Abstract
BCL6 corepressor like-1 (BCORL1) mutation has rarely been described in thyroid cancer or in association with BRAF mutations in any malignancy. However, we report a 49-year-old woman who had aggressive follicular variant papillary thyroid carcinoma (FV-PTC) with both the BRAF K601E and BCORL1 mutations. The patient underwent a total thyroidectomy for a 3.6 cm right thyroid nodule and a smaller lesion in the left lobe in 2007; both were FV-PTCs with no lymphovascular invasion or metastases. In 2015, a positron emission tomography–CT scan showed a small defect in the left posterior lateral fifth rib with mild increased hypermetabolic activity with standardised uptake value of 3.9 and another lesion in the right hip at the junction of the femoral neck and trochanter. Tumour biopsy and genetic analysis revealed an uncommon BRAF K601E and a rare BCORL1 mutation. While rare, we report a case of aggressive FV-PTC with both the BRAF K601E and BCORL1 mutations.
Keywords: thyroid disease, endocrine cancer, head and neck cancer
Background
Thyroid cancer is the most common endocrine malignancy.1 Papillary thyroid carcinoma (PTC) represents more than 80% of thyroid cancers,1 and BRAF (V600E) is the most frequent mutation to be associated with thyroid cancer.1–3 Afkhami et al2 reported that more than 45% of cases with papillary thyroid cancer had a BRAF mutation and the incidence of this mutation has been increasing lately.1 2 BRAF V600E is more likely to be associated with PTC with more aggressive pattern and worse prognosis.1 BRAF K601E is more likely to be associated with low-risk PTC, especially follicular variant PTC (FV-PTC), less often classic PTC and rarely, follicular adenoma and follicular carcinoma.1 2 4–14
BCL6 corepressor like-1 (BCORL1) is a transcriptional corepressor that promotes cellular migration and invasion via E-cadherin dysregulation.15 16 The role of BCORL1 mutation in cancer metastasis has been described in several cancers including breast cancer, acute myeloid leukaemia, hepatocellular carcinoma (HCC), retinoblastoma17 18 and non-small cell lung cancer (NSCLC).16 Over expression of this mutation could be inversely correlated with E-cadherin regulation which subsequently leads to poor prognosis and tumour metastasis.15 16 While the association of BCORL1 mutation with PTC and anaplastic thyroid cancer19 20 has been reported rarely, concomitant association of BCORL1 and BRAF mutations has not been reported in FV-PTC. Therefore, we report a case of aggressive FV-PTC with both the BRAF K601E and BCORL1 mutations.
Case presentation
In October 2007, a 49-year-old woman had a total thyroidectomy for a 3.6 cm right thyroid nodule and a smaller lesion in the left lobe; both were FV-PTCs with no lymphovascular invasion or lymph node metastases (figures 1 and 2). After that, she received 100 mCi of 131-Iodine. The patient denied a history of thyroid disease or radiation exposure other than dental X-rays. She had a family history of benign thyroid goitre. The patient had a previous medical history of arthritis, fibromyalgia and temporomandibular joint disorder.
Figure 1.
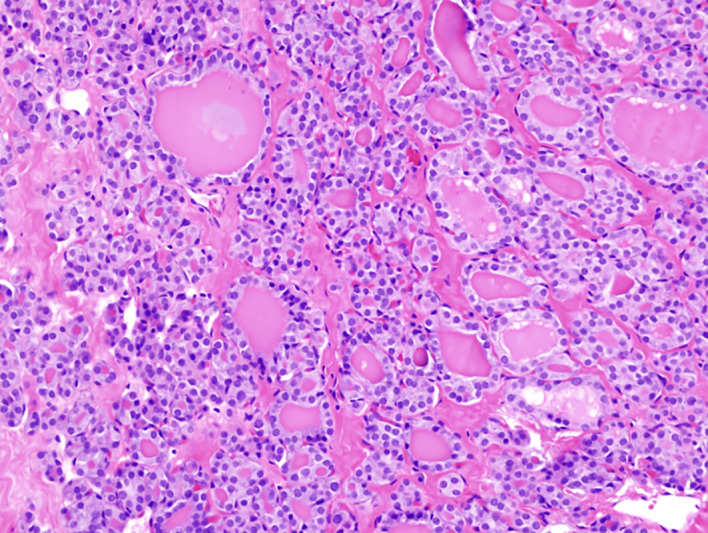
Primary thyroid tumour showing follicular growth pattern, 20× magnification.
