ABSTRACT
OBJECTIVE
This prospective, multicenter study evaluated the efficacy and safety of an acellular dermal matrix allograft, DermACELL (D-ADM; LifeNet Health, Virginia Beach, Virginia), in the treatment of large, complex diabetic foot ulcers (DFUs) that probed to tendon or bone.
METHODS
Inclusion criteria were Wagner grade 3 or 4 DFUs between 4 weeks and 1 year in duration. All participants received one application of D-ADM at baseline and could receive one additional application if wound healing arrested. Ulcers were assessed weekly for 16 weeks using a laser measuring device.
RESULTS
Sixty-one participants were enrolled, with an average wound area of 29.0 cm2; 59 of these ulcers showed exposed bone. The entire per-protocol population (n = 47) achieved 100% granulation. The mean time to 100% granulation was 4.0 weeks with an average of 1.2 applications of D-ADM. Mean percent wound area reduction was 80.3% at 16 weeks. Those DFUs 15 cm2 or smaller were substantially more likely to close than DFUs larger than 29 cm2 (P = .0008) over a 16-week duration. No complications were associated with the use of the studied matrix.
CONCLUSIONS
The D-ADM demonstrated the ability to rapidly reduce the size of large, complex DFUs with exposed bone. Some wounds did not completely heal by 16 weeks; however, the significant reduction in size suggests that these large, complex wounds may heal if given more time.
KEYWORDS: acellular dermal matrix, diabetes, diabetic foot ulcer, ulcer, wound healing
INTRODUCTION
Diabetic foot ulcers (DFUs) are a global concern, with an estimated incidence of 19% to 35% among people with diabetes.1 Between 9.1 and 26.1 million individuals suffer new DFUs annually, with reported infection rates as high as 50%.1,2 The consequences of an infected DFU include elevated risk of amputation and death, especially if the patient has peripheral artery disease or a renal comorbidity.1,2
Patients with DFUs suffer from a lower quality of life and are at an increased risk for depression than those with diabetes who do not have open wounds.3,4 Further, DFUs with exposed bone are especially prone to infection, which has been reported to increase amputation rates to as high as 92%.5,6 Exposed bone is at risk of osteomyelitis, which can also increase amputation rate, length of hospital stay, duration of antibiotic treatment, and length of time required for healing.7 Accordingly, large, complex DFUs with exposed bone or tendon are in great need of advanced treatments because they are very difficult to heal. Despite this, these “deep wound” ulcers are rarely included in clinical studies that help drive clinical decisions.
Complete wound closure may take many months for such large and deep DFUs. However, rapidly reducing the wound area and attaining 100% granulation reduce the risk of infection and, consequently, the risk of amputation. Reducing infection and amputation rates leads to lower healthcare costs and better quality of life. Advanced technologies that address large and deep DFUs are therefore important for the physical, mental, and financial well-being of patients.
One treatment option for large, complex DFUs with exposed bone or tendon is the application of a human acellular dermal matrix (ADM). The primary objective of this prospective study was to assess the ability of an ADM to reduce wound area, achieve 100% granulation, and promote closure of “deep wound” DFUs. Safety outcomes were also evaluated. Secondarily, this study provided an opportunity to assess the effect of baseline DFU area size on healing potential.
METHODS
Materials
The study ADM (DermACELL; LifeNet Health, Virginia Beach, Virginia; referred to here as D-ADM) undergoes a unique decellularization process that results in near-complete DNA removal, a ready-to-use product, and the ability to store the matrix at ambient temperatures. In addition, the graft is terminally sterilized in its final package to a sterility assurance level of 1 × 10-6. In a randomized controlled trial consisting of 168 participants, the D-ADM demonstrated excellent healing outcomes for more common DFUs (Wagner grade 1 and 2).8 In that study, Cazzell et al8 found that DFUs healed with an average of 1.1 D-ADM applications. The authors also found that the ulcer size was reduced significantly faster compared with treatment using conventional debridement and nonbiologic dressings. These results suggested that D-ADM might be efficacious for treating Wagner grades 3 and 4 ulcers.
Study Design
This study was a prospective, single-arm, multicenter open-label trial designed to evaluate the safety and efficacy of D-ADM in healing large, complex DFUs with exposed bone or tendon on the lower extremities (Clinical trial registration no. NCT03044132, http://ClinicalTrials.gov). The trial design, methods, and informed consent were reviewed and approved by Western Institutional Review Board (Puyallup, Washington).
