ABSTRACT
BACKGROUND:
The wound healing process includes inflammation, proliferation, and remodelling phases, the main features of which are inflammation, neoangiogenesis, and epithelialization. Hyperbaric oxygen therapy (HBOT) is one modality postulated to improve wound healing. The objective of this study was to determine whether HBOT could improve selected features of burn wound healing in an experimental rabbit model.
METHODS:
Researchers conducted an experimental study with 36 rabbits given second-degree burns. Subjects were separated into two groups: a control group (n = 18) and an intervention group that was given HBOT at 2.4 atmospheres absolute for 6 days (n = 18). The main outcome measure was wound healing.
RESULTS:
Compared with the control group, the HBOT group showed more robust inflammatory cells (P = .025) and epithelialization (P = .024), but no significant difference in angiogenesis (P = .442).
CONCLUSIONS:
The authors conclude that HBOT may improve second-degree burn healing by increasing inflammatory cell migration and re-epithelialization.
KEYWORDS: burn wound, hyperbaric oxygen therapy, rabbit model, second-degree burn, wound healing
INTRODUCTION
Burns are among the most devastating skin injuries, requiring complex management and accompanied by high morbidity and mortality. Acute thermal injuries requiring medical treatment affect nearly half a million Americans each year, with approximately 40,000 hospitalizations and 3,400 deaths.1 Hyperbaric oxygen therapy (HBOT) is the use of 100% oxygen at pressures greater than atmospheric pressure. The patient breathes 100% oxygen intermittently while the pressure of the treatment chamber is increased to greater than 1 atmosphere absolute (ATA). The treatment of burn wounds with HBOT was first investigated in the mid-1960s and gained attention in the decades following, but controversy remains over potential risks and costs.2 Recent work in rat models has shown that HBOT reduces healing time and improves scar appearance of burn injuries.3 Advancements in hyperbaric chambers have reduced the overall cost associated with treatment, and controlled clinical trials in humans are beginning to produce data supporting the conclusion that HBOT is safe and effective for improving burn wound healing.4,5
The postulated mechanisms of HBOT with beneficial effect on burn wounds include decreased edema formation because of hyperoxic vasoconstriction, increased collagen formation from accelerated glycosaminoglycan synthesis and hydroxylation, and improved phagocytic killing of bacteria with normoxia/hyperoxia.6 Further, HBOT may decrease morbidity, lessen the need for surgery (especially for second-degree burns), and reduce hospital lengths of stay.6 However, this modality is still not uniformly accepted.
The authors decided to conduct an experimental animal study to strengthen the evidence for HBOT especially in the treatment of second-degree burns. The purpose of the study was to assess the effects of HBOT on second-degree burn injury inflicted on rabbits in terms of inflammatory cell migration, angiogenesis, and epithelialization.
Declaration
This experimental study was approved by the Ethical Committee for Biomedical Research at the Universitas Sam Ratulangi, Manado, Indonesia. The data sets generated and/or analyzed during this study are available from the first author upon request.
METHODS
This study involved 36 New Zealand male rabbits with a mean weight of 1,200 to 1,400 g and contained in a quarantine area. A partial-thickness (second-degree) burn injury of 2 × 1 cm was inflicted on the back of subjects under general anesthesia using intramuscular administration of ketamine (100 mg/kg body weight) as the anesthetic. The anesthetized animals were exposed to direct flame for 5 to 7 seconds through a 1.5 × 1.5cm window in an asbestos network.
Subjects were assigned randomly to two groups, 18 rabbits in each. Group A (intervention group) was given HBOT therapy in 90-minute sessions of 2.4 ATA for 6 consecutive days, whereas group B (control group) received no treatment except Vaseline ointment on the surface of the wound. At day 14, all subjects were euthanized, and the burn area was excised and sent to the pathology laboratory for histologic examination. Standard tissue slices were prepared and painted with hematoxylin-eosin.
Histologic outcome measures included number of inflammatory cells, angiogenesis, and epithelialization. Angiogenesis was determined by counting the number of blood vessels per mm2. All microscopic measurements were carried out by an independent, blinded pathologist.
