ABSTRACT
OBJECTIVE
The purpose of this randomized open-label study was to investigate the effect of an oral nutrition supplement containing collagen peptides on stratum corneum hydration and skin elasticity.
METHODS
The study protocol was registered at the UMIN Clinical Trials Registry (UMIN 000027347). Once-a-day oral administration of a nutrition supplement containing collagen peptides (10.0 g) was instituted in 39 inpatients 65 years or older who were assigned to either the intervention or the control group using a block-randomization design. Stratum corneum hydration and skin elasticity were measured at baseline and at 2, 4, 6, and 8 weeks after the start of the intervention.
RESULTS
Mean stratum corneum hydration was significantly increased from 43.7 at baseline to 51.7 at postintervention week 8 in the intervention group (P = .001). Differences in skin elasticity from baseline were significant at postintervention week 6 (P = .026) and week 8 (P = .049).
CONCLUSIONS
Oral nutrition supplements containing collagen peptides may reduce skin vulnerability in older adults and thus prevent conditions such as skin tears.
KEYWORDS: collagen peptide, frailty, nutrition support, older adults, oral nutrition supplement, stratum corneum hydration, skin elasticity, skin aging
INTRODUCTION
As a result of the physiologic changes of aging, the dermis becomes thinner because of marked impairment in collagen metabolism.1 Consequently, skin becomes less elastic and may be vulnerable to damage. The possible causes of such aging-related changes include decreases in the number of fibroblasts,2 decreased collagen synthesis, and elevated collagen degradation.3 Aging-related decreases in stratum corneum hydration, impairment of sebaceous and sweat glands, and increases in transepidermal water loss (TEWL) have also been reported.4 Such changes impair skin’s barrier function and result in dry skin,5 and dry, thin skin is prone to damage caused by friction.
Such skin vulnerability is closely associated with impairment of physical function. Because vulnerable skin is one manifestation of frailty,6 good nutrition and exercise, in addition to topical skin care, are important to maintain the moisture and elasticity of the skin and thereby prevent injury. Vulnerable skin is also a risk factor for severe problems such as skin tears, dermatitis, and pressure injuries, which lower the quality of life in older adults. Such conditions may result in prolonged hospital stays and consequently lead to increases in medical expenses, which represents a considerable social concern.
Recently, improvements in stratum corneum hydration and skin elasticity via oral intake of collagen peptides (hydrolyzed collagen) have been reported in young women,7–10 but not in hospitalized older adults. Also, oral nutrition supplements (ONSs) containing collagen peptides have been shown to have therapeutic effects for pressure injuries,11 but whether they can protect the skin from pressure injuries and other skin problems is unknown. This study examined the effect of an ONS containing collagen peptides on stratum corneum hydration and skin elasticity as measured in the forearms in hospitalized older adults.