There were two study sites: one in North Carolina and one in California. Both are wound care centers with a comparable standard of care for large wounds (Wagner grades 3 and 4). This care plan includes surgical debridement, the addition of a cellular and/or tissue-based product (CTP), offloading with a boot, weekly office visits, and negative-pressure wound therapy (NPWT), if geographically available, until 100% granulation is achieved. Dressings were standardized across both sites.
After providing voluntary written informed consent, each individual was screened for eligibility based on the inclusion and exclusion criteria in Table 1. Study participants were evaluated during a screening period up to 7 days before receiving their baseline surgical debridement and treatment.
Table 1.
INCLUSION AND EXCLUSION CRITERIA

The primary endpoint was the time in weeks required for 100% wound granulation, which was defined as complete coverage of the exposed tendon and/or bone with collagen-rich connective tissue. Granulation was determined by the site investigator. Secondary endpoints were percent wound area reduction at 16 weeks, percent complete wound closure at 16 weeks, and the number of applications of D-ADM required to achieve 100% wound granulation. Complete wound closure was defined as (1) epithelium completely covering the entire wound with no bleeding or drainage of any kind, (2) freedom from the need for additional dressing, and (3) confirmation of closure at two consecutive study visits 2 weeks apart.9,10 The safety endpoint was the number of adverse events after D-ADM application including infection, hospitalization, and reoperation. All participants whose wounds had not yet healed were released from the study at the 16-week visit. If the wound healed at the 15- or 16-week visit, the participant remained in the study for a confirmatory visit 2 weeks later.
Procedure
Patients were consented and included if they presented with a Wagner 3 or 4 DFU that had no appreciable wound area reduction after being treated with advanced wound care for at least 4 weeks, and their ulcer required surgical debridement. Debridement was performed in the operating room per standard of care, which may have included the use of a scalpel, scissors, curette, rongeur, and/or hydrosurgical scalpel. Osteomyelitis was either treated or ruled out prior to the surgical debridement. Those with positive osteomyelitis were treated with conservative surgical intervention and/or antibiotics. The NPWT was permitted starting the day of the debridement procedure to achieve 100% granulation, but it was prescribed at only one study site. Many patients from the North Carolina site lived in rural areas where home health support services, such as NPWT, are not available.
The D-ADM was applied either in the operating room or the wound care clinic after debridement per the clinician’s standard of care. Meshed 4 × 4-cm and 5 × 7-cm D-ADM were attached onto the wound using sutures, staples, surgical tape, or bioglue. The D-ADM was then covered with standardized dressings, including a nonadherent contact layer. Bolster dressings were used for larger wounds to prevent the central area of the graft from tenting away from the wound bed. These were applied over the contact layer dressings to provide localized pressure. Bolster dressings could be any material that allowed for the evacuation of sanguineous or serous drainage while maintaining contact between the graft and the entire wound bed. The outer layer consisted of multilayer pressure dressings. The bolster and outer layer dressings were allowed to be reapplied if dressing changes were required between weekly clinic visits. Offloading with a removable boot was prescribed for all participants except one with a dorsal wound.
At investigator discretion, one additional application of D-ADM was allowed if (1) the wound required further coverage for exposed deep tissue, (2) there was less than 75% granulation tissue present after 4 weeks, or (3) less than 50% granulation tissue was present after 8 weeks.
Assessment
A validated laser measurement device (Silhouette Advanced Wound Assessment and Management System; Aranz Medical, Christchurch, New Zealand)11 was used to photograph and measure the wound area at baseline and at every clinic/wound center research visit. A generic, health-related quality of life assessment tool, the Medical Outcomes Study 36 Short-Form Health Survey (SF-36 version 2.0), was conducted prior to initial D-ADM application (baseline) and at study end. Circulation was measured with ankle-brachial index and/or Doppler arterial waveforms at the initial screening, week 4, week 8, week 12, and week 16 or termination. Adverse events and concurrent conditions that occurred during the study and were not present prior to study treatments were reported in detail on case report forms and followed to satisfactory resolution.
