population can access healthcare services.13 The main sources of finance for healthcare are government, households, employers, and nongovernmental organizations.
Government is the largest source of healthcare finance. Money is allocated to healthcare from tax revenue and largely funds the delivery of public sector healthcare services at the provincial level.13 The government also provides a tax benefit to those who purchase private healthcare through medical insurance companies. The second largest source of health finance is household spending. This financing takes place via contributions to a medical insurance company, direct out-of-pocket expenditures, and, to a limited extent, other forms of private insurance. Even households with private coverage make out-of-pocket payments for services that are not fully covered.13
Donabedian13,14 developed the structure-process-outcome model to measure quality of care. In this model, structure focuses on the qualifications of care providers, their tools and resources, and the physical/organizational setting of the facility. The second concept, process, refers to the interpersonal and technical aspects of treatment and best practice guidelines, and how these are implemented. Outcomes measure changes in patient symptoms and functioning. Implementing standardized care would improve not only the quality of care but also patient outcomes.
The current survey comprised an assessment of the current level of care patients with lower-leg venous ulcers receive using the Donabedian framework to evaluate the structure of the facility, processes implemented, and outcomes reached. This assessment could guide practitioners in addressing gaps in their application of evidence-based care into practice, resulting in improved outcomes. Figure 1 is a schematic representation of the application of the Donabedian model in this survey.
Figure 1.
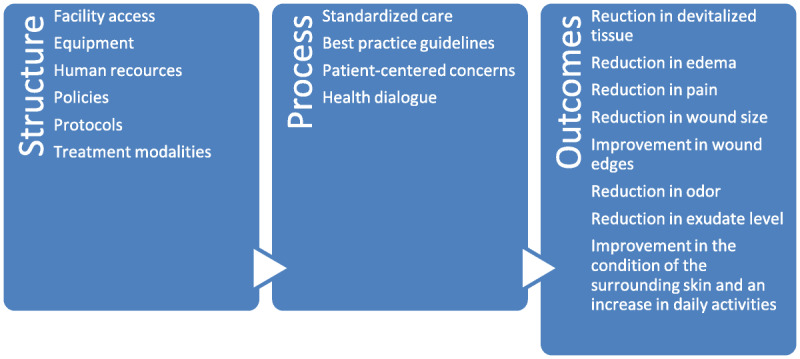
DONEBEDIAN SURVEY STRUCTURE
METHODS
A descriptive quantitative design was used to evaluate venous leg ulcer care in Gauteng. Two trained fieldworkers conducted structured interviews using a questionnaire to collect data on the first Donabedian concept of structure and extracted data from patient files about the process and outcomes using a checklist.
The initial questionnaire and checklist were designed by the first author to evaluate practice against standards based on international guidelines about the structure of the facility that delivers a wound care service.15 Published guidelines from the Wound Healing Society, Society of Vascular Surgeons, European Wound Management Association, and Wound Healing Association of Southern Africa were consulted, as well as the Dutch Venous Ulcer Guidelines and Australian and New Zealand clinical practice guidelines.8,9,16,17 A panel consisting of wound care experts and a biostatistician evaluated both initial drafts and determined content and face validity.
The questionnaire contained the following components to evaluate the structure of the facility: biographic data, access to the facility, equipment available in the facility, level of education of the staff at the facility, policies and protocols in place, and treatment modalities available.
The checklist examined processes by evaluating the assessment tools used (including patient assessment, history taking, diagnostic tests, wound bed assessments, and patient-centered concerns and health dialogue) to determine whether best practice guidelines were followed and evidence-based care was used. The checklist included the assessment of edema and infection, how infection was diagnosed and treated, what was used to clean the wounds, and the type of compression therapy used.
Finally, the outcome measures indicated any changes in health status according to the following criteria: reduction in devitalized tissue, edema, pain, wound size, malodor, and exudate level, as well as advancement of wound edges, increase in daily activities, and improvement in the surrounding skin condition.
Components relating to process and outcomes were evaluated for each patient file using the checklist for initial assessment, after a 3-week interval, and on completion of treatment to identify possible correlations. Based on the experience of the wound care experts, an interval of 3 weeks was used because this is the period during which most patients present with an infection after commencing treatment.