METHODS
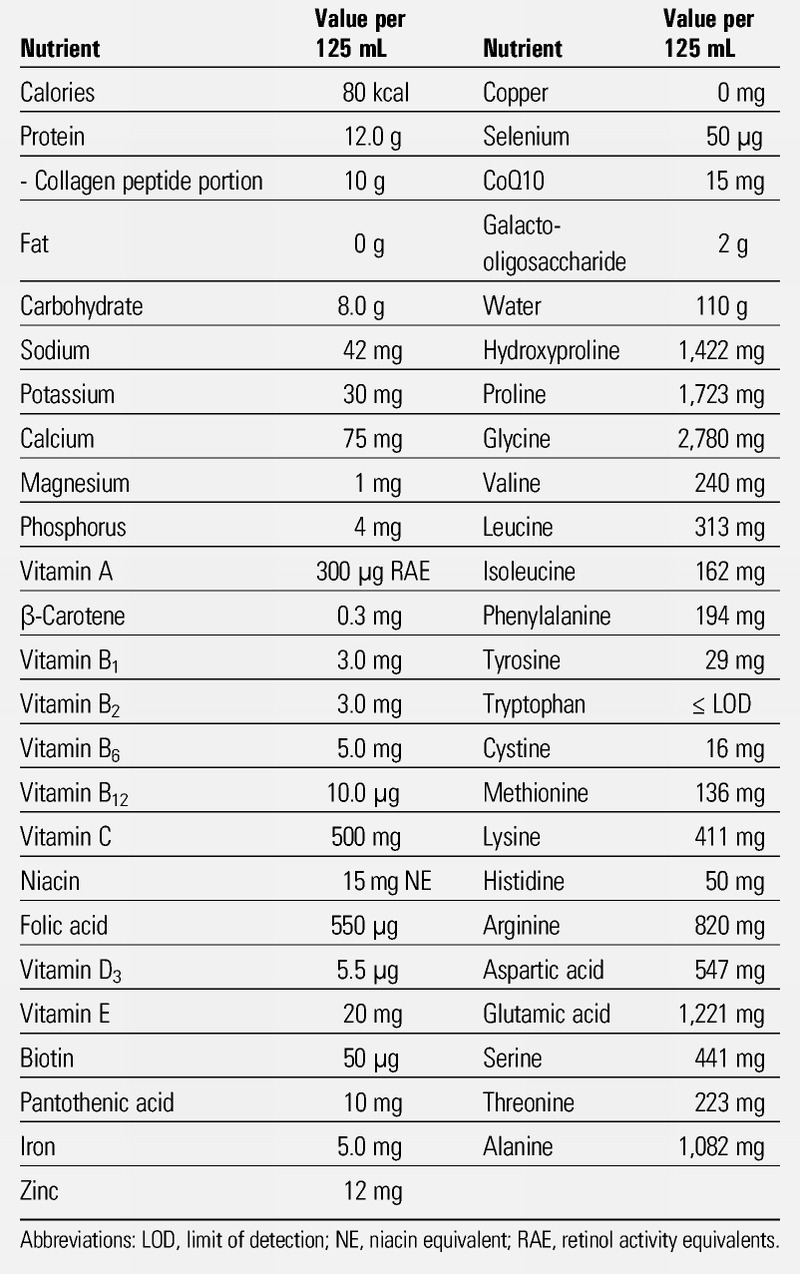
This study used V CRESC CP10 (Nutri Co, Ltd, Yokkaichi, Japan), which contains collagen peptides, vitamins, and minerals. Its nutrient composition is shown in Table 1. Collagen peptides account for 10.0 g of 12.0 g of total protein contained in this product. Thermal degradation of collagen yields gelatin, which can be further hydrolyzed to collagen peptides. Once ingested, collagen peptides are absorbed as free amino acids or enter the blood as dipeptides and tripeptides.12 Among them, the most abundant are prolyl-hydroxyproline and hydroxyprolyl-glycine.12,13
Table 1.
NUTRIENT COMPOSITION PER 125 ML OF THE SUPPLEMENT USED IN THE STUDY
Study Design
This randomized controlled open-label study investigated the effect of consuming an ONS containing collagen peptides for 8 consecutive weeks on the stratum corneum hydration and skin elasticity in the forearms of older adults.
In this study, an intervention group that received standard hospital meals with once-a-day administration of the ONS was compared with a control group that received standard hospital meals alone. Daily collagen peptide intake was 10.0 g in the intervention group. Standard hospital meals were slightly modified in the intervention group so that the increases in protein intake from the ONS were accounted for. Participants were assigned in a 1:1 manner to either the intervention group or the control group using a block-randomization design (block size of 8). Participating facilities were stratified, and an investigator independent of the participating facilities performed the randomization. Because the ONS was given to the intervention group only, this test was open label. The study protocol was registered at UMIN Clinical Trials Registry (UMIN 000027347).
Setting
The study was conducted at the rehabilitation wards of Minamino Hospital and an affiliated hospital (Eisei Hospital, Tokyo, Japan).
Ethics
This study was conducted in compliance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects and the Declaration of Helsinki (as revised in Brazil 2013). It was approved by the Eisei Hospital Ethics Committee and the Minamino Hospital Ethics Committee. All participants received detailed information about the individual parameters and a list of all the procedures involved. Written informed consent was obtained from all participants, and the anonymity of patients was preserved.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: inpatients older than 65 years and within 5 months of hospitalization who were able to consume 80% of the meals provided before the study.
The exclusion criteria were as follows: forearm skin requiring treatment or impeding measurement, chronic kidney disease, topical steroid therapy, any special skin care routine, aspiration pneumonitis within the past month, and ineligibility based on the physician’s judgment.