Statistics
All statistical analyses were calculated using the Stata program (StataCorp, College Station, Texas). Kaplan-Meier survival analysis and a log-rank test were used to estimate healing probabilities for stratified baseline wound areas of 15 cm2 or less, between 15 and 25 cm2, and 25 cm2 or larger. A Bonferroni correction was applied to the P value calculated through the log-rank test. A Cox proportional hazard model was used to assess the ability of baseline wound area size to predict healing outcomes. The proportional hazard assumption was met. A 95% confidence interval (CI) was calculated and included where appropriate. All statistical significance was analyzed with a two-sided α of .05. Continuous data are expressed as mean ± SD unless indicated otherwise. Safety and demographic analyses were presented using the intent-to-treat population. Efficacy analyses followed the published literature12 by presenting the per-protocol population first to provide a fair comparison.
RESULTS
Sixty-one participants were screened, and all were enrolled in the study as the intent-to-treat population. The two sites were located in Fresno, California (n = 45), and Rocky Mount, North Carolina (n = 16), which provided distinct geographic settings. There were no screening failures, but two patients were enrolled, treated with D-ADM, and included in the analyses even though each was on an immunosuppressant treatment regimen secondary to kidney transplant at the screening visit.
The mean participant age was 55 years, and 75.4% were male. The population included participants who identified as white (77.1%), African American (18.0%), Asian (3.3%), and Native American (1.6%). Preexisting conditions included diabetes mellitus type 2 (90.2%), hypertension (73.8%), hypercholesterolemia/hyperlipidemia (65.6%), peripheral neuropathy (42.6%), cardiovascular dysfunction (31.1%), chronic kidney disease (11.5%), diabetes mellitus type 1 (4.9%), and prediabetes (4.9%). In addition, nine participants (14.8%) had undergone at least one previous transmetatarsal amputation, and seven (11.5%) had at least one partial or full ray resection.
The ulcers were deep, with 59 of 61 probing to bone, and all wounds scored a Wagner grade 3 or 4. The average wound area was 29.0 ± 21.0 cm2 (maximum, 113.6 cm2). More than half of the DFUs had an area greater than 25 cm2, which is a common upper limit cutoff in published clinical studies. There were no significant changes in patient circulation status between the screening and termination visits. Most of the ulcers were located on the dorsal and plantar forefoot areas. See Table 2 for patient demographics and ulcer characteristics.
Table 2.
DEMOGRAPHICS AND ULCER CHARACTERISTICS
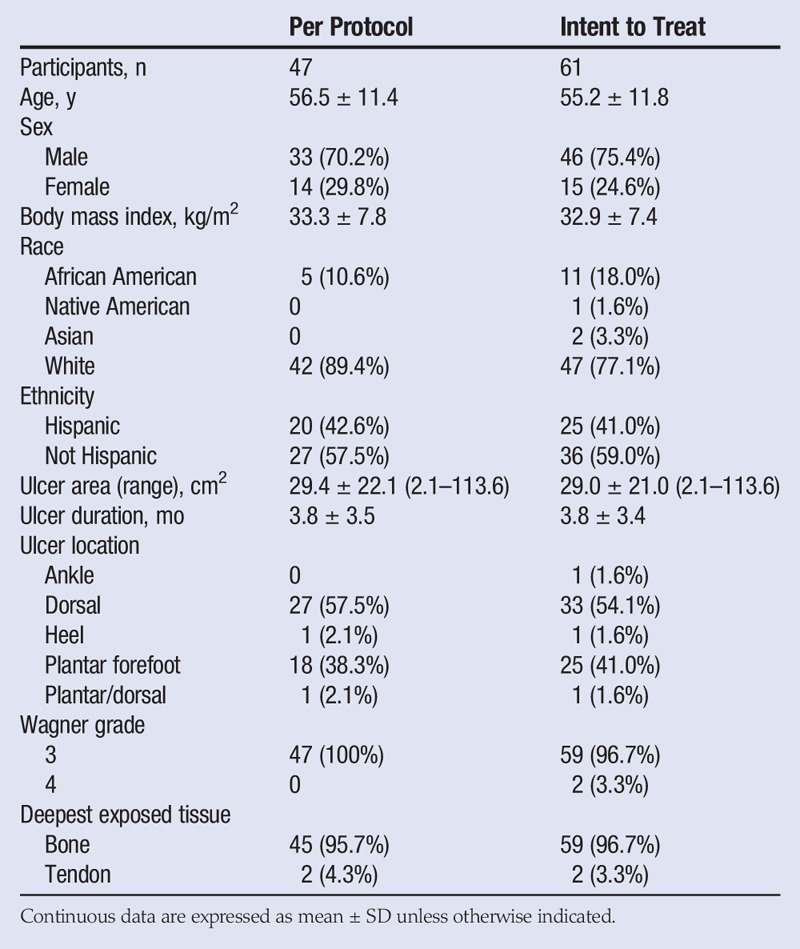
Fourteen participants did not complete the full 16 weeks of the study. Of these, 9 were withdrawn by an investigator for nongraft-related adverse events (8 of these participants required a surgical intervention that impacted the target wound area), 1 was nonadherent to the visit schedule and instructions, 1 withdrew consent to move out of state, 2 were lost to follow-up, and 1 died of an unrelated cause. This left 47 participants as the per-protocol population on which the clinical outcome analyses are based. The results for both populations are presented in Table 3.