Figure 2.
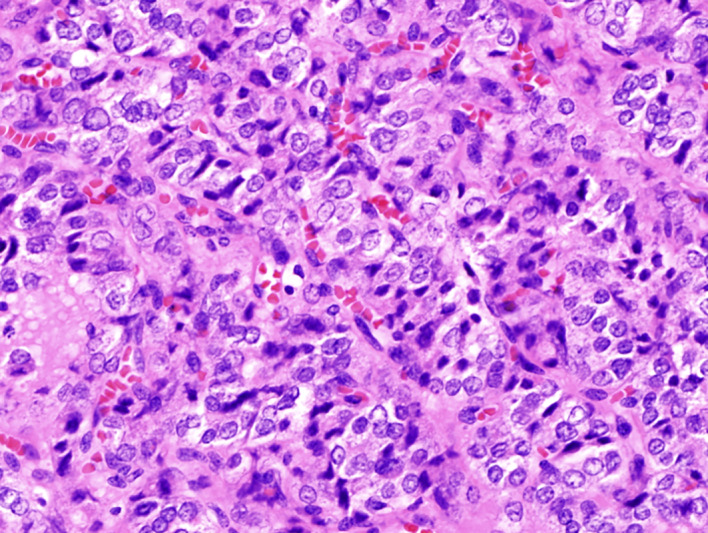
Primary tumour showing more solid growth pattern as well as more obvious papillary thyroid cancer features, 40× magnification.
Investigations
In 2009, serum thyroglobulin was <0.2 ng/mL and in 2013 was 4.3 ng/mL. Diagnostic 131-Iodine, positron emission tomography (PET)/CT and technetium bone scans in 2014 were negative and thyroglobulin was 14.3 ng/mL. In 2015, a PET–CT scan showed a small defect in the posterior/lateral aspect of the left fifth rib (figure 3) with mild increased hypermetabolic activity with standardised uptake value of 3.9. After a negative postrecombinant human thyrotropin (rhTSH) stimulated diagnostic radioiodide scan and inconclusive needle biopsy test, the patient underwent resection of the posterior/lateral aspect of the left fifth rib in early 2016. Immunohistochemistry was positive for thyroglobulin and weakly positive for thyroid transcription factor-1. Moreover, the tumour biopsy was consistent with her original thyroidectomy in 2007 (figure 4). Two months later, serum thyroglobulin was 13.7 ng/mL and 5 days after receiving 150 mCi of post-rhTSH radioiodine and taking low-iodine diet, there was a subtle uptake in the right hip at the junction of the femoral neck and trochanter. CT scan of the right femur showed a 7 mm lesion in the right femoral neck, and FDG–PET showed subtle uptake (figure 5) for which she received 5/5 2000 cGy palliative radiation therapy.
Figure 3.
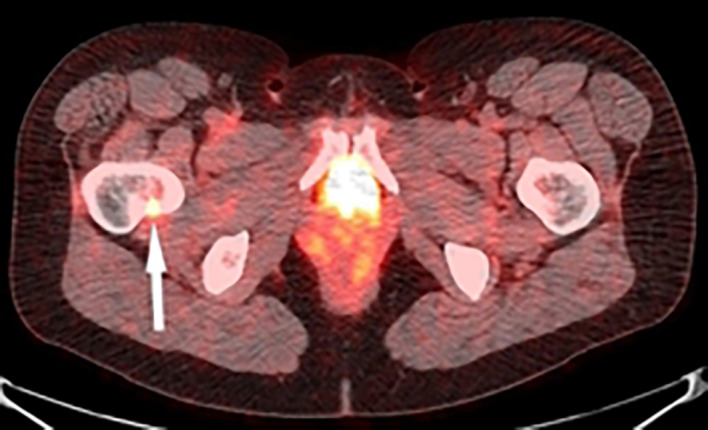
Positron emission tomography–CT scan of rib metastasis of follicular variant papillary thyroid carcinoma.