Measurements
The anterior forearm 10 cm distal to the elbow was used for assessment. This is because the arms are common sites of skin tears, which is the most frequent skin injury seen in the context of malnutrition and skin dryness.14 In patients with hemiparesis, the unaffected arm was used. Areas with visible blood vessels, ecchymosis, scarring, pigmented nevi, and hair were avoided. One blinded investigator performed all of the measurements. Stratum corneum hydration and skin elasticity were measured at baseline and at postintervention weeks 2, 4, 6, and 8.
Stratum corneum hydration was measured using a skin capacitance meter (Corneometer CM825+MPA580; Courage and Khazaka Electronic GmbH, Cologne, Germany) based on the principle that capacity changes according to skin hydration.15 This device measures the hydration of the epidermal layer to a depth of approximately 0.1 mm.16 Measurements were performed five times per participant at each time point, and the mean of three measurements excluding the largest and smallest was used for analysis.
Skin elasticity (R2) was measured using a cutometer (Cutometer CT580+MPA580; Courage and Khazaka Electronic GmbH).17–19 The depth of the portion of the skin surface suctioned into the probe opening (probe diameter, 6 mm; suction pressure, 400 mbar; suction time, 2 seconds; relaxation time, 2 seconds) was measured using prisms. Investigators obtained all 10 parameters, but the R2 parameter (indicating the recovery rate of the skin length after elongation and constriction) was used for analysis. An R2 value closer to 1.0 indicates greater elasticity; an R2 value of a completely elastic body is 1.0, and skin ranges from 0.3 to 0.5. Again, measurements were performed five times per participant at each time point, and the mean of three measurements excluding the largest and smallest was used for analysis.
Statistical Analysis
The sample size was determined based on a preliminary study using the R2 value as an outcome. A total of 44 participants were required to detect an R2 value greater than 0.1 in the intervention group than in the control group under the following conditions: no change in the control group, intraindividual correlation of 0.5, error variance of 0.1, α = .05, 1 − β = 0.8, and dropout rate of 10%.