To evaluate inflammation, stained and mounted sections of hematoxylin-eosin and CD68 (a monocyte/macrophage marker) were scanned for virtual microscopy at 40× magnification. Subsequently, using virtual microscopy software NDP (Hamamatsu Photonics, Hammatsu City, Japan), JPEG images of all sections except those stained with hematoxylin-eosin were taken at 2.5× magnification (10× digital zoom) and loaded into CellD software (Olympus Life Science, Hamburg, Germany) for computerized quantitative analysis. Thresholds for the number of pixels, hue, saturation, and intensity were set and verified by human eye.
Angiogenesis was examined by microvessel density using an established, validated protocol.7 Areas with the highest density of microvessels (vascular hotspots) were identified at low magnification of the stained sections using a light microscope. Within these areas, individually stained microvessels were counted at 400× magnification using a square grid graticule resulting in a field size of 0.0625 mm2. Transglutaminase-stained positive endothelial cells or endothelial cell clusters separated from microvessels and connective tissue elements were considered single countable microvessels; branching structures with continuity of the vessel were counted as one. In cases of discontinuity, structures were counted as two distinct vessels. Three fields per defect section were counted in the vascular hot-spots. Microvessel density was defined as the mean score from all three fields per mm2.
The epithelialized area was quantified by computerized morphometry. The burn areas were photographed at a fixed focus distance, using identical lighting on each occasion. A Minolta SRT MC-II camera (Konica Minolta, Tokyo, Japan) mounted on a stand and fitted with a 100-mm macro-objective lens, together with a uniform batch of AGEA 100 ASA color film, was used. The epithelialization areas were marked, and the photographs were then converted into a digital display using a computerized morphometric analysis system, and the wound area and areas of epithelialization were quantified (Image Measure Software; Phoenix Corporation, Seattle, Washington).
A Kolmogorov-Smirnov test was done to determine whether the two data sets were normally distributed. A Mann-Whitney U test was used to compare differences between the two groups. P < .05 was considered significant.
RESULTS
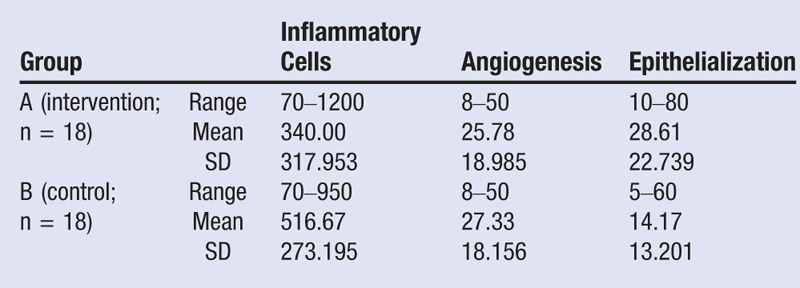
All subjects successfully completed the 14 days of observation and were euthanized. Histologic examination showed that group A (HBOT) had fewer inflammatory cells and angiogenesis and had more epithelialization compared with the control group (Table).
The Kolmogorov-Smirnov test showed that the data were not normally distributed, so the differences between the two groups were analyzed with a Mann-Whitney U test. There were significant differences between groups A and B in terms of inflammatory cells (P = .025) and epithelialization (P = .024; Figure), whereas there was no significant difference for angiogenesis (P = .442).
Figure.