Figure 4.
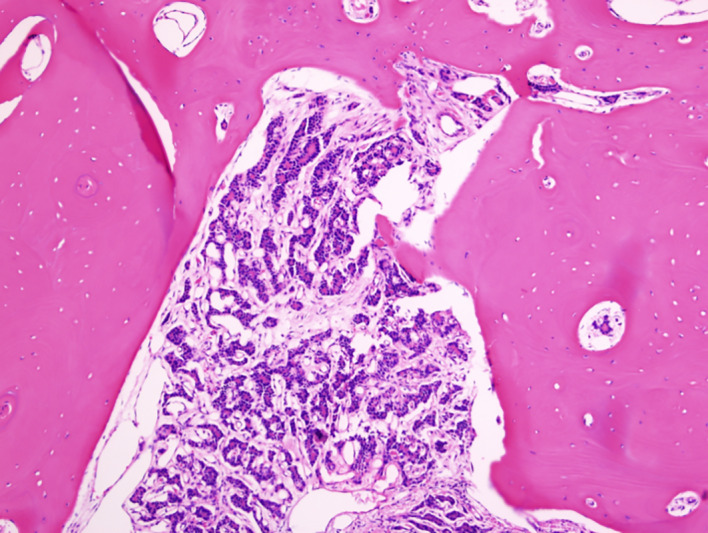
Rib metastasis, with follicles and colloid, 10× magnification.
Figure 5.
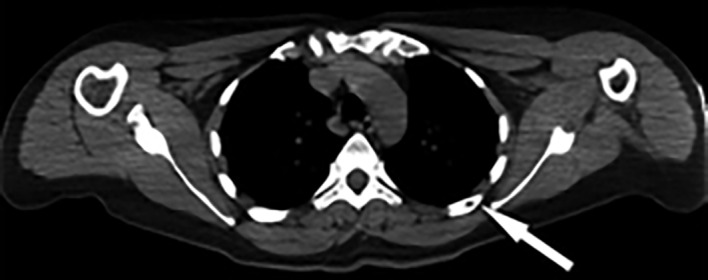
FDG–positron emission tomography/CT scan showing subtle uptake in right femoral neck metastasis.
Tumour from the original surgery was sent to FoundationOne (detects 328 cancer-related genes and 28 rearrangements) for genomic analysis and an uncommon BRAF K601E and a rare BCORL1 mutation were detected. In addition, there were variants of uncertain significance in the following genes: VEGFA; ARID1B (SWI/SNF); BRIP1 (interacts with BRCA1) and PREX2 (RAC1 and PTEN).
Treatment
In 2016, the patient underwent a resection of the posterior/lateral aspect of the left fifth rib and received 150 mCi of post-rhTSH radioiodine afterwards and was on low-iodine diet. She also received 5/5 2000 cGy palliative radiation therapy for the right femoral neck metastasis. The patient is currently on synthroid 150 mcg once a day, acetaminophen 500 mg once a day and escitalopram 5 mg once a day.
Outcome and follow-up
The patient was doing well until she had clival metastasis in 2018 for which she received radiation therapy and had an initial favourable response. In February 2019, the patient had a recurrence to the left iliac wing and acetabulum and right femoral neck for which she received 5/5 4000 cGy palliative radiation therapy. On 19 November 2019, FDG–PET/CT scan showed decreased activity in the clival metastasis and mixed changes in the iliac and femoral neck lesions. Due to some progression in the left iliac and right femoral neck metastases, palliative radiotherapy has been scheduled and denosumab is being considered to reduce the risk of hip fracture. A timeline of her clinical course is depicted in figure 6.
Figure 6.
