Description
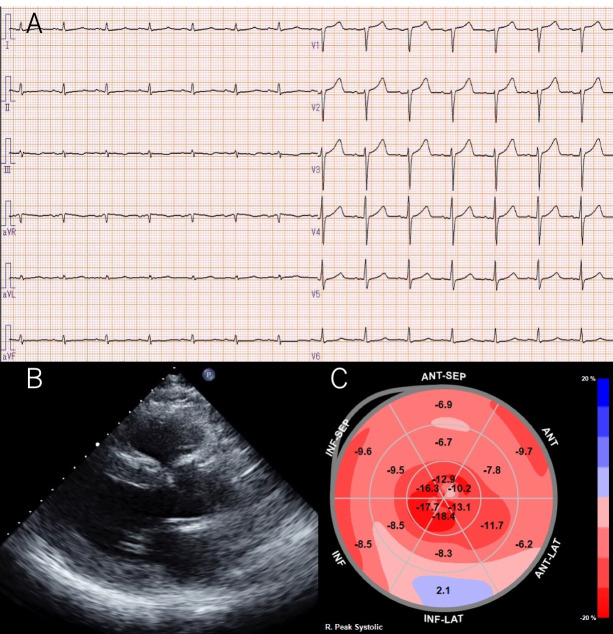
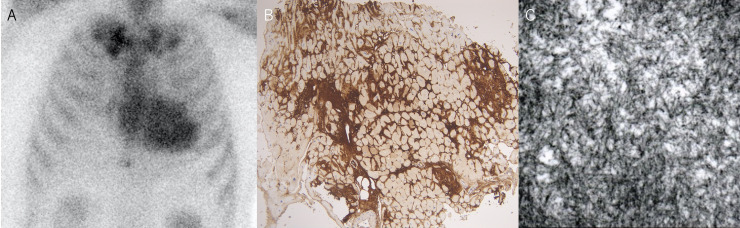
A 57-year-old man was diagnosed with paroxysmal atrial fibrillation (AF) and was admitted to our hospital for catheter ablation. He had been treated for hypertension for 10 years and was taking diuretics due to exertional dyspnoea (New York Heart Association class II) and lower leg oedema. He did not have carpal tunnel syndrome or spinal canal stenosis. Chest radiography revealed a cardiothoracic ratio of 51%, no pleural effusion and slight pulmonary congestion. A 12-lead ECG revealed a sinus rhythm with a heart rate of 73 beats/min and decreased voltage in the limb leads (figure 1A). Transthoracic echocardiography (TTE) revealed an increased left ventricular (LV) wall thickness (intraventricular septum, 16 mm; posterior wall thickness, 16 mm) (figure 1B). The LV ejection fraction was 61% with no regional LV wall motion abnormalities. Two-dimensional speckle-tracking echocardiography (STE) revealed a global longitudinal strain (LS) of −9.9% and a relative apical LS (=average apical LS/average basal LS+mid LS) of 1.9 with apical sparing (figure 1C). Bence-Jones protein and M-protein tests were negative. There were no abnormalities of free light chains (κ 40.6 mg/L, λ 32.6 mg/L, κ/λ 1.245). Technetium-99m pyrophosphate (99mTc-PYP) scintigraphy demonstrated myocardial uptake (Perugini grade 3) (figure 2A).1 Coronary angiography revealed no significant stenosis. The right ventricular endomyocardial biopsy specimens were positive for Congo red and direct fast scarlet staining and for transthyretin (TTR) immunostaining (figure 2B). Deposition of 10 nm diameter fine fibres was seen on electron microscopy (figure 2C). Genetic testing revealed no variant of the TTR gene variants. Based on these test results, the patient was diagnosed with wild-type amyloid transthyretin (ATTRwt) cardiac amyloidosis. His estimated glomerular filtration rate was 45.2 mL/min/body surface area and brain natriuretic peptide level was 119.4 pg/mL, corresponding to stage I according to the classification by Gillmore et al.2 After the introduction of tafamidis, the paroxysmal AF was successfully treated using pulmonary venous isolation with radiofrequency ablation.
Figure 1.