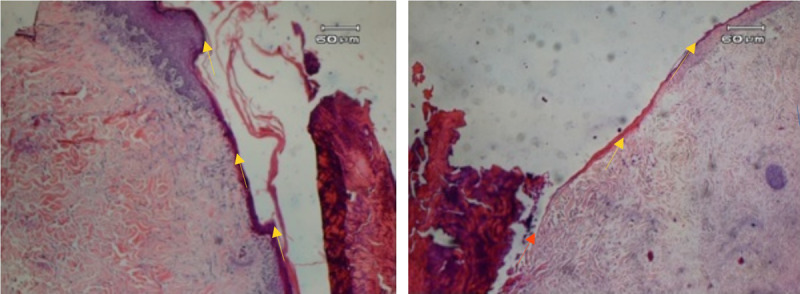
HISTOLOGIC VIEW OF EPITHELIALIZATION OF A SECOND-DEGREE BURN
The epithelialization line is the dark purple line (yellow arrow) on the edge of wound. The wound that received hyperbaric oxygen therapy is on the left; compare this with that of the control group (right). Epithelialization is more robust and thick on the left, whereas in the control group, epithelialization is very thin and even absent in certain areas (orange arrow).
Table.
COMPARISON OF OUTCOME MEASURES BETWEEN GROUPS
DISCUSSION
Burns are classified by depth into superficial (epidermal), partial-thickness, and full-thickness burns.8 The tissue damage is not just caused by the inciting event alone; prolonged inflammation results in an accumulation of cytotoxic cytokines and free radicals, along with neutrophil plugging of dermal venules. Increased vascular permeability and interstitial hydrostatic pressure augmentation lead to edema with vascular congestion. Hypercoagulability with thrombosis further impairs blood flow, whereas oxidative stress damages endothelial cells and compromises vascular patency. The main burden is a failure of oxygen and nutrient supply to injured cells.9
A number of studies have investigated the utility of various agents in modulating the mechanisms of burn wound progression. During HBOT, oxygen pressures higher than 1 ATA will increase the O2 gradient pressure to promote oxygen diffusion into tissue. Oxygen may also dissolve in plasma and may reach oxygen-deficient tissue through macro- and microcirculation.10,11
Inflammatory Cell Reduction
The number of inflammatory cells decreased after HBOT. A previous study by Thom12 showed that 2.8- to 3-ATA HBOT mediated inhibition of neutrophil β2 integrin adhesion. An early event associated with postischemic tissue reperfusion is the adherence of circulating neutrophils to vascular endothelium by β2 integrins. When animals or humans are exposed to hyperbaric oxygen for at least 45 minutes, the ability of circulating neutrophils to adhere to target tissues is temporarily inhibited,13 therefore reducing the number of inflammatory cells within the wound cavity. This hyperbaric oxygen-mediated inhibition of neutrophil β2 integrin adhesion also has been shown to ameliorate reperfusion injuries.14
The dose of oxygen pressure in this study was 2.4 ATA. This is lower than Bilic et al6 (2.5 ATA) and other studies, where the range of pressure was between 2 to 3 ATA; however, this lower dose showed the same effect in terms of number of inflammatory cells within the wound cavity. Future HBOT studies may explore the optimal cut-off point for hyperbaric dosing based on this finding.
Theoretically, HBOT does not inhibit neutrophil antibacterial function because the G protein-coupled “inside-out” pathway for activation (such as that triggered by endotoxin) remains intact, and actin S-nitrosylation is reversed as a component of this activation process.14 Although many fear that HBOT may temporarily inhibit the adherence of circulating neutrophils to target tissues, resulting in immunocompromise, the most compelling evidence comes from studies in sepsis models, where HBOT has a beneficial effect.15,16 A separate anti-inflammatory pathway for HBOT also involves impaired proinflammatory cytokine production by monocyte macrophages. This action has been shown in animal models and human beings.17,18 The effect on monocytes may be the basis for reduced levels of circulating proinflammatory cytokines under stress conditions.19
Decreased Angiogenesis
Regional angiogenic stimuli influence the efficiency of new blood vessel growth by local endothelial cells (termed angiogenesis), and these cells stimulate the recruitment and differentiation of circulating stem/progenitor cells to form new vessels (vasculogenesis).20 It is posited that HBOT has beneficial effects on these processes. Various studies with macrophages in culture, in wound fluid, using rat models, and in human volunteers have shown that HBOT increases active vascular endothelial growth factor (VEGF) production.21 There are various proposed mechanisms for how HBOT does so. One mechanism simply involves the correction of wound hypoxia. As part of the hemostatic process of wound healing, capillaries vasoconstrict, which increases the diffusion distance that oxygen must traverse to reach the endothelial cells. Given that VEGF requires oxygen, the greater diffusion distance decreases the amount of oxygen available to VEGF. The efficacy of HBOT is in the increase in oxygen partial pressure gradients between healthy and hypoxic tissues.