A small number of facilities (n = 5) were included in a pilot study using convenience sampling to validate the checklist and determine the feasibility of the study. Both the checklist and the questionnaire were adapted based on information obtained from the pilot study. Questions had to be streamlined, and the checklist was expanded to include different intervals of assessment. The pilot study tested the ease with which the newly developed tools could be administered during the survey, participant understanding of each item, and consistency in recording the results, among other things. The researcher incorporated the results from the training sessions and pilot study into the questionnaire and checklist, but none of the pilot data were included in the final analysis.
Study Population and Sampling
Figure 2 shows a schematic of the facility sampling process. The study population comprised 105 facilities that offer wound care within a 75-km radius of the researcher’s base in the following strata: private wound clinics (hospital-based or not), public outpatient wound clinics, pharmacies that deliver a wound care service, general practitioner (GP) practices, and private nursing practitioners who provide home-based care. The aim of including different strata was not to compare data gathered from the different providers, but to obtain a general view of current practices and generalize the results to the rest of the population.
Figure 2.
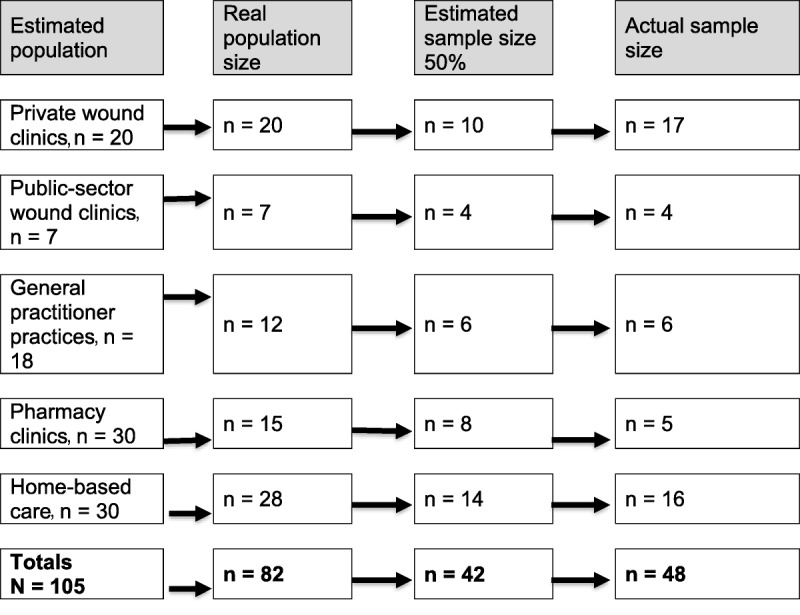
FACILITY SAMPLING SCHEMATIC
To reduce bias, only some facilities were selected as representative of each stratum. Facilities were listed alphabetically per stratum and numbered. First contact was made through an introductory phone call to establish willingness to participate and confirm contact details. After initial contact, only 82 facilities were actively practicing. Another list of these active facilities was compiled, and the final selection process resulted in 48 facilities.
Data Collection
Structured interviews were scheduled with the managers of the facilities that indicated they were willing to participate. On arrival at the facility, the fieldworker introduced herself and obtained written informed consent from the facility manager or practitioner in charge of the practice to conduct the interview and extract deidentified information from the patient files to complete the checklist. The fieldworker then proceeded with the structured interview using the questionnaire.
Clinical efficacy emphasizes the process of care, whereas an outcome-based measure focuses on outcomes reached and the patient’s response to the care provided.18 In this study, process and outcomes were measured using a checklist to extract data from files of patients who presented with lower-leg ulcers. The files were preselected by the unit manager and then supplied to the fieldworker. All of the facility managers received a condensed version of the study protocol that included inclusion/exclusion criteria. Inclusion criteria were files of patients with confirmed venous lower-leg ulcers who had completed care in the 6 months prior to the audit. Facility managers were asked to identify patients who fit the inclusion criteria, and from those, every other file was selected. The number of files extracted was calculated to provide at least a 60% sample of the total number of files supplied by the clinician.
Data Coding, Capturing, and Analysis
The data collected were coded by the first author, and numeric values were assigned to the checklist questions to aid analysis. An independent researcher captured the data in an electronic spreadsheet. A biostatistician checked all captured data and calculated descriptive statistics to describe the numeric data and frequency distributions used to summarize each variable.
