Abstract
Objective
To systematically review the literature exploring the associations between multimorbidity (the presence of two or more long-term conditions (LTCs)) and adverse clinical outcomes in patients with chronic kidney disease (CKD).
Design
Systematic review and meta-analysis.
Data sources
MEDLINE, EMBASE, CINAHL, Cochrane Library and SCOPUS (1946–2019). The main search terms were ‘Chronic Kidney Failure’ and ‘Multimorbid*’.
Eligibility criteria
Observational studies of adults over the age of 18 with CKD stages 3–5, that is, estimated glomerular filtration rate less than 60 mL/min/1.73 m2. The exposure was multimorbidity quantified by measures and the outcomes were all-cause mortality, renal progression, hospitalisation and cardiovascular events. We did not consider CKD as a comorbid LTC.
Data extraction and synthesis
Newcastle-Ottawa Scale for quality appraisal and risk of bias assessment and fixed effects meta-analysis for data synthesis.
Results
Of 1852 papers identified, 26 met the inclusion criteria. 21 papers involved patients with advanced CKD and no studies were from low or middle-income countries. All-cause mortality was an outcome in all studies. Patients with multimorbidity were at higher risk of mortality compared with patients without multimorbidity (total risk ratio 2.28 (95% CI 1.81 to 2.88)). The risk of mortality was higher with increasing multimorbidity (total HR 1.31 (95% CI 1.27 to 1.36)) and both concordant and discordant LTCs were associated with heightened risk. Multimorbidity was associated with renal progression in four studies, hospitalisation in five studies and cardiovascular events in two studies.
Limitations
Meta-analysis could only include 10 of 26 papers as the methodologies of studies were heterogeneous.
Conclusions
There are associations between multimorbidity and adverse clinical outcomes in patients with CKD. However, most data relate to mortality risk in patients with advanced CKD. There is limited evidence regarding patients with mild to moderate CKD, outcomes such as cardiovascular events, types of LTCs and regarding patients from low or middle-income countries.
PROSPERO registration number
CRD42019147424.
Keywords: chronic renal failure, dialysis, diabetic nephropathy & vascular disease, ischaemic heart disease, end stage renal failure
Strengths and limitations of this study.
This review is the first to synthesise the existing evidence on multimorbidity in patients with chronic kidney disease and it included a range of settings.
The outcomes of interest were chosen by researchers and these do not include all outcomes that are important to patients, for example, quality of life.
Two authors independently performed paper selection, data extraction and quality appraisal.
Meta-analysis was performed, but only included selected papers because of methodological heterogeneity of papers.
Introduction
Multimorbidity is the presence of two or more long-term conditions (LTC).1 In a Scottish study of 1.8 million patients, it was found to affect 23% of the whole population and in particular those from areas of lower socioeconomic status.2 It is a problem for individual patients because it is associated with complex treatment regimens that result in a high burden of treatment and reduced quality of life.3 For clinicians and health services, caring for these individuals represents a huge workload and equates to approximately two-thirds of healthcare spending.4 The current disease-orientated approaches of guidelines and healthcare are inadequate for patients with multiple LTCs and complex needs.5
Multimorbidity is more common in patients with chronic kidney disease (CKD) than any other LTC: for example, among 2.5 million Canadians, patients with CKD had more comorbid LTCs than patients with lung disease (mean 4.2 LTCs vs 2.8).6 The prevalence of CKD is around 12%7 and as this rises globally, the adverse effects of CKD and multimorbidity on quality of life are increasing.8 The leading cause of death in patients with CKD is cardiovascular disease and although this is partly related to risk factors common to both conditions, low estimated glomerular filtration rate (eGFR) and proteinuria are predictors of cardiovascular mortality.9 10 The higher cardiovascular risk observed among patients with CKD is independent of traditional atherosclerotic risk factors such as hypertension and dyslipidaemia, but the reasons for this and the influence of multimorbidity on CKD are incompletely understood. CKD and multimorbidity therefore occur together frequently and there are a number of issues common to both problems such as polypharmacy and significant treatment burden.11
We undertook this systematic review to establish the current evidence concerning associations between multimorbidity and adverse clinical outcomes in patients with CKD.
Materials and methods
The Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols guidelines were followed12 and this review was registered with the International Prospective Register of Systematic Reviews.