(A) A 12-lead ECG shows sinus rhythm with a heart rate of 73 beats/min and decreased voltage in the limb leads. (B) Transthoracic echocardiography reveals increased left ventricular wall thickness (intraventricular septum, 16 mm; posterior wall thickness, 16 mm). (C) Two-dimensional speckle-tracking echocardiography reveals a relative apical longitudinal strain (LS) (=average apical LS/average basal LS+mid LS) of 1.9 with apical sparing.
Figure 2.
(A) Technetium-99m pyrophosphate scintigraphy demonstrates myocardial uptake. (B) Right ventricular endomyocardial biopsy specimens are positive for transthyretin immunostaining. (C) Deposition of 10 nm diameter fine fibres is seen on electron microscopy.
ATTRwt amyloidosis is a systemic amyloidosis that is mainly recognised in elderly adults.3–5 In this case, the low voltage of limb leads on ECG, LV hypertrophy on TTE and LS apical sparing on STE raised the suspicion of cardiac amyloidosis.6 The 99mTc-PYP scintigraphy, TTR immunostaining and genetic testing findings confirmed ATTRwt amyloidosis. However, this patient is relatively younger than the patients with ATTRwt cardiac amyloidosis and there is a possibility that other rare TTR variants were associated with the ATTR cardiomyopathy in this case.7 Cardiac amyloidosis is associated with an increased risk for atrial arrhythmias. Tan et al reported that catheter ablation provides important symptomatic relief in patients with cardiac amyloidosis.8 Based on the prognostic staging system for ATTR amyloidosis, our patients were classified as stage I,2 and the paroxysmal AF was expected to respond well to catheter ablation. The development of effective therapeutic agents as well as the advancement in cardiac imaging, enabling early and accurate diagnosis, are expected to improve the prognosis of patients with ATTRwt cardiac amyloidosis.
Learning points.
Wild-type amyloid transthyretin (ATTRwt) cardiac amyloidosis is a form of amyloidosis associated with ageing.
We present a case of ATTRwt cardiac amyloidosis associated with paroxysmal atrial fibrillation that developed in a patient in his 50s. The development of effective therapeutic agents as well as the advancement in cardiac imaging, enabling early and accurate diagnosis, are expected to improve prognosis of patients with ATTRwt cardiac amyloidosis.
Footnotes
Contributors: JT, TO and NW were involved in the clinical management of the patient. All the authors contributed in drafting the article. JT and KT wrote and edited the manuscript. KT was responsible for overall supervision.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016;133:2404–12. 10.1161/CIRCULATIONAHA.116.021612 [DOI] [PubMed] [Google Scholar]
- 2.Gillmore JD, Damy T, Fontana M, et al. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J 2018;39:2799–806. 10.1093/eurheartj/ehx589 [DOI] [PubMed] [Google Scholar]
- 3.Sekijima Y. Transthyretin (ATTR) amyloidosis: clinical spectrum, molecular pathogenesis and disease-modifying treatments. J Neurol Neurosurg Psychiatry 2015;86:1036–43. 10.1136/jnnp-2014-308724 [DOI] [PubMed] [Google Scholar]
- 4.Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation 2012;126:1286–300. 10.1161/CIRCULATIONAHA.111.078915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakagawa M, Sekijima Y, Yazaki M, et al. Carpal tunnel syndrome: a common initial symptom of systemic wild-type ATTR (ATTRwt) amyloidosis. Amyloid 2016;23:58–63. 10.3109/13506129.2015.1135792 [DOI] [PubMed] [Google Scholar]
- 6.Koyama J, Minamisawa M, Sekijima Y, et al. Role of echocardiography in assessing cardiac amyloidoses: a systematic review. J Echocardiogr 2019;17:64–75. 10.1007/s12574-019-00420-5 [DOI] [PubMed] [Google Scholar]
- 7.Rowczenio DM, Noor I, Gillmore JD, et al. Online Registry for mutations in hereditary amyloidosis including Nomenclature recommendations. Hum Mutat 2014;35:E2403–12. 10.1002/humu.22619 [DOI] [PubMed] [Google Scholar]
- 8.Tan NY, Mohsin Y, Hodge DO, et al. Catheter ablation for atrial arrhythmias in patients with cardiac amyloidosis. J Cardiovasc Electrophysiol 2016;27:1167–73. 10.1111/jce.13046 [DOI] [PubMed] [Google Scholar]