Ethical Considerations
Approval for this research was obtained from the Health Sciences Research Ethics Committee of the University of the Free State (South Africa). Ethical principles were adhered to throughout the study. It was also registered with the Department of Health (GP2017RP16560), and permission to enter the facility and extract data from the files was obtained from each of the facilities.
Rigor
Newly developed, guideline-based, quality indicator index measurement tools are supported by evidence from studies conducted by Herberger et al19 on the quality of care for leg ulcers. The fieldworkers always had a copy of the research protocol with them to refer to the procedures if necessary. The primary author checked every completed data sheet for accuracy and comprehensiveness. Data clearance was done under the supervision of the biostatistician, who also performed the data analysis. The data interpretation and recommendations were verified by the biostatistician based on the study results.
RESULTS
Researchers audited 160 files representing each of the five strata using the checklist. In general, record keeping was very poor, and data recorded were incomplete. The authors applied the maxim that if something was not recorded it had not been done.
Structure
Figure 3 shows that many of the clinicians (61%, n = 48) attending to patients with lower-leg ulcers had no formal wound care training and that RNs were the main wound care providers.
Figure 3.
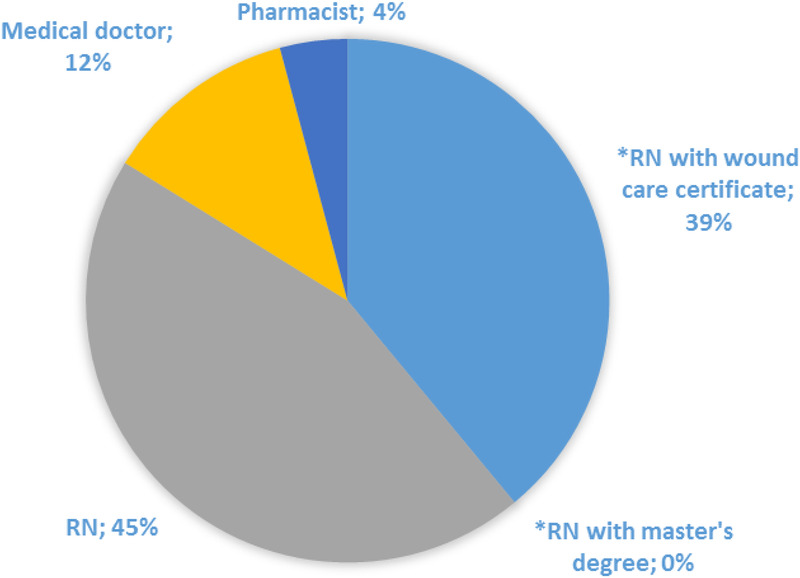
CLINICIAN LEVEL OF EDUCATION (N = 48)
Evaluating the structure of the facility also included assessing the clinical wound care experience of the clinicians attending to the patients within the facility. Figure 4 shows the distribution of years of clinical wound care experience. Within the 48 facilities, 11% of the practitioners had no clinical wound care experience. Further, only 37% (n = 48) of clinicians indicated that they used standards, guidelines, and protocols in their practice.
Figure 4.
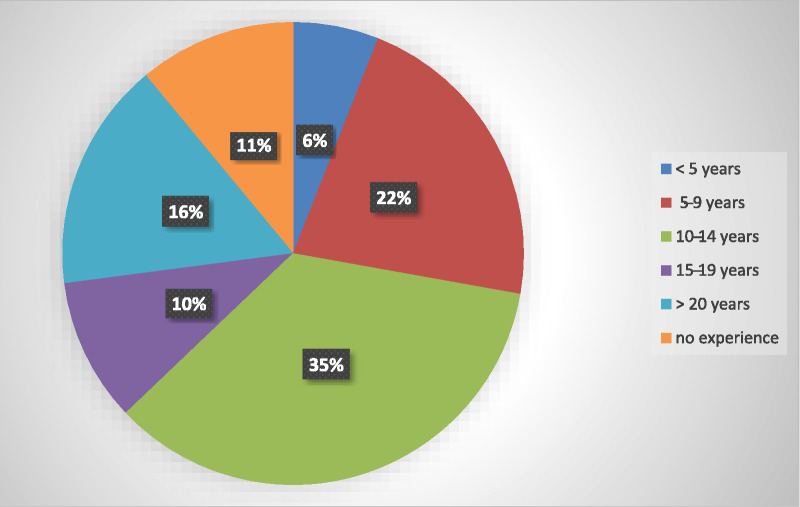
CLINICIAN YEARS OF WOUND CARE EXPERIENCE (N = 48)
Compression therapy is standard treatment of lower-leg ulcers of venous origin when the patient’s ABPI value is between 0.9 and 1.3.15 Ideally, an external pressure of 30 to 40 mm Hg is required to counteract the effects of venous insufficiency;20 however, 46% (n = 22) of the facilities did not have any form of compression available to treat patients presenting with venous lower-leg ulcers.
