Abstract
The purpose of this study was to investigate age-related differences in Achilles tendon loading during gait. Fourteen young (7F/7M, 26 ± 5 years) and older (7F/7M, 67 ± 5 years) adults without current neurological or orthopaedic impairment participated. Shear wave tensiometry was used to measure tendon stress by tracking Achilles tendon wave speed. The wave speed-stress relationship was calibrated using simultaneous tensiometer and force plate measures during a standing sway task. Tendon stress was computed from the force plate measures using subject-specific ultrasound measures of tendon moment arm and cross-sectional area. All subjects exhibited a highly linear relationship between wave speed squared and tendon stress (mean R2>0.9), with no significant age-group differences in tensiometer calibration parameters. Tendon wave speed was monitored during treadmill walking at four speeds (0.75, 1.00, 1.25, and 1.50 m/s) and used to compute the stress transmitted by the tendon. Relative to young adults, older adults exhibited 22% lower peak tendon wave speeds. Peak tendon stress during push-off in older adults (24.8 MPa) was 32% less than in the young adults (36.7 MPa) (p = 0.01). There was a moderate enhancement (+11%) in peak tendon stress across both groups when increasing speed from 0.75 to 1.50 m/s (main effect of speed, p = 0.01). Peak tendon loading during late swing did not differ between age groups (mean 3.8 MPa in young and 4.2 MPa in older adults). These age-related alterations in tendon tissue loading affect the mechanobiological stimuli underlying tissue remodeling, and thereby may alter the propensity for tendon injury and disease.
Keywords: tendon stress, tendon force, aging gait, subject-specific calibration, shear wave tensiometer
1. Introduction
The triceps surae are critical for modulating speed during walking, contributing to both forward propulsion and vertical support of the body during push-off [1]. Thus, it’s not surprising that diminished walking speed with aging is associated with reduced plantarflexor output [2–7]. However, even when speed is matched, it has been shown that older adults without neurological or orthopedic impairment adopt a gait strategy that requires lower ankle plantarflexion torque [5] and power [4,8] than that in young adults. This age-related decline in plantarflexor output becomes more pronounced at faster speeds [2,8–10].
The causes and ramifications of diminishing plantarflexor output with aging are not completely understood. Ankle torque is primarily produced by the triceps surae generating forces of 3–4 times body weight at faster walking speeds [11–13]. Age-related sarcopenia can result in the loss of ~30% of triceps surae muscle volume by age 70 [14,15], which reduces the ability of the triceps surae to generate such forces [16]. While muscle size is diminished, Achilles tendon cross-sectional area is maintained or slightly enhanced with normal aging [17]. As a result, diminished muscle force could substantially reduce the stress (force per unit area) that the aging tendon experiences. Changes in internal tissue loading could have ramifications for tissue maintenance mediated by mechanobiological stimuli [18] and thereby affect propensity for injury and disease [19].
The purpose of this study was to investigate age-related differences in Achilles tendon tissue loading during treadmill walking at a range of speeds. We leveraged recent advances in shear wave tensiometry, which allows for noninvasive measurement of stress in superficial tendons based on shear wave propagation speed [20]. We tested the hypotheses that: 1) older adults would exhibit lower tendon stress during walking, and 2) age-related differences in tendon stress would increase with walking speed.
2. Methods
2.1. Subjects
Fourteen healthy young (7F, 26 ± 5 years, 1.77 ± 0.11 m, 73.96 ± 15.19 kg) and 14 healthy older (7F, 67 ± 5 years, 1.75 ± 0.06 m, 71.90 ± 12.21 kg) adults participated in this study. All subjects self-reported that they could comfortably walk on a treadmill, that they were without current (past 6 months) orthopaedic or neurological impairment, and that they had no history of Achilles tendinopathy. The study protocol was approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board. After obtaining written consent, all subjects performed a 10 m walk test to assess preferred walking speed, where the middle 6 m was timed to allow distance for subjects to accelerate and decelerate.
2.2. Shear wave tensiometer
A shear wave tensiometer, consisting of a custom tapping device and accelerometer array in series, was secured over the right Achilles tendon with a self-adherent wrap. The piezo-actuated (PK4JQP2, Thorlabs, Newton, NJ) tapping device was driven by a 50 Hz square wave (50 cycles per second, 50% duty cycle) via an open-loop piezo controller (MDT694B, Thorlabs, Newton, NJ). The accelerometer array consisted of two miniature accelerometers (Model 352C23, PCB Piezotronics, Depew, NY) mounted 10 mm apart in a silicone mold (Mold Star 15 SLOW, Smooth-On, Macungie, PA). Accelerometery data were collected at 100 kHz and then bandpass filtered using a second-order, zero-lag Butterworth filter with 150 Hz and 5000 Hz cutoff frequencies. For each tap, we computed the time between wave arrival at the two accelerometers by finding the delay that maximized the normalized cross-correlation of the first millisecond after the tap between the first and second accelerometer signals. Sub-sample interpolation was performed using a local 3-point cosine fit of the normalized cross-correlation values [21]. Shear wave speed was calculated by dividing the distance between the accelerometers by the time delay. Performing this analysis for each tap resulted in a 50 Hz tendon wave speed signal.