Literature search
A comprehensive search strategy identified studies of patients with CKD that investigated the associations between multimorbidity and adverse clinical outcomes (see online supplementary file 1 for search terms). We included observational studies; in particular those using electronic healthcare records. There was no restriction on sample size. The databases searched included studies from 1946 to 2019. The search was limited to papers published in English. Databases searched were MEDLINE (OVID interface), EMBASE (OVID interface), CINAHL Complete (EBSCO interface), the Cochrane Library (OVID interface) and SCOPUS. Selected Medical Subject Headings were combined with keywords relating to multimorbidity and CKD to create a search strategy which was produced for use in MEDLINE and amended for use in the other databases, using controlled vocabulary, Boolean operators and search symbols. The search was carried out to include literature published up to 29 August 2019. The results were supplemented with searches of reference lists of included studies. Search data were stored and merged using Endnote X9 (Clarivate Analytics, Philadelphia, USA) and papers were shared and assessed using DistillerSR (Evidence Partners, Ottawa, Canada).
bmjopen-2020-038401supp001.pdf (2.9MB, pdf)
Inclusion criteria
We included empirical quantitative studies that contained data on associations between multimorbidity measures and all-cause mortality or additional outcomes in adults with CKD. We accepted any multimorbidity measure, which included simple counts of LTCs and comorbidity scoring systems. We did not consider CKD as a comorbid LTC because all of the patients in our papers had CKD. Additional outcomes were hospitalisation, cardiovascular events, cardiovascular deaths, heart failure hospitalisations and renal progression (40% reduction in eGFR, doubling of serum creatinine or initiation of renal replacement therapy (RRT)). Studies that analysed the relationship between a multimorbidity measure and any of our outcomes of interest were included in adults over the age of 18 with CKD stages 3–5, that is, eGFR less than 60 mL/min/1.73 m2 including those requiring RRT, that is, haemodialysis (HD), peritoneal dialysis (PD) or renal transplantation.
Exclusion criteria
Review articles, drug intervention studies, qualitative studies, case reports and conference abstracts were excluded. Studies with children or adolescents aged 18 or under, animals and individuals without CKD were excluded.
The study selection process was conducted by two reviewers (MS, AR). Title screening was followed by abstract and full paper review, where necessary. Any inter-reviewer disagreements were resolved by a third reviewer (PM).
Data extraction
As recommended by the Cochrane Handbook,13 data were extracted in a Population, Exposure, Comparator, Outcomes approach:
Population: We extracted data on the characteristics of study populations: country, sample size, follow-up time and setting, that is, CKD, HD, PD, renal transplant and conservative care.
Exposure: We extracted the multimorbidity measure used in each study and whether LTCs were categorised into different types for analysis.
Comparator: We extracted the details provided of comparator groups, that is, patients with CKD with less than two LTCs. We did not count CKD as an LTC.
Outcomes: We extracted details of the statistical analyses employed to evaluate the relationship between multimorbidity measure and outcomes. Risks were expressed as effect sizes with 95% confidence intervals (CIs), where available.
Data synthesis and analysis
Results were presented in a narrative format. Where possible, fixed effects meta-analysis was performed for the primary outcome, all-cause mortality. Previous systematic reviews including patients from the general population have demonstrated consistent associations between multimorbidity and mortality.14 We assumed the direction of effect of multimorbidity on mortality would be consistent across our studies, barring sampling errors and differences in sample size, and so we applied fixed effects models. However, random effects models were also performed as sensitivity analysis, as this approach would be more helpful if the participants in the included studies were inherently different. The generic inverse variance method was used where multimorbidity was expressed as a continuous variable and the Mantel-Haenszel method was used where multimorbidity was expressed as a categorical variable. Quantification of statistical heterogeneity was assessed by means of I2, which shows the percentage of total variation across studies due to heterogeneity.13 These analyses were carried out using RevMan V.5.3 (Cochrane Collaboration, Copenhagen, Denmark). Meta-analysis was limited by heterogeneous methodologies: variable multimorbidity measures, use of effect sizes (hazard ratios (HRs), risk ratios (RR), Kaplan-Meier curves) and the use of multimorbidity as a continuous and categorical variable. We therefore performed meta-analysis where several studies used similar methodologies. Data on numbers of deceased patients were not available for all studies and so we contacted study authors for their primary data. For meta-analysis and where necessary and possible, we calculated RRs for studies, comparing patients with multimorbidity to those without multimorbidity. HRs could not be calculated as there were no individual time-to-event data.
Quality appraisal
Two researchers conducted quality appraisal independently (MS, AR). Studies were assessed using an adapted Newcastle-Ottawa Scale (NOS) for quality assessment, as informed by the Cochrane Handbook13 (see online supplementary file 2). Studies were not excluded based on quality appraisal.
Patient and public involvement
No patients were involved.
Results
Search results
Figure 1 demonstrates the literature search flow. After the removal of duplicate papers, 1852 papers were identified. A total of 1756 papers were excluded as they were not relevant and so 96 full papers were screened and 26 papers met our eligibility criteria and were included in the review.15–40
Figure 1.