Process
When assessing processes including history taking, aspects such as smoking, body mass index, and anemia, all of which play a role in wound healing, were recorded in less than 30% (n = 48) of the files. A lack of baseline information could influence the quality of care delivered. Although the majority of files (92%, n = 147) indicated that an assessment tool was used, many of the elements thereof were not comprehensively assessed according to best available evidence. Pain, presence of varicose veins, previous treatment, and functioning of the calf muscle were assessed and recorded in more than 70% of the files.
Processes also included a physical examination, and as mentioned, an ABPI was recorded in only 30% (n = 48) of the patients. Compression therapy also seemed either underutilized or unhelpful in treating the underlying cause, or it was applied when the ABPI was not known, which is a medicolegal risk. According to the files, the required follow-up at 24 hours after application of compression was not routinely completed. The Table presents a summary of the data regarding the application of compression.
Table.
COMPRESSION APPLICATION DATA ANALYSIS
Some patient-centered concerns, such as pain, malodor, exudate, and social functioning, were assessed in 70% or more of the files, but general hygiene, religion, fear, anxiety, and financial issues were not addressed in any of the files. Although the descriptive CEAP (clinical, etiologic, anatomic, and pathophysiologic) classification of chronic venous insufficiency is a valuable communication and standardization tool, none of the clinicians reported using it.21
Clinicians who participated in this study neither adequately distinguished between superficial and deep infection nor implemented the correct treatment. The NERDS (Nonhealing wound, Exudate increase, Red friable granulation tissue, Debris, and Smell) and STONEES22 (Size increase, Temperature increase of 3° F or greater versus a contralateral site, Os, New skin breakdown, Edema/Erythema, Exudate increased, and Smell) mnemonics were included in the checklist to distinguish between superficial and deep infection. Although none of the files distinguished between superficial or deep infection, some of the signs and symptoms were described. Sixty-eight (42%) of the files (retrospectively) indicated three or more positive NERDS criteria, which is indicative of superficial infection. Of these, 31 (46%) indicated systemic antibiotics as choice of treatment, which is in violation of various guidelines.23 In 49 files (31%), three or more positive STONEES criteria were recorded. This is indicative of deep tissue infection, although again this was not explicitly stated in any files. Although 40% of the files indicated the use of wound swabs at assessment, there was a general tendency toward overuse of antimicrobial and systemic antibiotics in 40% to 60% of cases.
Finally, only 18% (n = 160) of the files indicated that the dressing applied adhered to guidelines advocating the use of a basic nonadherent absorbent dressing in conjunction with compression.24
Outcomes
Time to healing was recorded in 70 files (44%). In 36 of the 70 files (51%), this was 12 weeks, which is consistent with the expected healing trajectory mentioned by Sibbald et al.25 In 19 of the 70 files (27%), time to healing was between 13 and 24 weeks. However, 15 wounds (21%) took 25 weeks or more to heal.
Ultimately, of the 160 files audited, 61 (38%) indicated that the wounds did not heal. In 27 cases, an amputation was performed. (The high amputation rate was an unexpected finding, and thus was not listed among the initial outcome measures.) Unfortunately, the reason for amputations was not indicated; the question on the checklist merely asked “If the wound did not heal, what action was taken?”