2.3. Tensiometer Calibration
After walking on the treadmill for 5 minutes to warm-up the tendon [22], each subject performed a simple sway task on a force plate to facilitate subject-specific calibrations of the tensiometer. Subjects stood with their right foot on an in-ground force plate (BP400600–2000, AMTI, Watertown, MA) that sampled ground reaction forces at 1900 Hz. Subjects were asked to cyclically sway in the anteroposterior direction at 0.5 Hz guided by a metronome. Guidance was given as to sway forward and backwards while not allowing their heels or toes to leave the ground; all subjects were given time to practice the movement before collecting data. Eight motion capture cameras were used to track marker positions at 190 Hz (Eagle cameras, Cortex software, Motion Analysis, Rohnert Park, CA). Rigid body models were built by placing markers on the 1st and 5th metatarsal heads (on the shoe), medial and lateral malleoli, and medial and lateral femoral epicondyles and then tracking the segments dynamically using a 3-marker cluster (foot) or 4-marker cluster (thigh, shank) [23] (Fig 1). The pelvis was modelled and tracked using markers on the anterior and posterior superior iliac spine. Ankle plantarflexion angle and torque were calculated via standard inverse kinematics and dynamics calculations in Visual 3D (C-motion, Inc., Germantown, MD).
Figure 1:
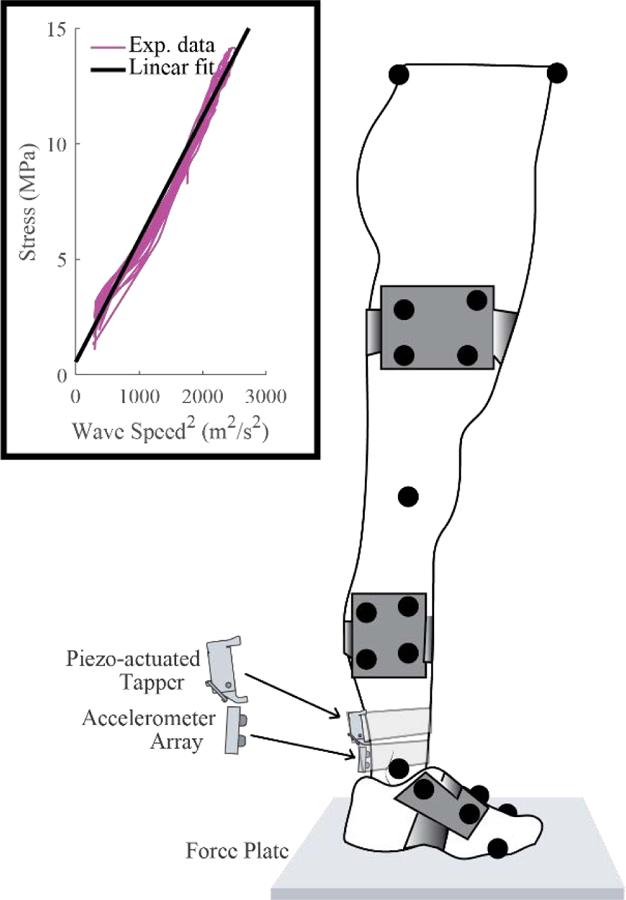
The shear wave tensiometer consisted of a piezo-actuated tapper and a two-accelerometer array, which was secured over the right Achilles tendon of each subject. To calibrate the tensiometers, subjects voluntarily swayed in the anteroposterior direction while tendon wave speed, ground reaction force, and motion data were collected. Tendon stress was estimated from ankle torque and linearly regressed against tendon wave speed squared. Inset figure shows calibration data from a representative subject.
Ultrasound imaging (SonixTOUCH Research, BK Medical, Peabody, MA) was used to assess tendon cross-sectional area. Cross-sectional images of the Achilles tendon were collected at the midpoint between the two accelerometers of the tensiometer. These images were segmented to compute the Achilles cross-sectional area using MATLAB R2019a (Mathworks, Natick, MA).
Tendon moment arm was measured using coupled motion capture and ultrasonography [13,24]. Subjects were positioned prone with their knee flexed 20° while their ankle was moved from about 10° of dorsiflexion to about 30° of plantarflexion with light but steady manual resistance to keep the tendon taught throughout the range of motion. Motion capture (190 Hz) and longitudinal ultrasound imaging of the Achilles tendon (19 Hz) were performed synchronously. A functional axis was then computed as the best-fit screw axis that described the foot motion with respect to the shank [25]. Ultrasound images were transformed into the motion capture reference frame, and the tendon line of action was defined as the midline of the tendon borders, which were manually identified in each image. The Achilles tendon moment arm at each frame was computed as the perpendicular distance between the tendon line of action and the functional axis [13]. A quadratic fit of the moment arm relative to ankle angle was then performed.
Ankle torque was used to compute the axial stress during calibration (cal), σcal, in the Achilles tendon throughout the sway tasks. We assumed that torque, T, was generated purely by the Achilles tendon, i.e., σcal = T/ [A·r(θ)] where A is the tendon cross-sectional area and r(θ) is the tendon moment arm as a function of the ankle dorsiplantarflexion angle, θ. Tibialis anterior (TA) electromyographic (EMG) signals were recorded during the sway tasks and used to identify periods of co-contraction, which typically occurred when subjects leaned backwards. TA EMG data were recorded at 1900 Hz using wireless sensors (Trigno™, DelSys, Inc. Boston, MA). Data were processed using a 10–500 Hz bandpass filter and signals were full wave rectified. Processed data were then passed through a 10 Hz lowpass filter to obtain a linear envelope and normalized to the maximum value of the trial signal. Instances during the sway task in which the TA was active (defined by a TA EMG signal greater than 20% of the maximum signal) were not used in the tensiometer calibration. In 5 subjects for which TA EMG activity was not sufficiently collected, data where σcal was less than 5 MPa were not included in the calibration.