Table 3.
CLINICAL OUTCOMES
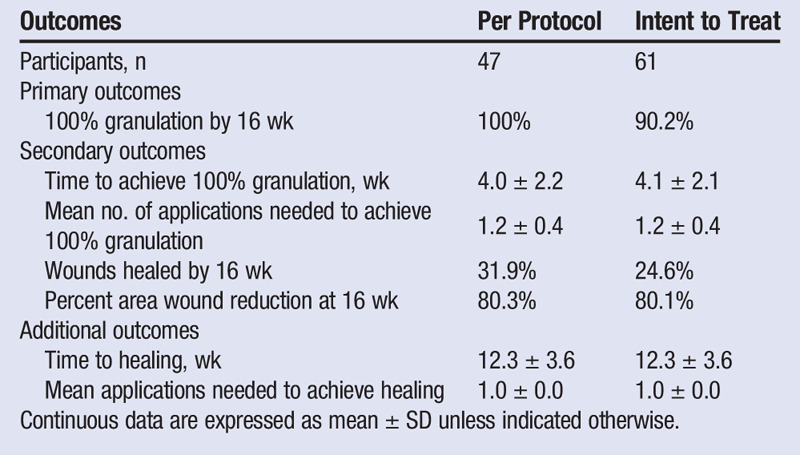
All participants in the per-protocol population achieved 100% granulation by 16 weeks. The mean time to 100% granulation was 4.0 weeks. Nine participants received a second application of D-ADM, resulting in an average of 1.2 applications. The mean percent wound area reduction of 80.3% showed a continuous and relatively consistent reduction in ulcer size from a mean baseline ulcer size of 29.0 ± 21.0 cm2 to 5.7 ± 8.9 cm2 by week 16 (Figure 1).
Figure 1.
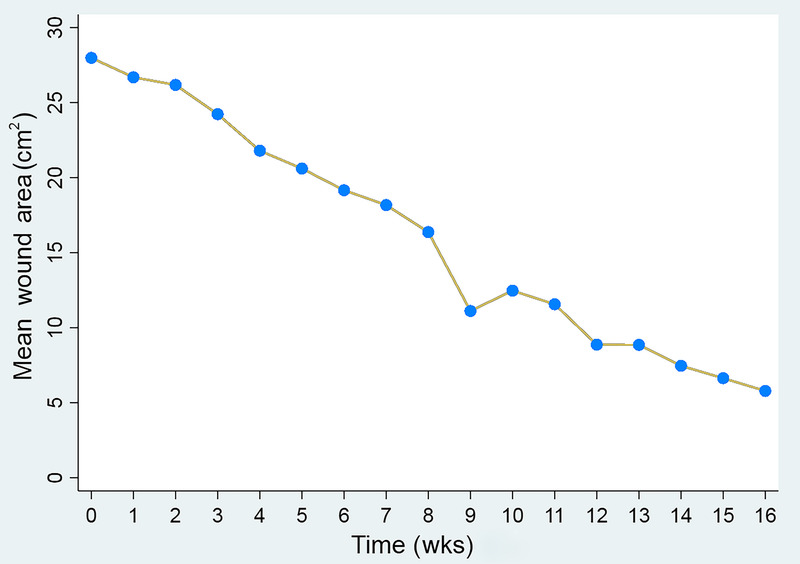
THE MEAN ULCER AREA DECREASED FROM A BASELINE OF 29 CM2 TO 5.7 CM2 OVER 16 WEEKS
Those DFUs 15 cm2 or smaller in area (n = 13) were 14 times more likely to heal than DFUs 29 cm2 or larger (n = 21; P = 0.0008, hazard ratio = 13.8, CI = 3.0–64.0). The DFUs that were 25 cm2 or smaller (n = 22), the typical inclusion cutoff for clinical studies, were 11 times more likely to heal than DFUs larger than 25 cm2 (n = 25, P = .002, hazard ratio = 11.0, CI = 2.5–48.8).