DISCUSSION
Guidelines, protocols, and algorithms are developed and designed to aid evidence-based practice.26 Weller and Evans4 state that evidence-based practice promotes a high quality of care, but the lack of guideline implementation results in practice variation and suboptimal care. Woo1 further points out that adequately trained clinicians are more likely to provide evidence-based care and there is a direct correlation between improved quality of care and clinicians who possess adequate knowledge. The application of evidence-based care not only contributes to improved outcomes, but also aids in more cost-effective care.20 Therefore, with clinicians who are adequately trained and apply evidence to practice, it is not only the patient who benefits, but also the payers and the community at large. Payers consider remuneration based on best practice guidelines, but these need to be constructed with expert opinion for modifying factors, taking patient preferences into account. Clinicians who are certified in wound care have a better understanding of wound care and deliver more consistent evidence-based care with improved outcomes, including reduced healing times and a smaller number of wound care rounds, which ultimately has a cost implication.1
Clinical experience is fundamental in acquiring the skills to apply theory to practice; therefore, continuous, self-directed learning regarding wound management could aid in improving clinical decision-making as well as the quality of care delivered.27 Within the 48 facilities selected for this review, 11% of the practitioners had no clinical wound care experience. Education is the cornerstone of wound care, and with diverse providers should come regulations requiring appropriate training and registration with regulatory bodies.
Comprehensive holistic assessment of the patient with a lower-leg ulcer is essential not only to identify the problem but also to consider barriers to healing, patient preferences, and an appropriate care plan that is accepted by the patient.28 Incomplete assessment and/or data collection influence quality of care and patient outcomes. Although more than 90% of the files reflected the use of assessment tools, the information on the tools was incomplete and lacked vital data that could correlate with suboptimal care in some instances.
Thorough documentation is a legislative requirement because it helps prevent litigation and aids in communication.20 From this study, it was clear that practitioners were not trained adequately in wound care, so they may not have had the required level of expertise to document properly. Incomplete records and the absence of documentation related to observations and treatment outcomes are strong evidence of negligence. Documenting the patient’s response to care and treatment outcomes is in itself an evaluation of the quality of care. Without careful, comprehensive documentation of treatment, outcomes and plans of care are impossible to justify in a court of law.
International guidelines dictate that peripheral arterial disease should be excluded in the diagnosis of lower-leg ulcers.9,16,17 A lack of equipment and education may have contributed to the fact that practitioners only assessed ABPI in 30% (n = 160) of cases, although it is worth noting that 66% (n = 48) of the facilities had handheld Dopplers available. Hanefeld et al18 state that the availability of equipment does not guarantee high-quality care, and it was clear from this survey that this was indeed the case at the facilities under study. Weller29 indicates a lack of confidence as one reason why practitioners may not routinely use Doppler assessments. There is a general underutilization of ABPI, overreliance on wound dressings, and a lack of understanding about compression therapy among practitioners.4 Time constraints and remuneration issues also contribute to this underutilization.27 Currently, medical payers in South Africa do not reimburse practitioners for ABPI measurements; however, when compression is applied without measuring ABPI, the risk of complications such as amputation and pressure damage increases, presenting a medicolegal risk.16
The inability of clinicians to distinguish between superficial and deep infection is concerning because the overprescribing of antimicrobials and antibiotics could contribute to an increase in antibiotic resistance and the cost of treatment. Infection is the most important contributor to delayed wound healing and, if diagnosed or treated insufficiently, it also contributes to cost increases and poor patient outcomes.29 Infection is also a contraindication to high compression. However, compression can be modified to still address edema; consequently, infection that is poorly assessed could result in unfavorable outcomes.30
Compression bandaging is the cornerstone of treatment in venous lower-leg ulcers and should be applied when indicated.26 From this survey, it was clear that compression was being underused or used inappropriately at the facilities under study. Once again, a lack of skill and knowledge among clinicians could prevent evidence-based care and result in detrimental outcomes.31 Partsch32 also concluded that there is a lack of knowledge regarding the use of compression and that training is needed.
In the current study, the outcomes showed that 27 cases (17%) resulted in amputations. The detrimental effects of amputation include an increase in cost, reduction in quality of life, and an increase in mortality.16 These amputations could be attributable to either inadequate assessment or suboptimal/incorrect treatment because of a lack of knowledge or lack of resources. More information is needed about this, and future studies could compare levels of service within the different sectors. For ethical reasons, researchers chose not to identify in which of the different strata the highest frequency of amputations was recorded, but this could be a motivation for further investigation into cause and prevention.