Changes in the primary outcome at each time point from baseline were obtained. An adjusted mixed model using participants as a random effect was used to test the interaction between time and group in longitudinal changes in measurement items. P < .05 was considered statistically significant. Differences in changes between groups were calculated, and 95% confidence intervals were estimated. The following variables were used for adjustment: facility, interaction between facility and time, demographic variables (age, sex, underlying diseases, Functional Independence Measure), nutrition condition (using the Mini Nutritional Assessment-Short Form and body mass index), baseline energy intake, protein intake (the proportion of energy intake from protein), and blood test results (serum albumin, total cholesterol, zinc, copper, alkaline phosphatase, urea nitrogen, creatinine, sodium, potassium, chloride, C-reactive protein, hemoglobin, white blood cells, and platelets). STATA version 13 software (Stata Corp, College Station, Texas) was used for statistical analysis.
RESULTS
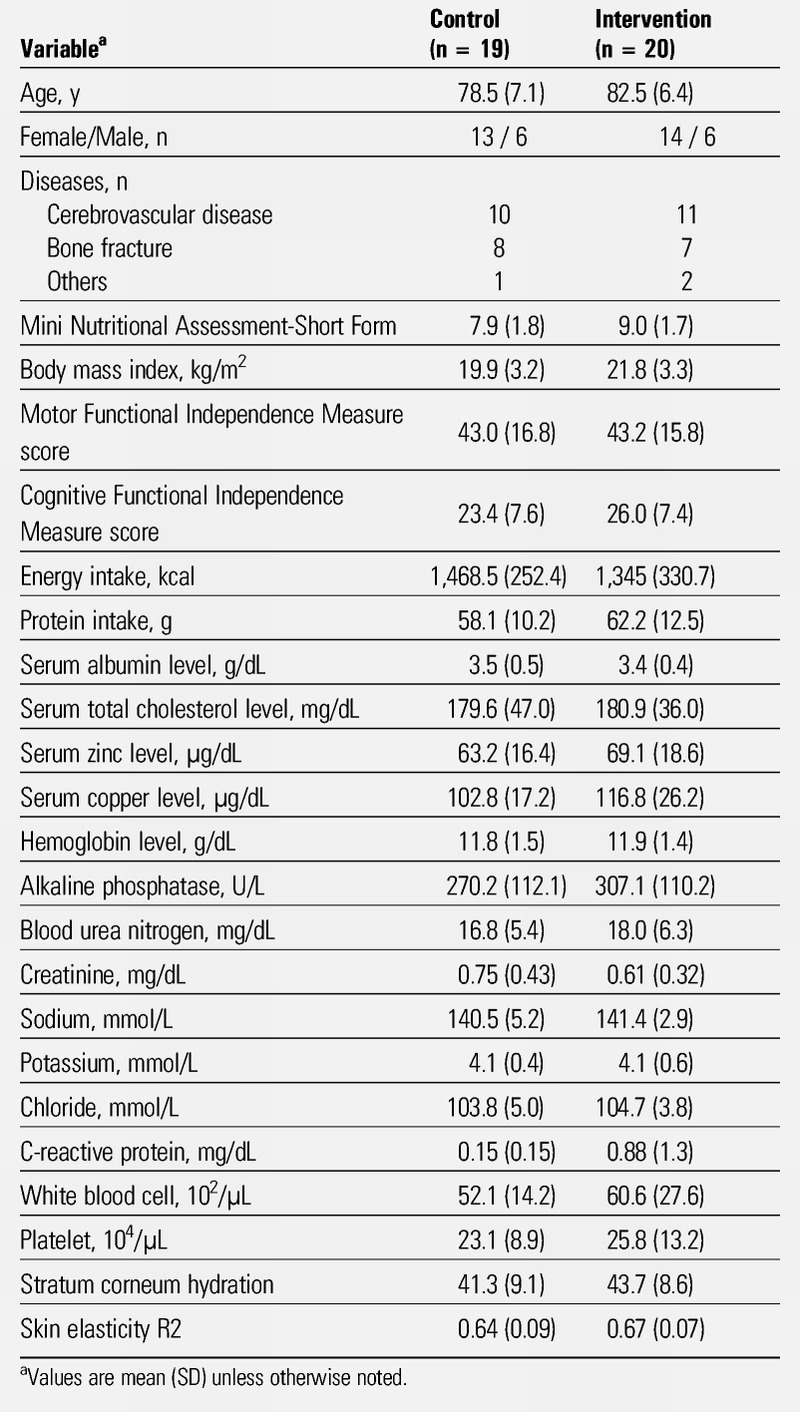
Of 46 participants who provided written consent, 45 satisfied the inclusion criteria and did not satisfy any of the exclusion criteria. Participants were randomly assigned to the intervention group (n = 23) and the control group (n = 22). Three participants were later removed from each group (2 because of disease progression and one because of discharge in each group; Figure 1). Accordingly, measurements in 20 participants in the intervention group and 19 in the control group were analyzed. Participant characteristics are shown in Table 2. There were no differences in the following baseline characteristics between the two groups: age, sex, Mini Nutritional Assessment-Short Form, body mass index, Functional Independence Measure, energy intake, protein intake, blood test results, skin elasticity, and stratum corneum hydration.
Figure 1.
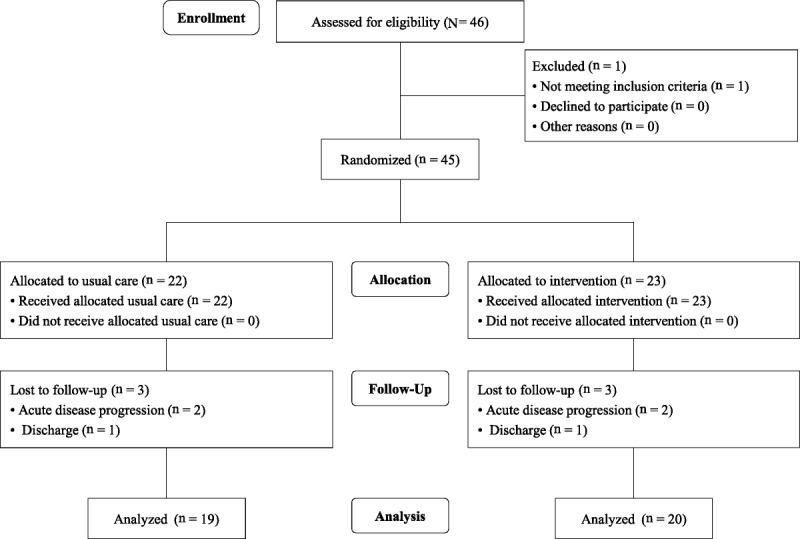
STUDY FLOW DIAGRAM
Table 2.