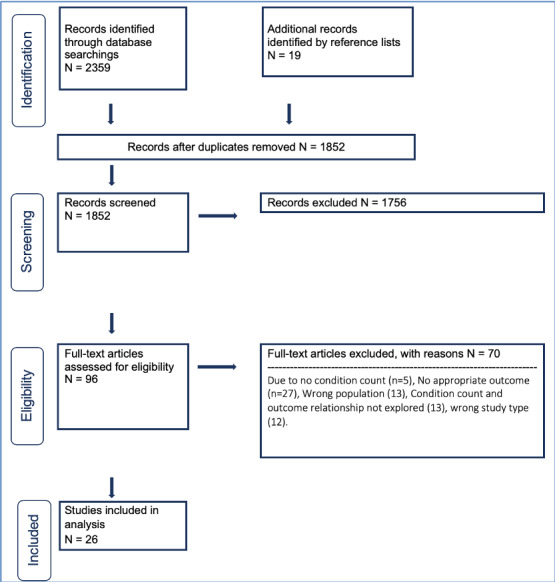
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Study characteristics
Table 1 lists the characteristics of the 26 included studies. The studies were published between 1995 and 2019 and all used a cohort design. The size of populations was between 69 and 821 334. Fourteen studies examined the subjects predominantly on dialysis15 17–22 25 27 30 33 35 40; five included patients with CKD stages 3–516 24 34 36 including two with mild CKD24 34; two involved patients with CKD stage 5 including those not on RRT or conservative care29 31; two included those receiving conservative care23 38; three included renal transplant recipients.26 32 39
Table 1.
Study characteristics
| Reference | Country | Setting | Sample size | Average follow-up (months) |
Outcome(s) | |
| Mortality | Others | |||||
| Dialysis | ||||||
| Beddhu et al15 | USA | HD/PD | 268 | 13.1 | ✔ | Hospitalisation |
| Chae et al17 | South Korea | HD | 456 | 40.6 | ✔ | |
| Chandna et al19 | UK | HD/PD | 292 | 63 | ✔ | Hospitalisation |
| Chandna et al18 | UK | CC/RRT | 844 | 58.7* | ✔ | |
| Davies et al21 | UK | PD | 97 | 30 | ✔ | |
| Davies et al20 | UK | PD | 303 | 72.0* | ✔ | |
| Di Iorio et al 22 | Italy | HD | 515 | 15 | ✔ | |
| Fried et al 25 | USA | PD | 268 | 16.9 | ✔ | |
| Hemmelgarn et al 27 | Canada | HD/PD | 237 | 26.3 | ✔ | |
| Park et al 30 | South Korea | HD | 24 738 | 47.7 | ✔ | |
| Rattanasompattikul et al33 | USA | HD | 893 | 72 | ✔ | |
| Shum et al35 | China | PD/CC | 157 | 23.5 | ✔ | Hospitalisation |
| van Manen et al37 | Netherlands | HD/PD | 589 | NK | ✔ | |
| Wu et al40 | Taiwan | HD/PD | 79 645 | NK | ✔ | |
| Non-RRT CKD | ||||||
| Bowling et al16 | USA | CKD 3–5 | 821 334 | 81.6 | ✔ | |
| Fraser et al24 | UK | CKD 3 | 1741 | 43.2 | ✔ | |
| Lee et al28 | Taiwan | CKD 3–5 | 1463 | 76.7 | ✔ | Renal progression |
| Lhotta et al29 | Austria | CKD 5 | 75 | 48 | ✔ | |
| Ritchie et al34 | USA | CKD/heart failure | 1974 | 32.6 | ✔ | Hospitalisation, HF hospitalisation, CV death |
| Tonelli et al36 | Canada | CKD 3–5 | 530 771 | 48 | ✔ | Hospitalisation, myocardial infarction |
| Transplant | ||||||
| Pérez Fernández et al31 | USA | Tx assessment | 2086 | NK | ✔ | |
| Grosso et al26 | Italy | Tx recipients | 223 | NK | ✔ | Renal progression |
| Pieloch et al32 | USA | Tx recipients | 100 261 | 36 | ✔ | Renal progression |
| Wu et al39 | USA | Tx recipients | 715 | 40.2 | ✔ | Renal progression |
| Conservative care | ||||||
| Ellam et al23 | UK | CC | 69 | 21* | ✔ | |
| Wong et al38 | UK | CC | 73 | 23.4* | ✔ | |
*Median survival.
CC, conservative care; CKD, chronic kidney disease; CV, cardiovascular; HD, haemodialysis; HF, heart failure; NK, not known; PD, peritoneal dialysis; RRT, renal replacement therapy; Tx, transplant.
Table 2 shows the number of studies using each multimorbidity measure and how the corresponding effect sizes were presented: as a categorical or a continuous variable. In addition to these, three studies examined more than one multimorbidity measure: comparing how effectively each measure predicted outcomes.22 27 37 Ten studies used the Charlson Comorbidity Index (CCI) or a modification of this scale (modified CCI).15 17 25 26 30 31 33 35 39 40 Seven studies used the number of LTCs, that is, condition count.16 23 24 28 29 36 38 Two studies used the Stoke comorbidity grade, which uses condition count to divide patients into low, intermediate and high grades.20 21 Two studies used the comorbidity severity score.18 19 One study compared those with CKD, diabetes and heart failure to those with just CKD and heart failure.34 One study used the Kidney Transplant Morbidity Index.32
Table 2.