A theoretical model and ex vivo data suggest that wave speed squared increases in proportion to axial tendon stress [20]. Tensiometers were calibrated for each subject by performing a least-squares linear fit between wave speed squared and σcal (Fig 1). The goodness of each fit was assessed by computing the root mean-squared error (RMSE) between the tendon stress obtained from ankle torque and that which was predicted from the measured wave speed. We also computed the coefficient of determination, R2, to ascertain the variance of the stress described by the wave speeds measured with the calibrated tensiometer.
2.4. Tendon loads during walking
Subjects walked on an instrumented treadmill (Bertec Corp., Columbus, OH) at four speeds in randomized order (0.75, 1.00, 1.25, and 1.50 m/s) with at least two 10-second trials collected at each speed. Achilles tendon wave speed was continuously monitored at 50 Hz throughout the walking trials. Ground reaction forces were recorded and used to identify heel strike events. Tendon wave speed data were extracted for individual gait cycles and resampled to 101 data points per cycle. Strides were removed from analysis if heel strike could not be detected due to feet crossing over onto opposite treadmill belts or if outliers were found in multiple data points throughout the stride. Achilles tendon stress was estimated from wave speed (Fig. 1) using a prediction model of the form:, where c is wave speed, β is the slope of the linear fit from calibration and is the minimum wave speed measured over all walking trials for a given individual, which approximated a zero-load state. Achilles tendon force was calculated as F = σΑ, where A is the tendon cross-sectional area. Force was then normalized to body weight.
2.5. Statistics
Cohen’s d parameters were used to evaluate the effect size of age group (young, older) on preferred walking speed, tendon cross-sectional area, tendon moment arm, and tensiometer calibration parameters. Peak Achilles tendon wave speed, stress and force were extracted from the stance and swing phases of each gait cycle and then averaged over all gait cycles at a given speed. Two-way mixed analyses of variance (ANOVAs) with Bonferroni post-hoc corrections were used to evaluate the effects of age group and walking speed on peak values during the stance and swing phases of the gait cycle. Significance for all ANOVAs was set at p = 0.05. Mean values are presented with standard deviations (SD) in parentheses (i.e., mean (SD)).
3. Results
3.1. Subject Characteristics
Any differences in mass, height, and preferred walking speed in our sample of young and older adults could be considered small based on the effect sizes (Table 1). Mean Achilles tendon moment arms for young adults (mean 43.68 mm) were not significantly different from those for older adults (43.59 mm) at a neutral 0° dorsi-plantarflexion ankle angle (p = 0.96) (Table 1). However, Achilles tendon cross-sectional areas were 17% greater on average in older adults (p = 0.01) (Table 1).
Table 1.
Subject characteristics (mean (SD)) with effect size calculation using Cohen’s d. Older adults did not exhibit significantly different tendon moment arms (presented at 0 degrees ankle angle) but did exhibit significantly larger tendon cross-sections than young adults.
| Young | Older | Cohen’s d | p-value | |
|---|---|---|---|---|
| Mass (kg) | 73.96 (15.19) | 71.90 (12.21) | 0.15 | - |
| Height (m) | 1.77 (0.11) | 1.75 (0.06) | 0.21 | - |
| Preferred speed (m/s) | 1.37 (0.14) | 1.38 (0.15) | 0.04 | - |
| CSA (mm2) | 60.13 (10.52) | 70.50 (9.73) | 1.02 | 0.01 |
| Moment Arm (mm) | 43.68 (4.49) | 43.59 (5.71) | 0.02 | 0.96 |
3.2. Tensiometer Calibration
Both young and older adults exhibited highly linear relationships (mean R2>0.90, range: 0.83 – 0.99) between tendon wave speed squared and stress in the calibration tasks (Table 2). The mean tensiometer calibration gain was slightly higher for the older adults (7.56 kPa·s2/m2) than for the young adults (6.83 kPa·s2/m2), though the difference was not significant (p = 0.45) (Table 2). The mean RMSE between measured tendon stress and calibrated wave speed predictions of tendon stress in the sway tasks was less than 1 MPa (Table 2).
Table 2.
Tensiometer calibrations (mean (SD)) were highly linear for both young and older adults, with no significant difference in calibration gains or fit metrics between groups and a small effect size.
| Young | Older | Cohen’s d | p-value | |
|---|---|---|---|---|
| Gain β (kPa*s2/m2) | 6.83 (2.22) | 7.56 (2.78) | 0.29 | 0.45 |
| R2 | 0.95 (0.03) | 0.94 (0.04) | 0.24 | 0.54 |
| RMSE (MPa) | 0.89 (0.53) | 0.80 (0.46) | 0.18 | 0.64 |
3.3. Tendon Loads during Walking
Tendon wave speed during walking was lower across all speeds in the older adults (Fig 2; see Supplemental Figure A-1 for means and standard deviations). No significant or important group-by-speed interaction effects were observed, so only the main effects of speed and of group are presented. Mean peak Achilles tendon wave speed in stance was significantly lower (main effect of speed, p < 0.01) in older adults than young adults (Table 3). Older adults exhibited a mean peak tendon wave speed of 58.5 m/s across speeds during stance, which was 22% lower than that seen in young adults. There was no significant effect of age group on peak tendon wave speed during swing phase (Table 4).