DEMOGRAPHIC CHARACTERISTICS OF PARTICIPANTS AT BASELINE
Measurement of Stratum Corneum Hydration
Changes in stratum corneum hydration in the intervention group and the control group are shown in Table 3 and Figure 2. There was a significant interaction between time and group affecting the stratum corneum hydration (P < .001). The mean values were 41.3 at baseline and 41.4 at postintervention week 8 in the control group. The mean value in the intervention group was significantly increased from 43.7 at baseline to 51.7 at postintervention week 8 (P = .001 [adjusted model]).
Table 3.
CHANGE IN STRATUM CORNEUM HYDRATION AND SKIN ELASTICITY (R2) BETWEEN THE INTERVENTION AND CONTROL GROUPS
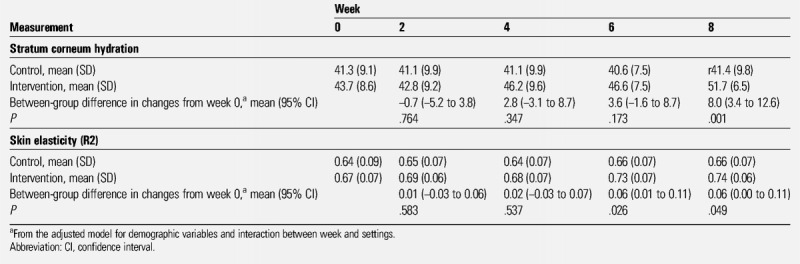
Figure 2.
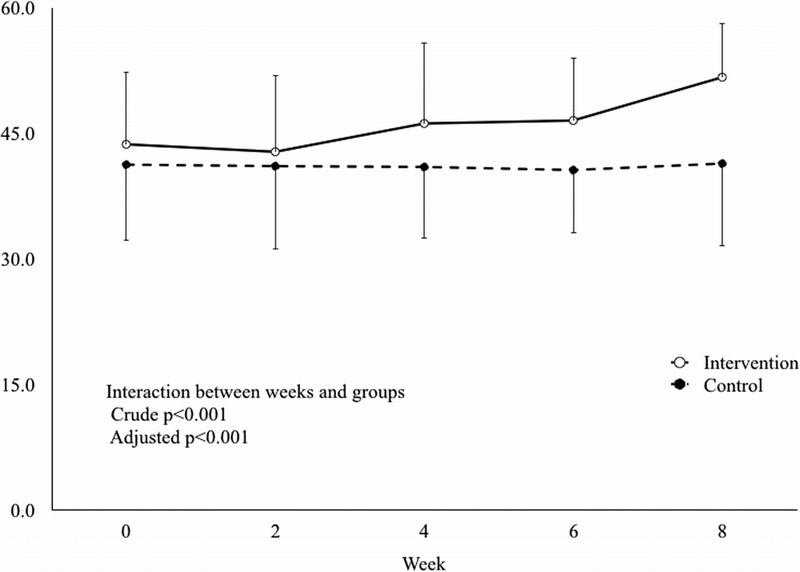
MEAN VALUES OF STRATUM CORNEUM HYDRATION BY WEEK
Measurement of Skin Elasticity
Changes in skin elasticity in the intervention group and the control group are shown in Table 3 and Figure 3. There was a significant interaction between time and group affecting skin elasticity (P = .005). The mean values in the control group were 0.64 at baseline and 0.66 at postintervention week 8. The mean value in the intervention group was significantly increased from 0.67 at baseline to 0.74 at postintervention week 8; differences from baseline were significant at postintervention week 6 (P = .026) and week 8 (P = .049 [adjusted model]).
Figure 3.