Studies using each multimorbidity measure
| Variable type | Multimorbidity measure: number of studies | ||||
| CCI | Condition count | CSS | KTMI | Heart failure and CKD versus heart failure, CKD and diabetes |
|
| Categorical | 6 | 4 | 1 | 1 | 1 |
| Continuous | 6 | 4 | 1 | 0 | 0 |
CCI, Charlson Comorbidity Index; CKD, chronic kidney disease; CSS, comorbidity severity score; KTMI, Kidney Transplant Morbidity Index.
All studies reported the effect of multimorbidity on all-cause mortality. Five studies reported the effect of multimorbidity on hospitalisation15 19 34 35 35 36 and four on renal progression.26 28 32 39 One study reported the effect of multimorbidity on heart failure hospitalisation and cardiovascular death34 and one study reported the effect of multimorbidity on myocardial infarction.36 Twelve studies expressed effect sizes using multimorbidity as a categorical variable,16–18 24 26 28 32–34 36 39 40 nine as a continuous variable15 19–21 25 29 30 35 38 and one as both.31 One study gave a narrative comparison of groups23 and two used Kaplan-Meier curves.27 37 Two studies categorised LTCs into types: both used concordant and discordant as types and one also specified mental health and chronic pain LTCs.16 36
Main findings
The results of the included studies were summarised in online supplementary file 3. Some papers did not provide adjusted HRs. To make it easier to compare the studies, we therefore quoted unadjusted HRs. Where multimorbidity was used as a categorical variable, 12 of 13 studies found that patients with multimorbidity had higher rates of mortality than patients without multimorbidity. In the one study that did not detect a difference, Lee et al’s primary outcome was renal progression.28 For all-cause mortality, the authors provided event rates and Kaplan-Meier curves but there were no HRs with adjustments for confounding variables.
Where multimorbidity was used as a continuous variable, 10 of 11 studies found that with each increase in multimorbidity measure, all-cause mortality was higher. In the one study to not detect a difference, Ellam et al was a study of just 69 conservatively managed patients.23
Of the four studies that reported renal progression, three were in renal transplant recipients.26 31 32 All four studies demonstrated higher rates of renal progression in patients with multimorbidity (HRs from each study 2.97 (95% CI 1.53 to 5.76), 2.44 (95% CI 1.19 to 5.02), 3.11 (95% CI 2.55 to 3.80), 1.42 (95% CI 1.02 to 1.97)). Renal progression was defined by graft loss or RRT initiation and one paper reported significant annual reductions in eGFR by increasing number of LTCs.28 Five studies reported rates of hospitalisation and all of these identified an association between multimorbidity and hospitalisation.15 19 34–36
One paper reported rates of heart failure hospitalisation and cardiovascular death34: patients with multimorbidity had higher rates of both outcomes than patients without multimorbidity. One paper reported higher rates of myocardial infarction in patients with multimorbidity.36
Two papers described the influence of concordant and discordant LTCs on adverse outcomes.16 36 These papers found that both types of LTC were associated with higher rates of mortality. One paper found that the rates of outcomes were higher in patients with at least one discordant LTC compared with patients with only concordant LTCs.16 No association was identified between mental health and chronic pain LTCs and myocardial infarction.36
Meta-analysis
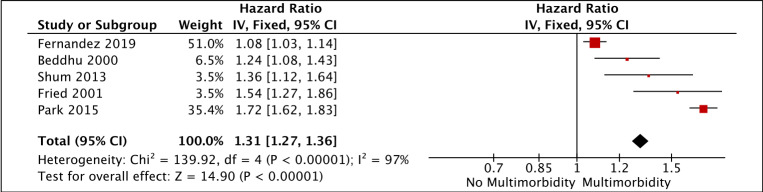
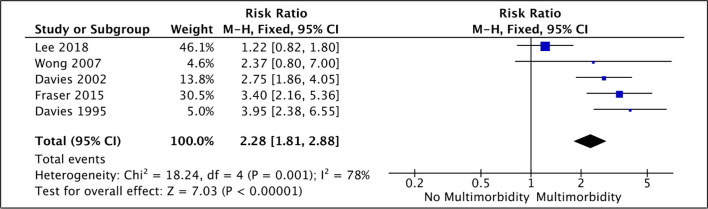
Data synthesis was problematic because each study reported different effect sizes for different categorical groups. We therefore performed meta-analysis for all-cause mortality where several studies used comparable methodologies. Figure 2 included studies that used CCI as a continuous variable, demonstrating that with each increase in CCI, the risk of mortality was higher (total HR 1.31 (95% CI 1.27 to 1.36)). All studies included in this meta-analysis had HRs available. Figure 3 included studies that used condition count as a categorical variable: demonstrating that patients with multimorbidity were at higher risk of mortality compared with patients without multimorbidity (total RR 2.28 (95% CI 1.81 to 2.88)). RR was used here because time-to-event data were not available for all these studies and so HRs could not be calculated. There was considerable statistical heterogeneity in the studies included in each meta-analysis (I2 97% in figure 2 and 78% in figure 3). Subgroup analyses were not possible such as for patients with mild to moderate CKD because there were inadequate studies. Where random effects models were fitted, there remained significant associations between multimorbidity and all-cause mortality (online supplementary file 4). For studies that used CCI as a continuous variable, the risk of mortality was higher for each increase in CCI (total HR 1.37 (95% CI 1.07 to 1.75)). For studies that used condition count as a categorical variable, patients with multimorbidity were at higher risk of mortality compared with patients without multimorbidity (total RR 2.53 (95% CI 1.57 to 4.07)).