Figure 2.
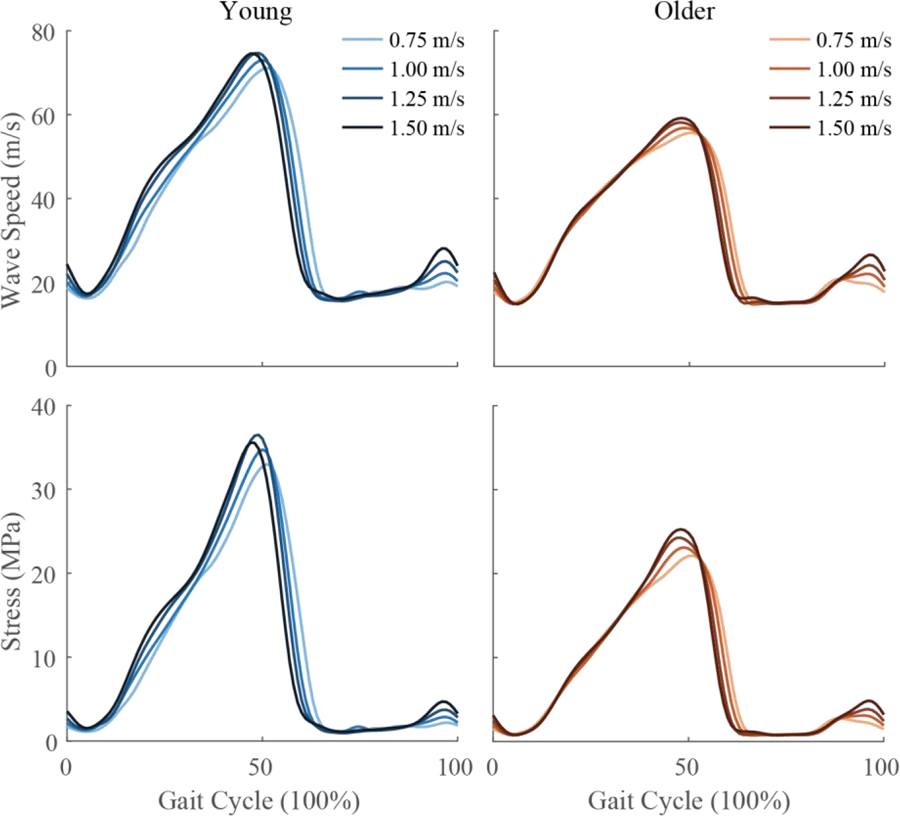
Ensemble averages of Achilles tendon wave speed (top row) and stress (bottom row) for young (left) and older (right) adults at four walking speeds. Stress was calculated from wave speed using calibration parameters from Table 2.
Table 3.
During stance phase, peak wave speed and stress (mean (SD)) were significantly lower in the older adults than those in the young adults (main effect p < 0.01, no significant group-by-speed interactions). Young adults tended to have higher forces than older adults, but the main effect of group on tendon force was not significant (p = 0.08). There was a significant main effect of speed in all three metrics (p < 0.01).
| Wave Speed (m/s) | |||
|---|---|---|---|
| Young | Older | Speed Mean | |
| 0.75 m/s | 72.5 (12.3) | 56.6 (9.1) | 64.5 (13.4) |
| 1.00 m/s | 74.2 (12.8) | 57.9 (10.2) | 66.1 (14.1) |
| 1.25 m/s | 75.8 (13.0) | 59.2 (10.4) | 67.5 (14.3) |
| 1.50 m/s | 75.5 (11.7) | 60.2 (10.7) | 67.9 (13.5) |
| Group Mean | 74.5 (12.2) | 58.5 (9.9) | |
| Stress (MPa) | |||
| Young | Older | Speed Mean | |
| 0.75 m/s | 34.8 (12.7) | 23.1 (9.1) | 29.0 (12.3) |
| 1.00 m/s | 36.6 (13.7) | 24.2 (9.6) | 30.4 (13.2) |
| 1.25 m/s | 38.2 (14.1) | 25.5 (10.6) | 31.8 (13.8) |
| 1.50 m/s | 37.2 (10.7) | 26.4 (11.1) | 31.8 (12.0) |
| Group Mean | 36.7 (12.5) | 24.8 (9.9) | |
| Force (BW) | |||
| Young | Older | Speed Mean | |
| 0.75 m/s | 2.87 (0.86) | 2.27 (0.80) | 2.57 (0.87) |
| 1.00 m/s | 2.97 (0.72) | 2.39 (0.88) | 2.68 (0.84) |
| 1.25 m/s | 3.11 (0.77) | 2.51 (0.95) | 2.81 (0.91) |
| 1.50 m/s | 3.08 (0.68) | 2.61 (1.06) | 2.84 (0.91) |
| Group Mean | 3.01 (0.75) | 2.44 (0.91) | |
Table 4.