Ulcers were divided into three groups for analysis using a Kaplan-Meier survival curve: those less than or equal to 15 cm2, those larger than 15 cm2 and up to 25 cm2, and those larger than 25 cm2 (Figure 2). These stratified groups had significantly different healing probabilities (P < .0001). The differences remained significant even after a Bonferroni correction was applied. In addition, substantial wound area reduction was observed at 16 weeks for each stratified group: 93.0% for those 15 cm2 or smaller, 95.9% for those between 15 and up to 25 cm2, and 76.5% for those larger than 25 cm2.
Figure 2.
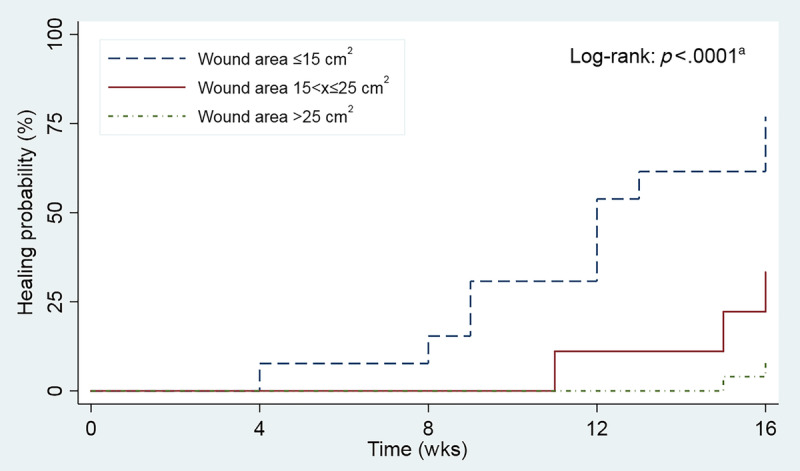
KAPLAN-MEIER SURVIVAL CURVE SHOWING HEALING PROBABILITY STRATIFIED BY ULCER SIZE
aStratified groups had significantly different healing probabilities (P < .0001).
In the intent-to-treat population, 42.6% (n = 20) of participants experienced at least one adverse event. Four involved cellulitis, two osteomyelitis, two sepsis, two Clostridium difficile colitis, one methicillin-resistant Staphylococcus aureus, one dry gangrene of toe, and eight an infection in the target ulcer. Participants with infection were treated with oral antibiotics. Severe infections were further treated with hospitalization for intravenous antibiotics or debridement. One participant had reapplication of D-ADM after the hospital debridement. The amputation rate for noninfected DFUs in this study was 1.6%.
Other adverse events observed included edema, vascular issues, hypercholesterolemia, necrosis, pain, and a single death for heart failure that was unrelated to treatment. Eighteen of the events were considered serious adverse events (SAEs). Seven of the participants with SAEs were removed from the study. The other eight remained through completion because their SAEs did not merit withdrawal. There were 17 hospitalizations among 15 participants during the study period. Two of the hospitalizations were attributed to chronic congestive heart failure and chronic obstructive pulmonary disease with pneumonia, and were considered unrelated to DFUs. Four participants required hospitalization and surgical debridement for other infected ulcers on the target foot. No adverse events directly related to D-ADM itself or the procedure to implant the graft were noted.
DISCUSSION
This study provides new insight into the care management of very large Wagner grades 3 and 4 ulcers. Despite the serious nature of these ulcers, 100% granulation occurred in a mean time period of 4 weeks. The full duration of this study was 16 weeks, which was based on outcomes realized in other DFU studies. However, these large, complex ulcers may require more time to heal. For example, the largest ulcer included in this study had a baseline wound area of 113.6 cm2. This ulcer shrank weekly and was reduced to 23.9 cm2, a substantial 89.7 cm2 reduction over 16 weeks (Figure 3). Similarly, 72% of per-protocol ulcers that did not completely close by 16 weeks had baseline areas larger than 25 cm2. These ulcers were 11 times less likely to heal than ulcers that were 25 cm2 or smaller (Figure 2). Notably, all wounds that healed completely needed only a single application of D-ADM.