Figure 2.
Mortality risk for each increase in Charlson Comorbidity Index (generic inverse variance method, fixed effects model).
Figure 3.
Mortality risk for patients with multimorbidity (Mantel-Haenszel method, fixed effects model).
Risk of bias
All studies selected patients with and without multimorbidity from the same cohort and used either secure medical records or structured interviews to collect data. Most studies included just one group of patients with CKD such as patients receiving HD and only three studies included patients with a true range of mild to severe CKD.16 28 36 All but two studies controlled for factors such as ischaemic heart disease, age or diabetes.18 23 Only one study made a statement about subjects who were lost to follow-up.27 However, as all the studies were based on healthcare databases, it is reasonable to assume complete or near-complete follow-up. All studies followed up patients for more than 1 year, but there was variation in the average length of follow-up (from 13.1 to 81.6 months). Four studies did not specify the average follow-up time but from their survival analyses, it was clear that patients were followed up for at least 1 year.26 31 37 40
The NOS score evaluation of each study was between five and seven stars (see online supplementary file 5). The two studies that did not control for confounding factors were ‘poor’ quality as per the Agency for Healthcare Research and Quality standards.18 23 41 The remainder were ‘good’ quality.15–17 19–22 24–40
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis to synthesise the existing evidence on the associations between multimorbidity and outcomes specific to patients with CKD. It is increasingly recognised that multimorbidity and the management of patients with disease clusters are challenging problems.42 The medical profession has been given a mandate to improve the care of patients affected by multimorbidity and to do so, improving our understanding of the issues will be fundamental. Multimorbidity has been studied in the general population, with clear associations reported between it and high rates of mortality.43 It is time for researchers to build a body of evidence about patients with kidney disease. Our review demonstrates that for patients with CKD, multimorbidity is associated with high rates of mortality, and the risk is higher with increasing numbers of LTCs. Unfortunately, the literature provides little detail beyond this association. Of the papers in the review, only two categorised LTCs and studied whether the type of LTCs influenced outcomes. Tonelli et al and Bowling et al found that concordant LTCs such as diabetes were associated with high rates of mortality, but so were discordant or unrelated LTCs like cancer and depression.16 36 Bowling et al found that the presence of one or more discordant LTCs conferred higher risk compared with patients with only concordant LTCs. This suggests that there are groups of patients in whom it is the number and the type of LTCs that put them at elevated risk. Further research is needed into what patterns or clusters of disease exist to help clinicians understand the risks faced by patients with CKD and multimorbidity.
Patients require clinicians to help with their overall health and quality of life, not just the status of individual LTCs. As seen in the Standardized Outcomes in Nephrology-Hemodialysis initiative, patients usually wish to understand the risks they face. However, there is often a mismatch between the outcomes regarded as important by patients to those emphasised in clinical guidelines.44 45 It is therefore imperative that we consider patient-oriented outcomes when studying multimorbidity and ensure that research leads to improvements in care for patients. A limitation of our review is that we did not summarise outcomes prioritised by patients. The merit in investigating multimorbidity in patients with CKD will be that patients and clinicians will have an improved understanding of the risks they face. They will therefore be able to prioritise particular interventions such as cardiovascular risk factor modification and vascular access creation.
Despite the methodological and clinical heterogeneity of the studies in our review, the findings are consistent with existing literature.11 We have confirmed associations between multimorbidity and adverse clinical outcomes in RRT and non-RRT settings, and in a range of countries. Twenty-one of 26 studies included patients with advanced CKD including those on RRT. However, it should be noted that there was no information available from low or middle-income countries. Mild to moderate CKD was also under-represented, despite this constituting 99% of the patients with CKD.46 Multimorbidity in patients with CKD from low and middle-income countries and in those with mild to moderate CKD should therefore be targets for future research. Only two studies assessed the influence of multimorbidity on cardiovascular outcomes.34 36 Cardiovascular morbidity and mortality is the most significant risk for patients with CKD and many of the LTCs that occur in patients with CKD are risk factors for cardiovascular events.10 Further research is therefore needed to explore how multimorbidity influences cardiovascular events in patients with CKD. Of the four studies that examined the influence of multimorbidity on renal progression, all but one were in patients with renal transplants. The study in non-transplant patients identified an association between multimorbidity and renal progression.28 This risk is a significant one, particularly for the patients who develop the need for RRT. Many patient cohorts around the world have ample follow-up data and so the influence of multimorbidity on renal progression in non-transplant cohorts should be studied in greater detail.