During swing phase, peak wave speed, stress, and force (mean (SD)) were not significantly different between age groups (main effect p > 0.05, no significant group-by-speed interactions). There was a significant main effect of speed in all three metrics (p < 0.001).
| Wave Speed (m/s) | |||
|---|---|---|---|
| Young | Older | Speed Mean | |
| 0.75 m/s | 21.8 (5.1) | 22.4 (6.5) | 22.1 (5.7) |
| 1.00 m/s | 23.0 (5.3) | 23.6 (6.2) | 23.3 (5.7) |
| 1.25 m/s | 26.0 (5.0) | 25.2 (6.2) | 25.6 (5.5) |
| 1.50 m/s | 28.9 (4.4) | 27.5 (5.7) | 28.2 (5.1) |
| Group Mean | 24.9 (5.6) | 24.7 (6.3) | |
| Stress (MPa) | |||
| Young | Older | Speed Mean | |
| 0.75 m/s | 2.7 (1.6) | 3.3 (3.2) | 3.0 (2.5) |
| 1.00 m/s | 3.1 (2.1) | 3.8 (3.3) | 3.4 (2.7) |
| 1.25 m/s | 4.1 (2.2) | 4.4 (3.8) | 4.3 (3.0) |
| 1.50 m/s | 5.1 (2.2) | 5.3 (3.9) | 5.2 (3.1) |
| Group Mean | 3.7 (2.2) | 4.2 (3.5) | |
| Force (BW) | |||
| Young | Older | Speed Mean | |
| 0.75 m/s | 0.21 (0.10) | 0.32 (0.30) | 0.27 (0.22) |
| 1.00 m/s | 0.25 (0.13) | 0.36 (0.31) | 0.30 (0.24) |
| 1.25 m/s | 0.33 (0.13) | 0.43 (0.35) | 0.38 (0.26) |
| 1.50 m/s | 0.41 (0.12) | 0.51 (0.35) | 0.46 (0.26) |
| Group Mean | 0.30 (0.14) | 0.41 (0.33) | |
Older adults exhibited significantly lower tendon stress (p = 0.01) and a tendency toward lower peak normalized tendon force (p = 0.08) in stance (Table 3). Peak stance phase stress averaged 24.8 MPa across speeds in older adults, which was 32% lower than that seen in young adults. There was a significant main effect of speed on both tendon stress and force in both stance (Table 3) and swing (Table 4) (p < 0.01).
4. Discussion
This study used shear wave tensiometry to investigate age-related differences in Achilles tendon loading during gait. This is the first study to use a superficial sensor to non-invasively compare tendon loading between groups of individuals during a dynamic task. In agreement with our first hypothesis, we observed a significant difference (−32%) in peak Achilles tendon stress during walking in older adults when compared to a young adult cohort. Our second hypothesis was rejected, as significant age-by-speed interactions were not observed for any metric of tendon loading tested. However, tendon stress was consistently lower in older adults across speeds during the stance phase. Diminished tissue loading may have important mechanobiological consequences that affect tendon health in older age [18].
Lower tissue stress in the older adults was in part attributable to an enlarged tendon cross-section. The older adults exhibited tendon cross-sections that were 17% larger on average than those in young adults, which is similar to results from previous studies [17,26]. While researchers have theorized that the increased cross-sectional area in aging tendon may be due to an increase in extracellular water within the tendon tissue caused by decreased loading [17,27], others have found the water content in the extracellular matrix decreases in aged tendon [19,28]. Individuals with tendinopathy have also exhibited greater tendon cross-sectional areas, in this case due to an increase in water content and accrued ground substance [29]. In a separate study using the same subjects, we used ultrashort echo time (UTE) magnetic resonance imaging (MRI) to assess the amount of free and bound water in young and older Achilles tendons [30]. That study revealed evidence of increased free water content in the Achilles tendons of older adults and indications of sub-clinical tendinopathy in 7 of 13 subjects [30]. Hence, it’s likely that water content was a contributor to the tendon cross-sectional area differences we observed.
Tendons are mechano-responsive structures where tenocytes are constantly stimulated by mechanical load and act to remodel and repair the tissue. The structural integrity and health of the tendon is dependent on a balance of mechanical loading that provides neither over- nor under-stimulation [18,31]. The mechanical stimulus experienced by tenocytes depends on the transformation of tendon loading to local tissue deformation. Aging tendon has been shown to exhibit greater compliance than young tendon, possibly as an adaptation to loading [17,32,33]. If older adults had a 30% lower tendon stress (as observed in this study) and 30% lower tendon modulus [32], then average axial tendon stretch may not change appreciably. However, overall tendon stretch arises from a combination of collagen fiber stretch and sliding between fibers, where the tenocytes are located [19]. There is growing evidence that the sliding behavior is altered with aging at the fibrillar [34], fascicular [35], and sub-tendon levels [35–37]. Our stress measures reflect the mean stress over the tendon cross-section and hence do not provide an indication of potential variations in loading among the sub-tendons of the individual triceps surae muscles. Decreased tenocyte stimulation could lead to an accumulation of collagen due to decreased matrix turnover. The resulting increase in cross-sectional area could be protective against acute injuries [38]. However, decreased turnover may come at the expense of tendon health (e.g., tendinopathy). Future investigations into non-uniform tendon loading and hierarchical tissue deformation will be needed to fully understand the mechanobiological implications of tendon loading on tissue health, injury, and disease.