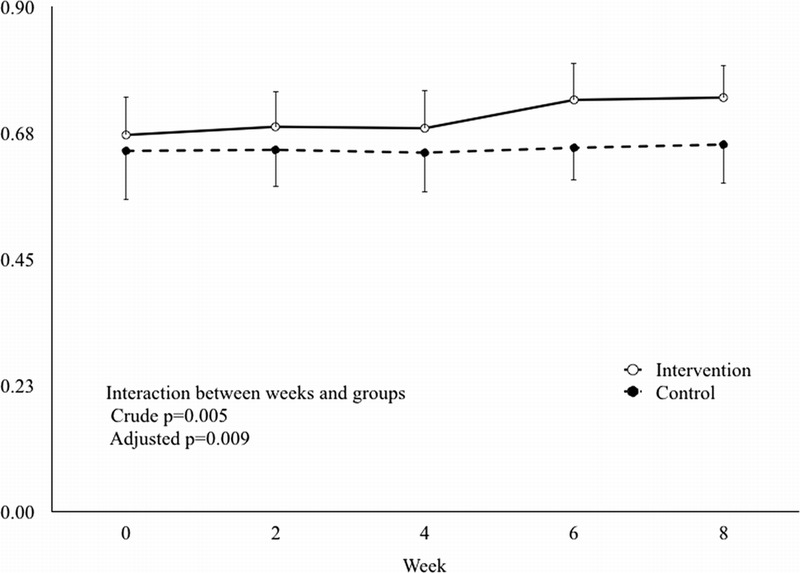
MEAN VALUES OF SKIN ELASTICITY BY WEEK
No adverse effects or events were reported. Further, no skin problems such as skin tears were reported in this study.
DISCUSSION
Collagen is a constituent protein of the epidermis, ligaments, tendons, bones, and cartilage and is the main component of the extracellular matrix. Collagen fibers in the epidermis are implicated in skin elasticity.20 Collagen metabolism in the skin is markedly impaired with age.1 This results in thinning of the skin and diminished skin elasticity in older adults. Also, the skin becomes drier with age.4 Dry, thin skin is susceptible to damage caused by friction. Therefore, resistance to external stimuli is impaired in the skin of older adults. Vulnerable skin is at risk of severe skin problems such as skin tears, dermatitis, and pressure injuries.
When oral collagen peptides are ingested, dipeptides (eg, prolyl-hydroxyproline and hydroxyprolyl-glycine) and tripeptides move into the blood, and free amino acids are absorbed. Hydroxyproline-containing peptides move into the blood particularly easily and remain in the blood for a long time.21 Such hydroxyproline-containing peptides induce the growth of skin fibroblasts and increase the production of hyaluronic acid,22 suggesting that these peptides are the active ingredients of the supplements that increase stratum corneum hydration and skin elasticity. Given that hydroxyproline is an amino acid specific to collagen and that prolyl-hydroxyproline and hydroxyprolyl-glycine are not derived from commonly consumed proteins, the effects of these peptides are characteristic of collagen peptides.12
The hydroxyproline content in granulation tissue was increased in rats that ingested collagen peptides, but not casein peptides, soybean peptides, or milk protein,23 indicating that collagen peptides are effective in improving the physical functioning of the skin. It is noteworthy that the hydroxyproline content in the skin was significantly increased in rats that ingested collagen peptides, but not in those that ingested gelatin,24 which suggests that the oral intake of high-molecular-weight collagen and gelatin is unlikely to increase the collagen content in the skin and improve its physical functioning.
In this study, stratum corneum hydration and skin elasticity increased significantly after 8 weeks in the intervention group compared with the control group. The mechanisms of such improvements are unknown, but the ingestion of the collagen peptides contained in ONSs may stimulate the growth of skin fibroblasts and the synthesis of hyaluronic acid. Improvement of stratum corneum hydration and/or skin elasticity by collagen peptides has been reported in recent years, and this study supports such findings.7–10
The ONS used in this study contained three major amino acids involved in collagen synthesis (proline, glycine, and hydroxyproline) and was abundant in antioxidants (vitamins A, C, and E and zinc). Vitamin C is a potent antioxidant that can neutralize and remove oxidants such as those found in environmental pollutants and after exposure to UV radiation. And the combination of these nutrients is known to suppress reactive oxygen species, which inhibit collagen synthesis in fibroblasts.25–27 Vitamin C is also essential for collagen synthesis because it promotes hydroxylation of proline and lysine—yielding hydroxyproline and hydroxylysine, respectively—to synthesize the collagen precursor procollagen.28 In addition to stabilizing the collagen molecule by hydroxylation, vitamin C also stimulates collagen mRNA production by fibroblasts.29,30 Based on this, vitamin C may have had some effect on the results of this study.