Figure 3.
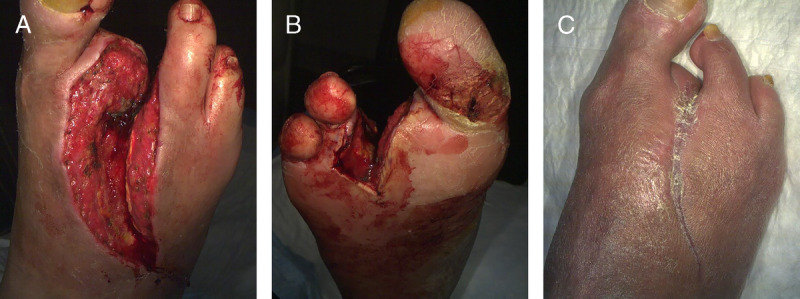
A AND B, SINGLE DIABETIC FOOT ULCER WITH EXPOSED BONE. C, ULCER AFTER SINGLE APPLICATION OF ACELLULAR DERMAL MATRIX
This ulcer reduced in area by 79.0% from 113.6 cm2 at baseline to 23.9 cm2 at week 16. Further, this ulcer achieved 100% granulation at week 3.
The significant percent wound area reduction in this study suggests that the large, complex wounds that did not heal by 16 weeks may have healed if given enough time. These findings underscore the difficulty in treating large DFUs and the importance of noting mean ulcer area when considering treatment options.
Infection is a principal concern with Wagner grades 3 and 4 ulcers. The 2012 Infectious Diseases Society of America Clinical Practice Guideline for the Diagnosis and Treatment of Diabetic Foot Infections provided the criteria for diagnosing and treating infections.13 In this study, 14.8% of target ulcers were infected. Infected ulcers not only take longer to heal, but also have a higher risk of amputation.5,6 By promoting rapid epithelialization and reducing the wound area, D-ADM may decrease the risk of amputation for infected ulcers.
In other studies, patients who had noninfected DFUs with bone exposure suffered amputation rates of 6.5% and 18.3%.5,12 The amputation rate for noninfected DFUs in this study was 1.6%. Notably, Armstrong et al5 reported that patients with infected, bone-exposed DFUs experienced a staggering 92% amputation rate over 6 months. In contrast, of the nine participants with infected DFUs in the present study, the amputation rate was only 11.1% over 16 weeks. Although none of the infected ulcers healed completely by 16 weeks, the safety of D-ADM with excellent limb salvage rates in the study timeframe was encouraging.
The North Carolina site, which did not use NPWT, had 70.0% of wounds heal, whereas the California site, which did use NPWT through granulation, had 21.6% healed wounds. There was no significant difference in wound size or time to granulation between the sites. Because it is generally accepted that NPWT is an important adjunctive therapy in deep wound treatment,14 these data may be relevant to those clinicians who cannot prescribe NPWT because of geographical or insurance constraints; very large, complex wounds can still heal despite a lack of access to all advanced wound care modalities. However, the sample size for the North Carolina site had fewer participants enrolled, precluding generalization.