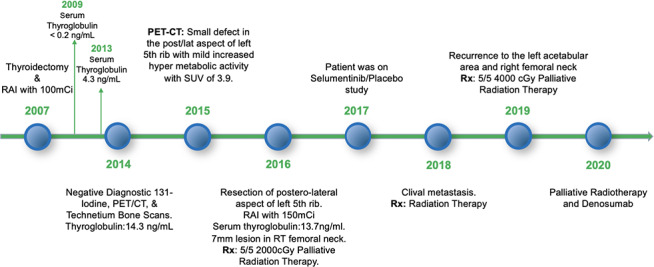
Timeline of patient’s clinical course.
Discussion
BRAF is a member of the RAF kinase family that promotes signalling via RAS–RAF–MAPK signal transduction which helps in the regulation of cellular proliferation and apoptosis.1 BRAF V600E is the most common mutation in PTC and presents in more than 50% of PTC cases.3 BRAF V600E mutation is an activating mutation on exon-15 where valine–glutamate substitution happens at amino acid 600. It is mainly associated with high-risk PTC with more aggressive clinical course, worse prognosis and distant metastasis,1 particularly when combined with a TERT promoter mutation.21
BRAF K601E mutation is an inframe mutation with either deletion or insertion of lysine to glutamate substitution at amino acid 601.2 BRAF K601E is not well described as much as BRAF V600E, however, BRAF K601E mutation is more linked with FV-PTC with more indolent clinical course, better outcomes and more conservative management.1 2 4–14
Trovisco et al reported that BRAF K601E is present in 7% of FV-PTC cases and few cases of follicular adenoma and carcinoma as well.4 22 Torregrossa et al stated that 80% of BRAF K601E mutations are found in early tumour stage thyroid cancers and 96% showed no extranodal extension or distant metastasis.1 Afkhami et al described that 5.3% of all BRAF mutations in thyroidectomy specimens were BRAF K601E; 93% of them were PTC predominantly FV-PTC, 3.4% were follicular thyroid carcinoma and 3.4% were follicular adenomas.2 Ninety-seven per cent were T1–T2 tumours, with no recurrences or metastases if only a single mutation was present. Barollo et al10 identified BRAF K601E in 4/347 BRAF-mutant tumours. Three were FV-PTC with no nodal metastases; one was poorly differentiated but also had a PIK3CA mutation.
BCORL-1 is a transcriptional corepressor that interacts with class II histone deacetylases by interacting with the amino terminus of c-terminal binding protein and decreasing the transcription of E-cadherin,15 16 23 an important transmembrane protein that mediates cell to cell adhesion.24
Multiple reports have described loss of E-cadherin expression and localisation in patients with advanced malignant tumours; moreover, it is associated with increased risk for tumour recurrence and metastasis.15 24
Tumour cells inhibit E-cadherin expression by a promoter methylation or upregulation of transcriptional repressors such as: SNAIL and SLUG which knock down the promoter.15 Loss of E-cadherin expression contributes to epithelial–mesenchymal transition, the main cause of metastasis in human cancer.15
To the best of our knowledge, there are no data about BCORL1 mutations in patients with PTC. Additionally, a literature search on the PubMed database through 7 November 2019 using the query ‘BCORL1 and cancer’ yielded 29 reports, none related to any type of thyroid cancers.
However, the role of BCORL1 in tumour metastasis has been reported in patients with HCC, acute myelogenous leukaemia, myelodysplastic syndrome and chronic myelomonocytic leukaemia.15 17 18 Yin et al reported that overexpression of BCORL1 in HCC patients leads to suppression of E-cadherin expression which subsequently facilitates cancer metastasis.15 Zhao et al stated that overexpression of BCORL1 in NSCLC is inversely linked to E-cadherin regulation and BCORL1 overexpression is correlated with worse prognosis and more tumour invasion.16
Xu et al25 recently reported that a small percentage of PTCs with low-risk histology (mostly FV-PTCs) had distant metastases, and 6/8 had more than one somatic mutation. Our patient, in addition to a BRAF K601E mutation, almost always associated with an indolent course, developed distant metastases. It is likely that the BCORL-1 mutation was responsible for the more aggressive behaviour, although any one or more of variants of unknown significance in four potentially oncogenic or tumour suppressive genes (ARID1B, BRIP1, PREX2, VEGFA) may have played a role. This case illustrates the value of more intensive interrogation of the molecular genotype of tumours, especially when the clinical cancer does not align with the histopathology and basic molecular characterisation.