We did not observe a more pronounced effect of age on tendon stress at higher walking speeds, as we hypothesized. While tendon stress did vary with walking speed in both young and older adults, the peak stress increased by 11% on average as walking speed was doubled from 0.75 to 1.5 m/s. The change in stress is 2.5-fold less than the fluctuation in peak ankle moment across that speed range (+28%). Variations in the Achilles tendon moment arm could contribute, in part, to the discrepancy. There is evidence of an increase in Achilles tendon moment arm with muscle loading in stance [24]. Further, peak dorsiflexion tends to decrease at faster walking speed, which would also act to increase the Achilles tendon moment arm [13]. It is also worth noting that while the difference in tendon stress between groups was ~30% (older < young), the difference in stride lengths between groups was only ~5% (older < young). Thus, differences in tendon loading between the groups were likely due to differences in force generation by the muscles rather than differences in stride length. Prior studies have found that triceps surae EMG and ankle power varies substantially with speed [13] and, further, that soleus EMG exhibits an amplified age-related decline when walking speed is increased [39]. The discrepancy between EMG and stress variations with speed likely reflects muscle-tendon dynamics. Varying walking speed affects the operating lengths and velocity of triceps surae muscle fascicles during the critical push-off phase of gait [40,41], thereby altering the ability of muscle-tendon units to produce force.
The current study was enabled by shear wave tensiometry, a new noninvasive approach to measure tendon stress during movement [20]. Following are relevant assumptions to consider when interpreting tensiometry results. Subject-specific calibration was used to transform the wave speed measures to Achilles tendon loading. Our approach for computing stress from torque relies on assumptions about muscle load sharing, which can affect the accuracy of the stress estimate. A prior tensiometer calibration study found that tensiometers predicted peak Achilles tendon stresses during walking to within 20% (on average) of those estimated from traditional inverse dynamics [13]. An important consideration with the new shear wave tensiometer device is whether simply wearing it may alter gait dynamics. We performed gait analyses on a subset of subjects walking with and without the tensiometer and found a slight decline in peak ankle power in young adults wearing the active tensiometer, while no change was seen in the older adults (See Supplemental Data B). As a result, the difference in tendon loading between age groups we observed using shear wave tensiometry may have been less substantial than the true age-related difference. Work is continuing to be done to enhance the human factors aspects of the tensiometer, and we believe this will reduce or eliminate the sensor’s effects on gait dynamics. We recognize that the accuracy of ultrasound-based methods for determining Achilles tendon CSA have been questioned [42,43]. However, we performed a sub-analysis on 20 subjects from this study (9 young, 11 older adults) and found that CSA measured using ultrasound-based methods and from magnetic resonance imaging was significantly correlated in this case (R2 = 0.81, p <0.001). Thus, this systematic effect should have minimal impact on our conclusions drawn from the between-group comparisons. Finally, only the right limb was tested during this study, regardless of limb dominance. However, only two young and three older adults were left leg dominant, and there were no observable trends associated with limb dominance.
In summary, we observed large and consistent differences in Achilles tendon loading between young and older adults that were evident over a broad range of walking speeds. Older adults exhibit lower Achilles tendon stress as a result of altered muscle force generation and an enlarged tendon cross-section. The effects of altered tissue loading on internal tissue deformations may be important for understanding the mechanobiological processes affecting tendon tissue health in older age.
Supplementary Material
Highlights:
Shear wave sensors can measure superficial tendon stress noninvasively
Achilles tendon stress is 32% lower in older than younger adults during walking
Achilles tendon loading is lower in older adults across a range of walking speeds
Diminished loads could alter mechanobiological stimuli underlying tendon remodeling
Acknowledgements:
We would like to thank Katie Knaus, Dr. Silvia Blemker, and Dr. Jason Franz for helpful discussion on this paper, Mikel Joachim for assistance with gait lab calibration, and Dr. Fang Liu for custom programming developed to process ultrasound images.