Limitations
First, this study was an open-label trial, and participants were not blinded to the intervention. However, objective measures were selected as endpoints, and the rater was blinded to mitigate the effect on the results. The aim of this study was to evaluate comprehensive effectiveness from a clinical perspective, but not the efficacy of a single nutrient, so the ONS containing collagen peptides was not compared against one without collagen peptides.
Second, TEWL was not measured because such measurements are generally not very accurate, and TEWL is strongly correlated with stratum corneum hydration in the clinical setting. Third, because this study included only 39 participants, the statistical power of some analyses may have been insufficient.
Finally, no participant in this study developed a skin tear, but the 3-month cumulative incidence was reported to be 3.8% in Japan.14 A future large-scale study is required to test the effectiveness of oral collagen peptides in preventing skin problems; as such, skin tears were not the primary outcome in this study.
CONCLUSIONS
A multicenter open-label randomized controlled study was conducted to examine the effect of 8-week oral administration of an ONS containing 10.0 g of collagen peptides on stratum corneum hydration and skin elasticity in 39 older adult inpatients in convalescent rehabilitation wards. Stratum corneum hydration and skin elasticity were significantly higher in the intervention group that consumed the ONS than in the control group that did not. This indicates that ONSs containing collagen peptides can reduce the vulnerability of older adult skin, helping to prevent problems such as skin tears. The precise effects of oral collagen peptides in preventing skin problems require further study.
REFERENCES
- 1.Mays PK, McAnulty RJ, Campa JS, Laurent GJ. Age-related changes in collagen synthesis and degradation in rat tissues. Importance of degradation of newly synthesized collagen in regulating collagen production. Biochem J 1991;276:307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varani J, Warner RL, Gharaee-Kermani M, et al. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol 2000;114(3):480–6. [DOI] [PubMed] [Google Scholar]
- 3.Sottile J, Mann DM, Diemer V. Regulation of collagenase and collagenase mRNA production in early- and late-passage human diploid fibroblasts. J Cell Physiol 1989;138:281–90. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi Y, Sultana R. Skin aging. Biomedical Gerontol 2008;32:15–9. [Google Scholar]
- 5.Tezuka T. Senile changes of the epidermis. Nagoya Med J 2000;43:121–7. [Google Scholar]
- 6.Iizaka S. Frailty and body mass index are associated with biophysical properties of the skin in community-dwelling older adults. J Tissue Viability 2018;27(3):141–5. [DOI] [PubMed] [Google Scholar]
- 7.Sugihara F, Inoue N, Wang X. Clinical effects of ingesting collagen hydrolysate on facial skin properties. A randomized, placebo-controlled, double-blind trial. Jpn Pharmacol Ther 2015;43:67–70. [Google Scholar]
- 8.Matsumoto H, Ohara H, Ito K, Nakamura Y, Takahashi S. Clinical effects of fish type I collagen hydrolysate on skin properties. ITE Lett 2006;7:386–90. [Google Scholar]
- 9.Patrícia Maia Campos MBG, Meloo MO, Calixto LS, Fossa MM. An oral supplementation based on hydrolyzed collagen and vitamins improves skin elasticity and dermis echogenicity: a clinical placebo-controlled study. Clin Pharmacol Biopharm 2015;4:142. [Google Scholar]
- 10.Borumand M, Sibilla S. Effects of a nutritional supplement containing collagen peptides on skin elasticity, hydration and wrinkles. J Med Nutr Nutraceut 2015;4(1):47–53. [Google Scholar]
- 11.Yamanaka H, Okada S, Sanada H. A multicenter, randomized, controlled study of the use of nutritional supplements containing collagen peptides to facilitate the healing of pressure ulcers. J Nutr Intermed Metab 2017;8:51–9. [Google Scholar]
- 12.Iwai K, Hasegawa T, Taguchi Y, et al. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J Agric Food Chem 2005;53:6531–6. [DOI] [PubMed] [Google Scholar]