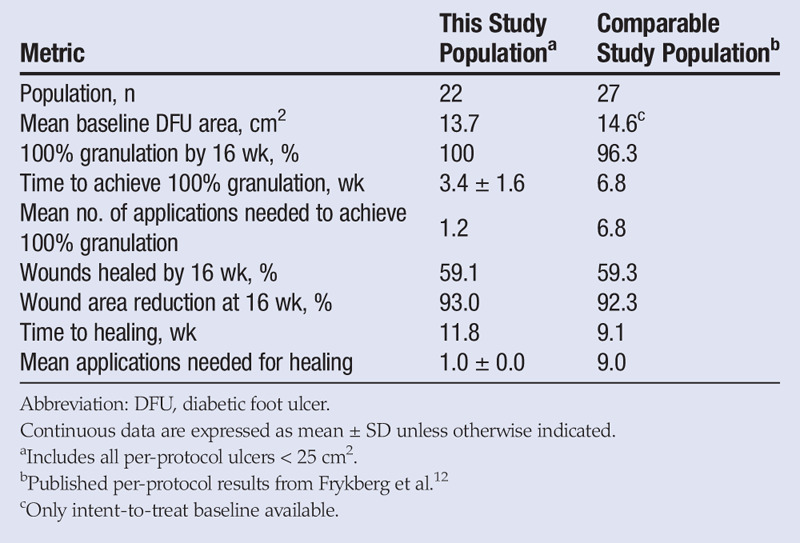
Few clinical trials include “deep wound” ulcers. This is likely attributable to the difficulty in healing these wounds as well as the greater risk of infection and subsequent amputation. Wagner grades 3 and 4 ulcers are the first and second most significant predictors of a DFU failing to heal.15 A recent study of a viable cryopreserved human placental membrane (GrafixCORE; Osiris Therapeutics, Inc, Columbia, Maryland) included deep tissue DFUs, but with a much smaller mean area of 14.6 cm2.12 To provide as fair a comparison as possible, the authors of this study have prepared a secondary analysis of the data by removing all ulcers larger than 25 cm2; this provided a mean area similar to related studies (13.7 cm2; Table 4). This cutoff is not completely equivalent because Frykberg et al12 included some ulcers larger than 25 cm2, but it does allow for a meaningful comparison of populations with similar mean wound areas and levels of exposed tissue. Further, a typical and broad exclusion requirement of ulcer areas larger than 25 cm2 ensures that individual results are not subject to selection bias. The application of both D-ADM and viable cryopreserved human placental membrane (vCHPM) resulted in 100% granulation, but vCHPM took twice as long and required almost six times as many applications as D-ADM to achieve this outcome. Both CTPs had the same healing rate at 16 weeks, but vCHPM required nine times as many mean applications to achieve that same healing rate as D-ADM, suggesting that D-ADM is more cost-effective and less labor-intensive.
Table 4.
COMPARISON OF CELLULAR AND/OR TISSUE-BASED PRODUCTS ON COMPARABLE DEEP DFUS
Limitations
A major limitation of this study was the lack of a control arm. The authors believe that the efficacy of the D-ADM was supported because of rapid granulation and wound area reduction. Although it is not possible to make direct comparisons to results from standard of care, the outcomes using D-ADM can be compared with results reported in the literature. For example, the product’s efficacy was supported by the average number of applications required to induce granulation (1.2) and healing (1.0). These averages are in sharp contrast to the 9.0 and 6.8 mean applications, respectively, reported for a vCHPM.12
Another major limitation was that the study follow-up terminated after 16 weeks, which provided an insufficient length of time for the extremely large ulcers to heal. Future studies that include Wagner grades 3 and 4 DFUs would benefit from a longer study duration.
In addition, hemoglobin A1c levels were recorded for only 16 patients (mean hemoglobin A1c = 8.0%) because that information was charted well before informed consent was obtained for the other participants. Because that information was not available, study authors collected concomitant medications and antidiabetic regimens for those 59 participants with a diagnosis of diabetes: 13 were treated with 1 or more oral agents, 20 with insulin, 23 with both insulin and oral agents, and 2 with diet and exercise.
The biases encountered for natural recovery or healing and use of adjunctive therapies were considered during protocol design. The protocol required the target wounds to have shown little response to standard-of-care wound therapies for at least 4 weeks prior to the screening visit. Criteria also dictated that the wounds required aggressive surgical debridement in addition to a documented need for the use of a CTP. Hyperbaric oxygen treatments were not allowed as an adjunct treatment during the trial. Those participants who required additional surgical intervention on the target limb during the treatment period were withdrawn from the trial.
An additional limitation was that the analyses primarily focused on per-protocol participants. The focus on per-protocol participants was done to ensure fair and accurate comparisons with the previously published literature on DFUs with exposed bone.12 However, the per-protocol population does include all patients who completed the trial regardless of their compliance. For example, one per-protocol patient was consistently nonadherent to offloading, NPWT, and dressing changes. This not only hindered healing, but also resulted in the participant’s wound area increasing in size compared with baseline. This patient’s outcome not only highlights the importance of treatment adherence, but also represents an issue that clinicians encounter and is therefore included in the analyses.