Increased Epithelialization
Hyperbaric oxygen therapy theoretically influences the epithelialization of second-degree burns by minimizing the destructive effect of hypoxia and encouraging a faster rate of mitosis of epithelial cells and cell migration to the wound cavity. In practice, however, previous studies have established conflicting results about whether HBOT increases the rate of epithelialization in second-degree burns.22–24 Korn et al22 stated that epithelial cells may survive without oxygen for a while, but cannot divide and migrate. It is possible that HBOT increases the oxygenation of hypoxic tissue that may not survive without additional oxygen during ischemia.25 The present study demonstrated faster epithelialization in the intervention group versus the control group, which supports the conclusion that adequate oxygen is mandatory for wound healing.
Limitations and Recommendations for Future Research
Angiogenesis begins during the proliferation phase, which can continue for up to 3 weeks after injury. However, this study revealed no significant difference in angiogenesis between the HBOT and control groups. This may be because the rabbits were euthanized after 14 days, when the proliferation phase was still in progress. It is possible that a study conducted for a longer period of time would result in significant differences.
More trials to assess the clinical efficacy of HBOT in humans are needed to determine the optimum dose of ATA and how many sessions are needed. Like any medical therapy, the risk and benefits of HBOT must be carefully weighed. An untreated pneumothorax is the only absolute contraindication to HBOT.
CONCLUSIONS
These results show that HBOT has a beneficial effect on burn wound healing by reducing edema and ensuring there is adequate oxygen in microcirculation. It may speed up epithelialization and suppress unnecessary inflammation that could negatively affect normal wound healing. With further research, HBOT may become an adjuvant therapy to surgery.
REFERENCES
- 1.Gibran NS, Wiechman S, Meyer W, et al. American Burn Association consensus statements. J Burn Care Res 2013;34:361–5.23835626 [Google Scholar]
- 2.Cianci P, Sato R. Adjunctive hyperbaric oxygen therapy in the treatment of thermal burns: a review. Burns 1994;20:5–14. [DOI] [PubMed] [Google Scholar]
- 3.Selcuk CT, Ozalp B, Durgun M, et al. The effect of hyperbaric oxygen treatment on the healing of burn wounds in nicotinized and nonnicotinized rats. J Burn Care Res 2013;34:e237–43. [DOI] [PubMed] [Google Scholar]
- 4.Cianci P, Slade JB, Jr, Sato RM, Faulkner J. Adjunctive hyperbaric oxygen therapy in the treatment of thermal burns. Undersea Hyperb Med 2013;40:89–108. [PubMed] [Google Scholar]
- 5.Eskes A, Vermeulen H, Lucas C, Ubbink DT. Hyperbaric oxygen therapy for treating acute surgical and traumatic wounds. Cochrane Database Syst Rev 2013;12: CD008059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilic I, Petri NM, Bezic J, et al. Effects of hyperbaric oxygen therapy on experimental burn wound healing in rats: a randomized controlled study. Undersea Hyperb Med 2005:32(1):1–9. [PubMed] [Google Scholar]
- 7.Grassmann JP, Schneppendahl J, Hakimi AR, et al. Hyperbaric oxygen therapy improves angiogenesis and bone formation in critical sized diaphyseal defects. J Orthop Res 2015;33(4):513–20. [DOI] [PubMed] [Google Scholar]
- 8.Friedstat J, Endorf FW, Gibran NS. Burns. In: Schwartz’s Principles of Surgery. 10th ed. New York, NY: McGraw-Hill Education; 2015. [Google Scholar]