Patient’s perspective.
I have been dealing with thyroid cancer since 2007 and underwent thyroidectomy. The cancer came back to my bones many years later. At the beginning, I was very scared because the doctors did not know how to locate my lesions but the lab results showed the signs of recurrence. When the doctors finally located my lesions in the ribs. I was told that I have an unusual thyroid cancer with two mutations that do not seem to be together in one patient before.
I have been dealing with doctors ever since. Even though my thyroid cancer seems to be more aggressive than what I thought, I still feel great that I only feel some pain more like arthritis. I still think that I am much better than most of other cancer patients. I am optimistic and will never lose hope that doctors will find the right treatment for my cancer.
Most importantly, I would like to thank my husband, friends and family for their support. I also feel a depth of gratitude for all of my doctors who helped me through this journey.
Learning points.
This is a case of an aggressive follicular variant papillary thyroid carcinoma (FV-PTC) with a rare association with BRAF K601E and BCL6 corepressor like-1 (BCORL1) mutations.
This case illustrates the value of more intensive interrogation of the molecular genotype of tumours, especially when the clinical course does not align with the histopathology and basic molecular characterisation.
When a case of aggressive course of FV-PTC is found, it is important to further investigate to determine the type of mutation associated with the tumour.
Physicians should be educated about the possible association of BCORL-1 mutation and thyroid cancer.
Acknowledgments
The authors would like to thank Dr Alan Ho for providing records on patient’s recent status.
Footnotes
Contributors: DA wrote the case report, performed substantial contributions to the manuscript design and took the patient consent for publication. AL revised the manuscript and performed substantial contribution to the follow-up of the patient. QZ selected the pathology figures and interpreted all the biopsy findings. TM edited the case presentation. RS revised the manuscript and provided a critical feedback. AL, TM and RS were responsible for the management of the case.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Torregrossa L, Viola D, Sensi E, et al. Papillary thyroid carcinoma with rare exon 15 BRAF mutation has indolent behavior: a single-institution experience. J Clin Endocrinol Metab 2016;101:4413–20. 10.1210/jc.2016-1775 [DOI] [PubMed] [Google Scholar]
- 2.Afkhami M, Karunamurthy A, Chiosea S, et al. Histopathologic and Clinical Characterization of Thyroid Tumors Carrying the BRAF(K601E) Mutation. Thyroid 2016;26:242–7. 10.1089/thy.2015.0227 [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014;159:676–90. 10.1016/j.cell.2014.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trovisco V, Vieira de Castro I, Soares P, et al. Braf mutations are associated with some histological types of papillary thyroid carcinoma. J Pathol 2004;202:247–51. 10.1002/path.1511 [DOI] [PubMed] [Google Scholar]
- 5.Pennelli G, Vianello F, Barollo S, et al. BRAF(K601E) mutation in a patient with a follicular thyroid carcinoma. Thyroid 2011;21:1393–6. 10.1089/thy.2011.0120 [DOI] [PubMed] [Google Scholar]
- 6.Schulten H-J, Salama S, Al-Mansouri Z, et al. Braf mutations in thyroid tumors from an ethnically diverse group. Hered Cancer Clin Pract 2012;10:10. 10.1186/1897-4287-10-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JY, Kim WY, Hwang TS, et al. Braf and Ras mutations in follicular variants of papillary thyroid carcinoma. Endocr Pathol 2013;24:69–76. 10.1007/s12022-013-9244-0 [DOI] [PubMed] [Google Scholar]