The intent-to-treat population included nine participants withdrawn for nongraft-related events, of which two ulcers did not have a decrease in size over time. One of these two patients was diagnosed at week 9 with osteomyelitis that required a resection impacting the target ulcer (change in area from 5.9 cm2 to 7.6 cm2 at exit). The other participant was removed at week 2 for osteomyelitis in the target limb that required a first ray resection that impacted the target ulcer (change in area from 13.6 cm2 to 13.7 cm2 at exit). The average decrease in ulcer area was 33.1% during the time these nine participants were under treatment. Study authors acknowledge that the wide CIs for baseline wound size on healing (Table 3) indicate that a larger population is needed to calculate a more precise interval. However, the lower ends of the ranges still suggest that ulcer size has a significant impact on healing. Further study is warranted.
Last, the study population had an unusually poor level of health for inclusion in a clinical trial; however, their preexisting vascular and renal conditions are representative of patients seen in “real-world” clinics, and the study authors believe that their outcomes are an important and beneficial addition to the literature.
CONCLUSIONS
The D-ADM demonstrated the ability to rapidly reduce the size of large, complex DFUs with exposed bone when used as part of a treatment regimen for “deep wound” DFUs. Amputation risk was low compared with other studies. The results of this study demonstrate the safety and efficacy of using D-ADM to treat Wagner grades 3 and 4 DFUs to promote wound closure and rapidly reduce even very large wound areas in patients with multiple comorbidities.
REFERENCES
- 1.Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017;376(24):2367–75. [DOI] [PubMed] [Google Scholar]
- 2.Hobizal KB, Wukich DK. Diabetic foot infections: current concept review. Diabet Foot Ankle 2012;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nabuurs-Franssen MH, Huijberts MS, Nieuwenhuijzen Kruseman AC, Willems J, Schaper NC. Health-related quality of life of diabetic foot ulcer patients and their caregivers. Diabetologia 2005;48(9):1906–10. [DOI] [PubMed] [Google Scholar]
- 4.Ragnarson Tennvall G, Apelqvist J. Health-related quality of life in patients with diabetes mellitus and foot ulcers. J Diabetes Complications 2000;14(5):235–41. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system: the contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998;21(5):855–9. [DOI] [PubMed] [Google Scholar]
- 6.Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Harkless LB, Boulton AJM. A comparison of two diabetic foot ulcer classification systems: the Wagner and the University of Texas wound classification systems. Diabetes Care 2001;24(1):84–8. [DOI] [PubMed] [Google Scholar]
- 7.Mutluoglu M, Sivrioglu AK, Eroglu M, et al. The implications of the presence of osteomyelitis on outcomes of infected diabetic foot wounds. Scand J Infect Dis 2013;45(7):497–503. [DOI] [PubMed] [Google Scholar]
- 8.Cazzell S, Vayser D, Pham H, et al. A randomized clinical trial of a human acellular dermal matrix demonstrated superior healing rates for chronic diabetic foot ulcers over conventional care and an active acellular dermal matrix comparator. Wound Repair Regen 2017;25(3):483–97. [DOI] [PubMed] [Google Scholar]
- 9.Snyder DL, Sullivan N, Schoelles KM. Skin Substitutes for Treating Chronic Wounds. Agency for Healthcare Research and Quality: Rockville, MD; 2012. [PubMed] [Google Scholar]
- 10.Food and Drug Administration Wound Healing Clinical Focus Group. Guidance for industry: chronic cutaneous ulcer and burn wounds—developing products for treatment. Wound Repair Regen 2006;9(4):258–68. [DOI] [PubMed] [Google Scholar]
- 11.Davis KE, Constantine FC, Macaslan EC, Bills JD, Noble DL, Lavery LA. Validation of a laser-assisted wound measurement device for measuring wound volume. J Diabetes Sci Technol 2013;7(5):1161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frykberg RG, Gibbons GW, Walters JL, Wukich DK, Milstein FC. A prospective, multicentre, open-label, single-arm clinical trial for treatment of chronic complex diabetic foot wounds with exposed tendon and/or bone: positive clinical outcomes of viable cryopreserved human placental membrane. Int Wound J 2017;14(3):569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012;54(12):e132–73. [DOI] [PubMed] [Google Scholar]
- 14.Panayi AC, Leavitt T, Orgill DP. Evidence based review of negative pressure wound therapy. World J Dermatol 2017;6(1):1–16. [Google Scholar]
- 15.Fife CE, Horn SD, Smout RJ, Barrett RS, Thomson B. A predictive model for diabetic foot ulcer outcome: the wound healing index. Adv Wound Care (New Rochelle) 2016;5(7):279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]