- 9.Shupp JW, Nasabzadeh TJ, Rosenthal DS, Jordan MH, Fidler P, Jeng JC. A review of the local pathophysiologic bases of burn wound progression. J Burn Care Res 2010;31(6):849–73. [DOI] [PubMed] [Google Scholar]
- 10.Sahni T, Singh P, John MJ. Hyperbaric oxygen therapy: current trends and applications. J Assoc Physicians India 2003;51:280–4. [PubMed] [Google Scholar]
- 11.Delaney JS, Montgomery DL. How can hyperbaric oxygen contribute to treatment? Phys Sports Med 2001:29(3):77–84. [DOI] [PubMed] [Google Scholar]
- 12.Thom SR. Hyperbaric oxygen—its mechanism and efficacy. Plast Reconstr Surg 2011;127(Suppl1):131S–41S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalns J, Lane J, Delgado A, et al. Hyperbaric oxygen exposure temporarily reduces mac-1 mediated functions of human neutrophils. Immunol Lett 2002;83:125–31. [DOI] [PubMed] [Google Scholar]
- 14.Thom SR, Bhopale VM, Mancini DJ, Milovanova TN. Actin S-nitrosylation inhibits neutrophil beta2 integrin function. J Biol Chem 2008;283(16):10822–34. [DOI] [PubMed] [Google Scholar]
- 15.Buras JA, Holt D, Orlow D, Belikoff B, Pavlides S, Reenstra WR. Hyperbaric oxygen protects from sepsis mortality via an interleukin-10–dependent mechanism. Crit Care Med 2006;34(10):2624–9. [DOI] [PubMed] [Google Scholar]
- 16.Thom SR, Lauermann MW, Hart GB. Intermittent hyperbaric oxygen therapy for reduction of mortality in experimental poly-microbial sepsis. J Infect Dis 1986;154(3):504–10. [DOI] [PubMed] [Google Scholar]
- 17.Lahat N, Bitterman H, Yaniv N, Kinarty A, Bitterman N. Exposure to hyperbaric oxygen induces tumor necrosis factor-alpha (TNF-alpha) secretion from rat macrophages. Clin Exp Immunol 1995;102:655–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benson RM, Minter LM, Osborne BA, et al. Hyperbaric oxygen inhibits stimulus-induced proinflammatory cytokine synthesis by human blood-derived monocyte-macrophages. Clin Exp Immunol 2003;134:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fildissis G, Venetsanou K, Myrianthefs P, Karatzas S, Zidianakis V, Baltopoulos G. Whole blood pro-inflammatory cytokines and adhesion molecules post-lipopolysaccharides exposure in hyperbaric conditions. Eur Cytokine Netw 2004;15(3):217–21. [PubMed] [Google Scholar]
- 20.Tepper OM, Capla JM, Galiano RD, et al. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow–derived cells. Blood 2005;105:1068–77. [DOI] [PubMed] [Google Scholar]
- 21.Buckley CJ, Cooper JS. Hyperbaric, Angiogenesis. StatPearls. Treasure Island, FL: StatPearls Publishing; 2018. [Google Scholar]
- 22.Korn HN, Wheeler ES, Miller TA. Effect of hyperbaric oxygen on second degree burn wound healing. Arch Surg 1977;112(6):732–7. [DOI] [PubMed] [Google Scholar]
- 23.Shoshani O, Shupak A, Barak A, et al. Hyperbaric oxygen therapy for deep second degree burns: an experimental study in the guinea pig. Br J Plast Surg 1998;51(1):67–73. [DOI] [PubMed] [Google Scholar]
- 24.Perrins D. Failed attempt to limit tissue destruction in scalds of pig skin with HBO2. In: Wada J, Iwa T, eds. Book of Proceedings of the Fourth International Congress on Hyperbaric Medicine. Baltimore, MD: Lippincott, Williams & Wilkins; 1970. [Google Scholar]
- 25.Niezgoda JA, Cianci P, Folden BW, Ortega RL, Slade JB, Storrow AB. The effect of hyperbaric oxygen therapy on a burn wound model in human volunteers. Plast Reconstr Surg 1997;99:1620–5. [PubMed] [Google Scholar]