The studies included in our review are heterogeneous. Clinical heterogeneity is evident in the range of populations studied: stage 3 CKD, HD, PD, transplant and conservative care. There are high levels of methodological and statistical heterogeneity. There is no consensus as to which multimorbidity measure should be used, and which measure is the most effective at predicting adverse outcomes.47 CCI was the most commonly used measure, although a number of modifications have been made for use in populations with CKD. Three studies included in this review compared different multimorbidity measures. CCI was found to effectively predict mortality risk, with other scoring systems performing comparably and none superior to the rest. Although our work demonstrates that various multimorbidity measures are associated with adverse clinical outcomes, we have not identified the best multimorbidity measure for risk prediction.
It has been recognised that there are fewer randomised controlled trials (RCTs) to assess the efficacy of interventions in patients with CKD than in other medical specialties and that patients with CKD are often excluded from RCTs.48 49 Furthermore, patients with advanced CKD that are included in RCTs are not representative of the wider population of those with CKD.50 Similar observations have been made in other fields, whereby subjects with multimorbidity are under-represented in trials of novel interventions.51 Therefore, to improve outcomes for patients with CKD, both epidemiological studies and RCTs need to account for the range of multimorbidity in patients with CKD. A strength of our review is that it brings together information about the effects of multimorbidity in patients with CKD from various settings to create a comprehensive picture of the effects on different outcomes. Although the studies are challenging to summarise given the heterogeneity, the data are ample and clinically acceptable and therefore likely to be correct. Meta-analysis was performed with data from only 10 studies. The data from 16 studies, including those with large sample sizes, therefore did not contribute to full data analysis. If a uniform multimorbidity measure were agreed and established in guidelines, the comparability and synthesis of data in future would be improved. The evaluation of the effects of types of LTCs on outcomes was limited because only two studies examined this issue. A key focus of future research should therefore be what patterns of multimorbidity or disease clusters exist in groups of patients with CKD.
In conclusion, this review provides evidence of associations between multimorbidity and heightened risk of adverse clinical outcomes in patients with CKD. Our findings emphasise the need for further research into the details of how multimorbidity influences different outcomes. In particular, evidence gaps exist for patients with mild to moderate CKD, for outcomes other than mortality such as renal progression and cardiovascular events, for patients with CKD from low and middle-income countries and for the patterns of multimorbidity that contribute to heightened risk.
Supplementary Material
Acknowledgments
We thank Paul Cannon of the University of Glasgow Library for assistance with the literature search.
Footnotes
Twitter: @sullivanmk8, @alastairrankin1?lang=en, @BhauteshJani, @FrancesMair, @drpaddymark
Contributors: MS, AR, BJ, FM and PM contributed to conceptualisation, appraisal of results, writing (review and editing) and manuscript approval. MS, AR and PM performed data analysis. MS and AR performed data extraction. MS prepared the original manuscript draft.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: The results presented in this paper have not been published previously in whole or part.
Competing interests: PM reports personal fees and non-financial support from Vifor, personal fees from AstraZeneca, grants from Boehringer Ingelheim, personal fees and non-financial support from Pharmacosmos, personal fees from Janssen, personal fees from Novartis, personal fees from Pfizer, personal fees from Bristol Myers Squibb, personal fees and non-financial support from Napp, outside the submitted work.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data sharing is not applicable as no data sets were generated and/or analysed for this study. All relevant data are included in the article or uploaded as supplementary information.