Declaration of Interest: Funding provided by NIH HD092697, AG051748, and AG000213. J.A.M. and D.G.T. are co-inventors on a patent application for tensiometer technology. The other authors have no declarations of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].McGowan CP, Neptune RR, Kram R, Independent effects of weight and mass on plantar flexor activity during walking: implications for their contributions to body support and forward propulsion., J. Appl. Physiol 105 (2008) 486–94. 10.1152/japplphysiol.90448.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kerrigan DC, Todd MK, Della Croce U, Lipsitz LA, Collins JJ, Biomechanical gait alterations independent of speed in the healthy elderly: Evidence for specific limiting impairments, Arch. Phys. Med. Rehabil 79 (1998) 317–322. 10.1016/S0003-9993(98)90013-2. [DOI] [PubMed] [Google Scholar]
- [3].McGibbon CA, Krebs DE, Effects of age and functional limitation on leg joint power and work during stance phase of gait., J. Rehabil. Res. Dev 36 (1999) 173–82. 10.1016/j.cbpc.2010.01.009. [DOI] [PubMed] [Google Scholar]
- [4].Winter D, Patla AE, Frank JS, Walt SE, Biomechanical walking pattern changes in the fit and healthy elderly., Phys. Ther 70 (1990) 340–347. 10.1093/ptj/70.6.340. [DOI] [PubMed] [Google Scholar]
- [5].DeVita P, Hortobagyi T, Age causes a redistribution of joint torques and powers during gait., J. Appl. Physiol 88 (2000) 1804–1811. 10.1177/0893318915601161. [DOI] [PubMed] [Google Scholar]
- [6].Kulmala JP, Korhonen MT, Kuitunen S, Suominen H, Heinonen A, Mikkola A, Avela J, Which muscles compromise human locomotor performance with age?, J. R. Soc. Interface 11 (2014). 10.1098/rsif.2014.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Franz JR, Kram R, Advanced age and the mechanics of uphill walking: A joint-level, inverse dynamic analysis, Gait Posture 39 (2014) 135–140. 10.1016/j.gaitpost.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cofré LE, Lythgo N, Morgan D, Galea MP, Aging modifies joint power and work when gait speeds are matched, Gait Posture 33 (2011) 484–489. 10.1016/j.gaitpost.2010.12.030. [DOI] [PubMed] [Google Scholar]
- [9].Silder A, Heiderscheit B, Thelen DG, Active and passive contributions to joint kinetics during walking in older adults, J. Biomech 41 (2008) 1520–1527. 10.1016/j.jbiomech.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Judge JO, Davis RB, Ounpuu S, Step length reductions in advanced age: the role of ankle and hip kinetics., J. Gerontol. A. Biol. Sci. Med. Sci 51 (1996) M303–12. http://www.ncbi.nlm.nih.gov/pubmed/8914503. [DOI] [PubMed] [Google Scholar]
- [11].Giddings VL, Beaupré GS, Whalen RT, Carter DR, Calcaneal loading during walking and running, Med. Sci. Sports Exerc 32 (2000) 627–634. 10.1097/00005768-200003000-00012. [DOI] [PubMed] [Google Scholar]
- [12].Finni T, Komi PV, Lukkariniemi J, Achilles tendon loading during walking: Application of a novel optic fiber technique, Eur. J. Appl. Physiol. Occup. Physiol 77 (1998) 289–291. 10.1007/s004210050335. [DOI] [PubMed] [Google Scholar]
- [13].Keuler E, Loegering I, Martin J, Roth J, Thelen D, Shear Wave Predictions of Achilles Tendon Loading during Human Walking, Sci. Rep (2019) 1–9. 10.1038/s41598-019-49063-7. [DOI] [PMC free article] [PubMed]
- [14].Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R, Aging of skeletal muscle: a 12-yr longitudinal study., J. Appl. Physiol 88 (2000) 1321–6. 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- [15].Janssen I, Heymsfield SB, Wang Z, Ross R, Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr, J Appl Physiol 116 (2014) 1342 10.1152/japplphysiol.zdg-1052-corr.2014.Janssen. [DOI] [PubMed] [Google Scholar]
- [16].Franz JR, The Age-Associated Reduction in Propulsive Power Generation in Walking, Exerc. Sport Sci. Rev 44 (2016) 129–136. 10.1249/JES.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stenroth L, Peltonen J, Cronin NJ, Sipila S, Finni T, Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo, J. Appl. Physiol 113 (2012) 1537–1544. 10.1152/japplphysiol.00782.2012. [DOI] [PubMed] [Google Scholar]
- [18].Pizzolato C, Lloyd DG, Zheng MH, Besier TF, Shim VB, Obst SJ, Newsham-West R, Saxby DJ, Barrett RS, Finding the sweet spot via personalised Achilles tendon training: The future is within reach, Br. J. Sports Med 53 (2019) 11–12. 10.1136/bjsports-2018-099020. [DOI] [PubMed] [Google Scholar]
- [19].Tuite DJ, Renström PAFH, O’Brien M, The aging tendon, Scand. J. Med. Sci. Sports 7 (2007) 72–77. 10.1111/j.1600-0838.1997.tb00122.x. [DOI] [PubMed] [Google Scholar]
- [20].Martin JA, Brandon SCE, Keuler EM, Hermus JR, Ehlers AC, Segalman DJ, Allen MS, Thelen DG, Gauging force by tapping tendons, Nat. Commun 9 (2018) 1–9. 10.1038/s41467-018-03797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cespedes I, Methods for Estimation of Subsample Time Delays of Digitized Echo Signals, Ultrason. Imaging 17 (1995) 142–171. 10.1006/uimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- [22].Hawkins D, Lum C, Gaydos D, Dunning R, Dynamic creep and pre-conditioning of the Achilles tendon in-vivo, J. Biomech 42 (2009) 2813–2817. 10.1016/j.jbiomech.2009.08.023. [DOI] [PubMed] [Google Scholar]