- 13.Shigemura Y, Akaba S, Kawashima E, Park EY, Nakamura Y, Sato K. Identification of a novel food-derived collagen peptide, hydroxyprolyl-glycine, in human peripheral blood by pre-column derivatisation with phenyl isothiocyanate. Food Chem 2011;129:1019–24. [DOI] [PubMed] [Google Scholar]
- 14.Sanada H, Nakagami G, Koyano Y, Iizaka S, Sugama J. Incidence of skin tears in the extremities among elderly patients at a long-term medical facility in Japan: a prospective cohort study. Geriatr Gerontol Int 2015;15(8):1058–63. [DOI] [PubMed] [Google Scholar]
- 15.Fluhr JW, Kuss O, Diepgen T, et al. Testing for irritation with a multifactorial approach: comparison of eight non-invasive measuring techniques on five different irritation types. Br J Dermatol 2001;145:696–703. [DOI] [PubMed] [Google Scholar]
- 16.Marcon AFVS, Wagemaker TAL, Maia Campos PMBG. Rheology, clinical efficacy and sensorial of a silicone-based formulation containing pearl extract. Biomed Biopharm Res 2014;11:247–55. [Google Scholar]
- 17.Enomoto DN, Mekkes JR, Bossuyt PM, Hoekzema R, Bos JD. Quantification of cutaneous sclerosis with a skin elasticity meter in patients with generalized scleroderma. J Am Acad Dermatol 1996;35:381–7. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues L. EEMCO guidance to the in vivo assessment of tensile functional properties of the skin. Part 2; instrumentation and test modes. Skin Pharmacol App Skin Physiol 2001;14:52–67. [DOI] [PubMed] [Google Scholar]
- 19.Dobrev H. Application of Cutometer area parameters for the study of human skin fatigue. Skin Res Technol 2005;11:120–2. [DOI] [PubMed] [Google Scholar]
- 20.Tsukahara K, Nakagawa H, Moriwaki S, Takema Y, Fujimura T, Imokawa G. Inhibition of ultraviolet-B-induced wrinkle formation by an elastase-inhibiting herbal extract: implication for the mechanism underlying elastase-associated wrinkles. Int J Dermatol 2006;45:460–8. [DOI] [PubMed] [Google Scholar]
- 21.Ohara H, Matsumoto H, Ito K, Iwai K, Sato K. Comparison of quantity and structures of hydroxy-proline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. J Agric Food Chem 2007;55:1532–5. [DOI] [PubMed] [Google Scholar]
- 22.Ohara H, Saito S, Matsumoto H, et al. Effect of collagen-derived hydroxyproline containing peptides in cultured human dermal fibroblast. J Dermatol Sci 2007;47:102. [Google Scholar]
- 23.Ito R, Sugitani M, Inagaki H, Setoguchi Y, Uchida H, Ito T. Effect of orally administered collagen peptide on granulation tissue collagen content in an aged rat model. J Jpn Soc Food Sci Technol 2013;60(6):278–85. [Google Scholar]
- 24.Nishimoto S, Hiura N, Sato R, Suzuki K, Asano R. Effect of oral administration of gelatin and collagen peptides on the hydroxyproline content of rats skin. J Jpn Soc Food Sci Technol 2002;49(3):199–202. [Google Scholar]
- 25.Lin JY, Selim MA, et al. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J Am Acad Dermatol 2003;48:866–74. [DOI] [PubMed] [Google Scholar]
- 26.Darr D, Dunston S, Faust H, Pinnell S. Effectiveness of antioxidants (vitamin C and E) with and without sunscreens as topical photoprotectants. Acta Derm Venereol 1996;76:264–8. [DOI] [PubMed] [Google Scholar]
- 27.Dreher F, Gabard B, Schwindt DA, Maibach HI. Topical melatonin in combination with vitamins E and C protects skin from ultraviolet-induced erythema: a human study in vivo. Br J Dermatol 1998;139:332–9. [DOI] [PubMed] [Google Scholar]
- 28.May JM, Qu ZC. Transport and intracellular accumulation of vitamin C in endothelial cells: relevance to collagen synthesis. Arch Biochem Biophys 2005;434:178–86. [DOI] [PubMed] [Google Scholar]
- 29.Duarte TL, Cooke MS, Jones GD. Gene expression profiling reveals new protective roles for vitamin C in human skin cells. Free Radic Biol Med 2009;46:78–87. [DOI] [PubMed] [Google Scholar]
- 30.Tajima S, Pinnell SR. Ascorbic acid preferentially enhances type I and III collagen gene transcription in human skin fibroblasts. J Dermatol Sci 1996;11:250–3. [DOI] [PubMed] [Google Scholar]