References
- 1.NICE Multimorbidity: clinical assessment and management: National Institute for health and care excellence, 2017: 23. [Google Scholar]
- 2.Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37–43. 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 3.Harris MF, Dennis S, Pillay M. Multimorbidity: negotiating priorities and making progress. Aust Fam Physician 2013;42:850–4. [PubMed] [Google Scholar]
- 4.Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition--multimorbidity. JAMA 2012;307:2493–4. 10.1001/jama.2012.5265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mair FS, May CR. Thinking about the burden of treatment. BMJ 2014;349:g6680. 10.1136/bmj.g6680 [DOI] [PubMed] [Google Scholar]
- 6.Tonelli M, Wiebe N, Manns BJ, et al. Comparison of the complexity of patients seen by different medical subspecialists in a universal health care system. JAMA Netw Open 2018;1:e184852. 10.1001/jamanetworkopen.2018.4852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coresh J. Update on the burden of CKD. J Am Soc Nephrol 2017;28:1020–2. 10.1681/ASN.2016121374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kassebaum NJ, Arora M, Barber RM, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the global burden of disease study 2015. The Lancet 2016;388:1603–58. 10.1016/S0140-6736(16)31460-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011;79:1341–52. 10.1038/ki.2010.536 [DOI] [PubMed] [Google Scholar]
- 10.Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 2015;3:514–25. 10.1016/S2213-8587(15)00040-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraser SDS, Taal MW. Multimorbidity in people with chronic kidney disease: implications for outcomes and treatment. Curr Opin Nephrol Hypertens 2016;25:465–72. 10.1097/MNH.0000000000000270 [DOI] [PubMed] [Google Scholar]
- 12.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;349:g7647 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 13.Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for systematic reviews of interventions. Cochrane Database Syst Rev 2019;10:Ed000142. 10.1002/14651858.ED000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nunes BP, Flores TR, Mielke GI, et al. Multimorbidity and mortality in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr 2016;67:130–8. 10.1016/j.archger.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 15.Beddhu S, Bruns FJ, Saul M, et al. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med 2000;108:609–13. 10.1016/s0002-9343(00)00371-5 [DOI] [PubMed] [Google Scholar]
- 16.Bowling CB, Plantinga L, Phillips LS, et al. Association of multimorbidity with mortality and healthcare utilization in chronic kidney disease. J Am Geriatr Soc 2017;65:704–11. 10.1111/jgs.14662 [DOI] [PubMed] [Google Scholar]
- 17.Chae J-W, Song CS, Kim H, et al. Prediction of mortality in patients undergoing maintenance hemodialysis by Charlson comorbidity index using ICD-10 database. Nephron Clin Pract 2011;117:c379–84. 10.1159/000321525 [DOI] [PubMed] [Google Scholar]
- 18.Chandna SM, Da Silva-Gane M, Marshall C, et al. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant 2011;26:1608–14. 10.1093/ndt/gfq630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandna SM, Schulz J, Lawrence C, et al. Is there a rationale for rationing chronic dialysis? a hospital based cohort study of factors affecting survival and morbidity. BMJ 1999;318:217–23. 10.1136/bmj.318.7178.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies SJ, Phillips L, Naish PF, et al. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant 2002;17:1085–92. 10.1093/ndt/17.6.1085 [DOI] [PubMed] [Google Scholar]
- 21.Davies SJ, Russell L, Bryan J, et al. Comorbidity, urea kinetics, and appetite in continuous ambulatory peritoneal dialysis patients: their interrelationship and prediction of survival. Am J Kidney Dis 1995;26:353–61. 10.1016/0272-6386(95)90657-6 [DOI] [PubMed] [Google Scholar]
- 22.Di Iorio B, Cillo N, Cirillo M, et al. Charlson comorbidity index is a predictor of outcomes in incident hemodialysis patients and correlates with phase angle and hospitalization. Int J Artif Organs 2004;27:330–6. 10.1177/039139880402700409 [DOI] [PubMed] [Google Scholar]
- 23.Ellam T, El-Kossi M, Prasanth KC, et al. Conservatively managed patients with stage 5 chronic kidney disease--outcomes from a single center experience. QJM 2009;102:547–54. 10.1093/qjmed/hcp068 [DOI] [PubMed] [Google Scholar]
- 24.Fraser SDS, Roderick PJ, May CR, et al. The burden of comorbidity in people with chronic kidney disease stage 3: a cohort study. BMC Nephrol 2015;16:193. 10.1186/s12882-015-0189-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fried L, Bernardini J, Piraino B. Charlson comorbidity index as a predictor of outcomes in incident peritoneal dialysis patients. Am J Kidney Dis 2001;37:337–42. 10.1053/ajkd.2001.21300 [DOI] [PubMed] [Google Scholar]
- 26.Grosso G, Corona D, Mistretta A, et al. Predictive value of the Charlson comorbidity index in kidney transplantation. Transplant Proc 2012;44:1859–63. 10.1016/j.transproceed.2012.06.042 [DOI] [PubMed] [Google Scholar]
- 27.Hemmelgarn BR, Manns BJ, Quan H, et al. Adapting the Charlson comorbidity index for use in patients with ESRD. Am J Kidney Dis 2003;42:125–32. 10.1016/s0272-6386(03)00415-3 [DOI] [PubMed] [Google Scholar]