- 8.Kim WY, Ko YS, Hwang TS, et al. A case of multifocal papillary thyroid carcinoma consisting of one encapsulated follicular variant with BRAF K601E mutation and three conventional types with BRAF V600E mutation. Korean J Pathol 2013;47:293–8. 10.4132/KoreanJPathol.2013.47.3.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho U, Oh WJ, Bae JS, et al. Clinicopathological features of rare BRAF mutations in Korean thyroid cancer patients. J Korean Med Sci 2014;29:1054–60. 10.3346/jkms.2014.29.8.1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barollo S, Pezzani R, Cristiani A, et al. Prevalence, tumorigenic role, and biochemical implications of rare BRAF alterations. Thyroid 2014;24:809–19. 10.1089/thy.2013.0403 [DOI] [PubMed] [Google Scholar]
- 11.Monti E, Bovero M, Mortara L, et al. Braf mutations in an Italian regional population: implications for the therapy of thyroid cancer. Int J Endocrinol 2015;2015:1–7. 10.1155/2015/138734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murugan AK, Qasem E, Al-Hindi H, et al. Classical V600E and other non-hotspot BRAF mutations in adult differentiated thyroid cancer. J Transl Med 2016;14:204. 10.1186/s12967-016-0958-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macerola E, Torregrossa L, Ugolini C, et al. BRAFK601EMutation in a Follicular Thyroid Adenoma: A Case Report. Int J Surg Pathol 2017;25:348–51. 10.1177/1066896916688083 [DOI] [PubMed] [Google Scholar]
- 14.Schopper HK, Stence A, Ma D, et al. Single thyroid tumour showing multiple differentiated morphological patterns and intramorphological molecular genetic heterogeneity. J Clin Pathol 2017;70:116–9. 10.1136/jclinpath-2016-203821 [DOI] [PubMed] [Google Scholar]
- 15.Yin G, Liu Z, Wang Y, et al. BCORL1 is an independent prognostic marker and contributes to cell migration and invasion in human hepatocellular carcinoma. BMC Cancer 2016;16:103. 10.1186/s12885-016-2154-z [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Zhao X, Tuo H, Si M, et al. [Expression and clinical significance of BCL6 corepressor-like 1 in non-small cell lung cancer]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2015;31:1677–81. [PubMed] [Google Scholar]
- 17.de Rooij JDE, van den Heuvel-Eibrink MM, Hermkens MCH, et al. Bcor and BCORL1 mutations in pediatric acute myeloid leukemia. Haematologica 2015;100:e194–5. 10.3324/haematol.2014.117796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto Y, Abe A, Emi N. Clarifying the impact of polycomb complex component disruption in human cancers. Mol Cancer Res 2014;12:479–84. 10.1158/1541-7786.MCR-13-0596 [DOI] [PubMed] [Google Scholar]
- 19.Pitt SC, Hernandez RA, Nehs MA, et al. Identification of Novel Oncogenic Mutations in Thyroid Cancer. J Am Coll Surg 2016;222:1036–43. 10.1016/j.jamcollsurg.2015.12.047 [DOI] [PubMed] [Google Scholar]
- 20.Pozdeyev N, Gay LM, Sokol ES, et al. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin Cancer Res 2018;24:3059–68. 10.1158/1078-0432.CCR-18-0373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xing M, Liu R, Liu X, et al. Braf V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol 2014;32:2718–26. 10.1200/JCO.2014.55.5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trovisco V, Soares P, Preto A, et al. Type and prevalence of BRAF mutations are closely associated with papillary thyroid carcinoma histotype and patients' age but not with tumour aggressiveness. Virchows Arch 2005;446:589–95. 10.1007/s00428-005-1236-0 [DOI] [PubMed] [Google Scholar]
- 23.Pagan JK, Arnold J, Hanchard KJ, et al. A novel corepressor, BCoR-L1, represses transcription through an interaction with CtBP. J Biol Chem 2007;282:15248–57. 10.1074/jbc.M700246200 [DOI] [PubMed] [Google Scholar]
- 24.Canel M, Serrels A, Frame MC, et al. E-cadherin-integrin crosstalk in cancer invasion and metastasis. J Cell Sci 2013;126:393–401. 10.1242/jcs.100115 [DOI] [PubMed] [Google Scholar]
- 25.Xu B, Tuttle RM, Sabra MM, et al. Primary thyroid carcinoma with low-risk histology and distant metastases: clinicopathologic and molecular characteristics. Thyroid 2017;27:632–40. 10.1089/thy.2016.0582 [DOI] [PMC free article] [PubMed] [Google Scholar]