Limitations
Study limitations included the lack of accurate and comprehensive record keeping in the audited files, which influenced the quality of the data collected and the ability to assess the stated outcome measures. The physical clinic setting was also not inspected, and availability of standard operating procedures and equipment was only assessed through an interview. Outcome measures were not quantified and could have contributed to more comprehensive data collection.
Further, sampling was challenging because contact details in the initial lists were incomplete, which made contact with the facilities difficult. Some of the practices withdrew and could not be replaced, and the large pharmacy groups declined participation. Gaining access to public clinics was challenging and required registration with the Department of Health, as well as obtaining consent from each of the department heads and the CEO of each facility, which had to be done in person.
CONCLUSIONS
As a specialty, wound care has evolved over the last 10 to 15 years and requires clinicians who can effectively apply evidence-based care in practice, make effective clinical decisions, and are committed to delivering a high standard of care through continuous self-directed, lifelong learning. From this survey, it is evident that not all clinicians providing wound care in Gauteng are adequately educated, best practice guidelines are not fully implemented, and the consequences or outcomes may be detrimental to patients. This highlights the need for improved legislation and regulation for practitioners who deliver wound care services. It is recommended that medical payers consider remuneration actions based on best practice guidelines. Future research could include the development of a model to improve the translation of evidence-based care into practice or identify and address barriers that might influence this.
The hippopotamus is a large semiaquatic mammal found in East African countries. Hippos can weigh up to 3,200 kg, and they spend most of their time in rivers and lakes with only their eyes, nose, and ears sticking out of the water. They seem harmless but are one of the most dangerous animals in Africa. They lurk under the water but can swim very fast, and their jaws are said to be able to snap a canoe in half; because of this, the metaphor “the ears of the hippo” is used in Afrikaans to refer to a problem that is not fully appreciated. This survey can be compared to the “ears of the hippo” because the bulk of this healthcare problem is yet to be addressed. Lower-leg ulcer care is only one component of specialized wound care, and if guidelines are not adhered to in one area, one could hypothesize that this carries into other aspects of wound management.
Footnotes
The authors have disclosed no financial relationships related to this article. Submitted May 22, 2019; accepted in revised form August 16, 2019.
REFERENCES
- 1.Woo KY. Trends in wound management. Adv Skin Wound Care 2013;26(12):538–42. [DOI] [PubMed] [Google Scholar]
- 2.Mosti G, Iabichella ML, Partsch H. Compression therapy in mixed ulcers increases venous output and arterial perfusion. J Vasc Surg 2012;55(1):122–8. [DOI] [PubMed] [Google Scholar]
- 3.O’ Brien M, Lawton JE, Conn CR, Ganley HE. Best practice wound care. Int Wound J 2011;8:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weller C, Evans S. Venous leg ulcer management in general practice. Aust Fam Physician 2012;41(5):331–7. [PubMed] [Google Scholar]
- 5.Anderson I. Venous leg ulcers in context. J Community Nurs 2012;26(4):31–4. [Google Scholar]
- 6.Sieggreen M, Kline RA. Venous disease and lymphedema management. In: Wound Care Essentials: Practice Principles. Ayello E, Baranoski S, eds. 4th ed Philadelphia, PA: Wolters Kluwer; 2015:272. [Google Scholar]
- 7.Kirsner RS, Vivas AC. Lower-extremity ulcers: diagnosis and management. Br J Dermatol 2015;173(2):379–90. [DOI] [PubMed] [Google Scholar]
- 8.Franks PJ, Barker J. Document summary: management of patients with venous lower leg ulcers. J Wound Care 2016;16(1):81–8. [DOI] [PubMed] [Google Scholar]