- 28.Lee W-C, Lee Y-T, Li L-C, et al. The number of comorbidities predicts renal outcomes in patients with stage 3–5 chronic kidney disease. J Clin Med 2018;7:493 10.3390/jcm7120493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lhotta K, Zoebl M, Mayer G, et al. Late referral defined by renal function: association with morbidity and mortality. J Nephrol 2003;16:855–61. [PubMed] [Google Scholar]
- 30.Park JY, Kim M-H, Han SS, et al. Recalibration and validation of the Charlson comorbidity index in Korean incident hemodialysis patients. PLoS One 2015;10:e0127240. 10.1371/journal.pone.0127240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pérez Fernández M, Martínez Miguel P, Ying H, et al. Comorbidity, frailty, and Waitlist mortality among kidney transplant candidates of all ages. Am J Nephrol 2019;49:103–10. 10.1159/000496061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pieloch D, Dombrovskiy V, Osband AJ, et al. The kidney transplant morbidity index (KTMI): a simple prognostic tool to help determine outcome risk in kidney transplant candidates. Prog Transplant 2015;25:70–6. 10.7182/pit2015462 [DOI] [PubMed] [Google Scholar]
- 33.Rattanasompattikul M, Feroze U, Molnar MZ, et al. Charlson comorbidity score is a strong predictor of mortality in hemodialysis patients. Int Urol Nephrol 2012;44:1813–23. 10.1007/s11255-011-0085-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ritchie C, Ekundayo OJ, Muchimba M, et al. Effects of diabetes mellitus in patients with heart failure and chronic kidney disease: a propensity-matched study of multimorbidity in chronic heart failure. Int J Cardiol 2009;134:330–5. 10.1016/j.ijcard.2008.12.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shum CK, Tam KF, Chak WL, et al. Outcomes in older adults with stage 5 chronic kidney disease: comparison of peritoneal dialysis and conservative management. J Gerontol A Biol Sci Med Sci 2014;69:308–14. 10.1093/gerona/glt098 [DOI] [PubMed] [Google Scholar]
- 36.Tonelli M, Wiebe N, Guthrie B, et al. Comorbidity as a driver of adverse outcomes in people with chronic kidney disease. Kidney Int 2015;88:859–66. 10.1038/ki.2015.228 [DOI] [PubMed] [Google Scholar]
- 37.van Manen JG, Korevaar JC, Dekker FW, et al. How to adjust for comorbidity in survival studies in ESRD patients: a comparison of different indices. Am J Kidney Dis 2002;40:82–9. 10.1053/ajkd.2002.33916 [DOI] [PubMed] [Google Scholar]
- 38.Wong CF, McCarthy M, Howse MLP, et al. Factors affecting survival in advanced chronic kidney disease patients who choose not to receive dialysis. Ren Fail 2007;29:653–9. 10.1080/08860220701459634 [DOI] [PubMed] [Google Scholar]
- 39.Wu C, Evans I, Joseph R, et al. Comorbid conditions in kidney transplantation: association with graft and patient survival. J Am Soc Nephrol 2005;16:3437–44. 10.1681/ASN.2005040439 [DOI] [PubMed] [Google Scholar]
- 40.Wu P-H, Lin Y-T, Lee T-C, et al. Predicting mortality of incident dialysis patients in Taiwan--a longitudinal population-based study. PLoS One 2013;8:e61930. 10.1371/journal.pone.0061930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.AHRQ Customer Service Standards Agency for healthcare research and quality. Rockville, MD, 2018. https://www.ahrq.gov/contact/standards.html [Google Scholar]
- 42.Whitty CJM, MacEwen C, Goddard A, et al. Rising to the challenge of multimorbidity. BMJ 2020;368:l6964. 10.1136/bmj.l6964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jani BD, Hanlon P, Nicholl BI, et al. Relationship between multimorbidity, demographic factors and mortality: findings from the UK Biobank cohort. BMC Med 2019;17:74. 10.1186/s12916-019-1305-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evangelidis N, Tong A, Manns B, et al. Developing a set of core outcomes for trials in hemodialysis: an international Delphi survey. Am J Kidney Dis 2017;70:464–75. 10.1053/j.ajkd.2016.11.029 [DOI] [PubMed] [Google Scholar]
- 45.Tong A, Manns B, Hemmelgarn B, et al. Establishing core outcome domains in hemodialysis: report of the standardized outcomes in Nephrology-Hemodialysis (SONG-HD) consensus workshop. Am J Kidney Dis 2017;69:97–107. 10.1053/j.ajkd.2016.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–47. 10.1001/jama.298.17.2038 [DOI] [PubMed] [Google Scholar]
- 47.Stirland LE, González-Saavedra L, Mullin DS, et al. "Measuring multimorbidity beyond counting diseases: systematic review of community and population studies and guide to index choice." 2020;368:m160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Konstantinidis I, Nadkarni GN, Yacoub R, et al. Representation of patients with kidney disease in trials of cardiovascular interventions: an updated systematic review. JAMA Intern Med 2016;176:121–4. 10.1001/jamainternmed.2015.6102 [DOI] [PubMed] [Google Scholar]
- 49.Smyth B, Haber A, Trongtrakul K, et al. Representativeness of randomized clinical trial cohorts in end-stage kidney disease. JAMA Intern Med 2019;179:1316 10.1001/jamainternmed.2019.1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strippoli GFM, Craig JC, Schena FP. The number, quality, and coverage of randomized controlled trials in nephrology. J Am Soc Nephrol 2004;15:411–9. 10.1097/01.ASN.0000100125.21491.46 [DOI] [PubMed] [Google Scholar]
- 51.Hanlon P, Hannigan L, Rodriguez-Perez J, et al. Representation of people with comorbidity and multimorbidity in clinical trials of novel drug therapies: an individual-level participant data analysis. BMC Med 2019;17:201. 10.1186/s12916-019-1427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-038401supp001.pdf (2.9MB, pdf)