- 9.Harding K, Dowsett C, Fias L, Jelnes R, Mosti G. Consensus recommendations: simplifying venous leg ulcer management. Wounds International 2015. http://multimedia.3m.com/mws/media/1082808O/wounds-international-consensus-document.pdf. Last accessed October 10, 2019. [Google Scholar]
- 10.Regmi S, Regmi K. Best practice in the management of venous leg ulcers. Nurs Stand 2012;26(32):56, 58, 60 passim. [DOI] [PubMed] [Google Scholar]
- 11.Hanson D, Langemo D, Thompson P, Anderson J, Swanson K. Providing evidence-based care for patients with lower-extremity cellulitis. Wound Care Advisor 2015;4(3). [Google Scholar]
- 12.Andrews E, Langley G. A qualitative description of current practice in the management of burn wounds. Wound Heal South Africa 2015;8(2):59–65. [Google Scholar]
- 13.Donabedian A. Evaluating the quality of medical care. Milbank Mem Fund Q 1966;44(4):166–203. [PubMed] [Google Scholar]
- 14.Donabedian A. The quality of care how can it be assessed? JAMA 1988;260:1743–8. [DOI] [PubMed] [Google Scholar]
- 15.Probst S, Seppanen A, Gerber V, Hopkins A, Rimdeika R, Gethin G. EWMA document: home care-wound care: overview, challenges and perspectives. J Wound Care 2014;23 Suppl 5a:S1–41. [DOI] [PubMed] [Google Scholar]
- 16.Weir G, Smart H, Marle K Van, et al. WHASA consensus document on the management of lower limb ulcers. Wound Heal South Africa 2015;8(1):6–16. [Google Scholar]
- 17.The Australian Wound Management Association Australian and New Zealand Clinical Practice Guideline for Prevention and Management of Venous Leg Ulcers. October 2011. www.nzwcs.org.nz/images/luag/2011_awma_vlug.pdf. Last accessed November 22, 2019.
- 18.Hanefeld J, Powell-Jackson T, Balabanova D. Understanding and measuring quality of care: dealing with complexity. Bull World Heal Organ 2017;95(June 2016):368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herberger K, Heyer AK, Blome C, et al. Development and use of guideline-derived quality indicators for community lymphoedema. J Eur Acad Dermatology Venereol 2013;27(2):227–34. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher J, Moffat C, Partsch H, Vowden C, Vowden P. Principles of compression in venous disease. Wounds Int 2013;1(1):22. [Google Scholar]
- 21.Kistner RL, Eklof BG. Classification and etiology of chronic venous disease. In: Gloviczki P, ed. Handbook of Venous and Lymphatic Disorders. 4th Ed Boca Raton, FL: CRC Press; 2017:2645–3243. [Google Scholar]
- 22.Sibbald RG, Woo K, Ayello EA. Increased bacterial burden and infection: NERDS and STONES. Wounds UK 2007;3(2):25–46. [DOI] [PubMed] [Google Scholar]
- 23.Swanson T, Angel D, Sussman G, Cooper R. Wound Infection in Clinical Practice. London, England: Wounds International; 2016. [Google Scholar]
- 24.Broussard KC, Powers JG. Wound dressings: selecting the most appropriate type. Am J Clin Dermatol 2013;14(6):449–59. [DOI] [PubMed] [Google Scholar]
- 25.Sibbald RG, Goodmam L, Reneeka P. Wound bed preparation. J Cutan Aesthet Surg 2013;17(S1):S12–S22. [DOI] [PubMed] [Google Scholar]
- 26.Tomson CR V, van der Veer SN. Learning from practice variation to improve the quality of care. Clin Med (Lond) 2013;13(1):19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes RG. Evaluation of patient care standards, guidelines and protocols. In: Evaluation of Health Care Quality in Advanced Practice Nursing. New York: Springer Publishing Company, LLC; 2012:2562–3056. [Google Scholar]
- 28.Franks P, Barker J, Collier J, Gethin G. The management of patients with venous leg ulcers. J Wound Care 2016;25(6):S1–67. [DOI] [PubMed] [Google Scholar]
- 29.Weller C. Venous leg ulcer management in general practice. Austr Fam Phys 2012;41(5). [PubMed] [Google Scholar]
- 30.Kolluri R. Management of venous ulcers. Tech Vasc Interv Radiol 2014;17(2):132–8. [DOI] [PubMed] [Google Scholar]
- 31.Muldoon J. Chronic ulcers of the lower limb. In: Flanagan M, ed. Wound Healing and Skin Integrity: Principles and Practice. Oxford: Wiley-Blackwell; 2013. [Google Scholar]
- 32.Partsch H. Clinical practice Inelastic compression by bandages: effective, but requiring education. Wounds Int 2017;8(3):6–9. [Google Scholar]