- [23].Collins TD, Ghoussayni SN, Ewins DJ, Kent JA, A six degrees-of-freedom marker set for gait analysis: Repeatability and comparison with a modified Helen Hayes set, Gait Posture 30 (2009) 173–180. 10.1016/j.gaitpost.2009.04.004. [DOI] [PubMed] [Google Scholar]
- [24].Rasske K, Thelen DG, Franz JR, Variation in the human Achilles tendon moment arm during walking, Comput. Methods Biomech. Biomed. Engin 20 (2017) 201–205. 10.1080/10255842.2016.1213818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Siston RA, Daub AC, Giori NJ, Goodman SB, Delp SL, Evaluation of methods that locate the center of the ankle for computer-assisted total knee arthroplasty, Clin. Orthop. Relat. Res (2005) 129–135. 10.1097/01.blo.0000170873.88306.56. [DOI] [PubMed]
- [26].Magnusson SP, Beyer N, Abrahamsen H, Aagaard P, Neergaard K, Kjaer M, Increased Cross-sectional Area and Reduced Tensile Stress of the Achilles Tendon in Elderly Compared With Young Women, 58 (2003) 123–127. [DOI] [PubMed] [Google Scholar]
- [27].Kinugasa R, Hodgson JA, Edgerton VR, Shin DD, Sinha S, Reduction in tendon elasticity from unloading is unrelated to its hypertrophy, J. Appl. Physiol 109 (2010) 870–877. 10.1152/japplphysiol.00384.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ippolito E, Natali PG, Postacchini F, Accinni L, De Martino C, Morphological, immunochemical, and biochemical study of rabbit achilles tendon at various ages., J. Bone Joint Surg. Am 62 (1980) 583–98. [PubMed] [Google Scholar]
- [29].Arya S, Kulig K, Tendinopathy alters mechanical and material properties of the Achilles tendon, J. Appl. Physiol 108 (2010) 670–675. 10.1152/japplphysiol.00259.2009. [DOI] [PubMed] [Google Scholar]
- [30].Loegering IF, Denning SC, Johnson KM, Liu F, Lee KS, Thelen DG, Ultrashort Echo Time (UTE) Imaging Reveals a Shift in Bound Water That is Sensitive to Sub-clinical Tendinopathy in Older Adults, Skeletal Radiol (n.d.). [DOI] [PMC free article] [PubMed]
- [31].Thampatty BP, Wang JHC, Mechanobiology of young and aging tendons: In vivo studies with treadmill running, J. Orthop. Res 36 (2018) 557–565. 10.1002/jor.23761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Onambele GL, Narici MV, Maganaris CN, Calf muscle-tendon properties and postural balance in old age, J. Appl. Physiol 100 (2006) 2048–2056. 10.1152/japplphysiol.01442.2005. [DOI] [PubMed] [Google Scholar]
- [33].Mian OS, Thom JM, Ardigò LP, Minetti AE, Narici MV, Gastrocnemius muscle-tendon behaviour during walking in young and older adults, Acta Physiol 189 (2007) 57–65. 10.1111/j.1748-1716.2006.01634.x. [DOI] [PubMed] [Google Scholar]
- [34].Muench J, Thelen DG, Henak CR, Interfibrillar shear behavior is altered in aging tendon fascicles, Biomech. Model. Mechanobiol accepted (2019). [DOI] [PMC free article] [PubMed]
- [35].Thorpe CT, Udeze CP, Birch HL, Clegg PD, Screen HRC, Capacity for sliding between tendon fascicles decreases with ageing in injury prone equine tendons: A possible mechanism for age-related tendinopathy?, Eur. Cells Mater 25 (2012) 48–60. 10.22203/eCM.v025a04. [DOI] [PubMed] [Google Scholar]
- [36].Franz JR, Thelen DG, Depth-dependent variations in Achilles tendon deformations with age are associated with reduced plantarflexor performance during walking, J. Appl. Physiol 119 (2015) 242–249. 10.1152/japplphysiol.00114.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Franz JR, Thelen DG, Imaging and simulation of Achilles tendon dynamics: Implications for walking performance in the elderly, J. Biomech 49 (2016) 1403–1410. 10.1016/j.jbiomech.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Svensson RB, Heinemeier KM, Couppé C, Kjaer M, Magnusson SP, Effect of aging and exercise on the tendon, J. Appl. Physiol 121 (2016) 1353–1362. 10.1152/japplphysiol.00328.2016. [DOI] [PubMed] [Google Scholar]
- [39].Schmitz A, Silder A, Heiderscheit B, Mahoney J, Thelen DG, Differences in lower-extremity muscular activation during walking between healthy older and young adults, J. Electromyogr. Kinesiol 19 (2009) 1085–1091. 10.1016/j.jelekin.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lichtwark GA, Wilson AM, Interactions between the human gastrocnemius muscle and the Achilles tendon during incline, level and decline locomotion., J. Exp. Biol 209 (2006) 4379–88. 10.1242/jeb.02434. [DOI] [PubMed] [Google Scholar]
- [41].Orselli MIV, Franz JR, Thelen DG, The effects of Achilles tendon compliance on triceps surae mechanics and energetics in walking, J. Biomech 60 (2017) 227–231. 10.1016/j.jbiomech.2017.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bohm S, Mersmann F, Schroll A, Mäkitalo N, Arampatzis A, Insufficient accuracy of the ultrasound-based determination of Achilles tendon cross-sectional area, J. Biomech 49 (2016) 2932–2937. 10.1016/j.jbiomech.2016.07.002. [DOI] [PubMed] [Google Scholar]
- [43].McCrum C, Leow P, Epro G, König M, Meijer K, Karamanidis K, Alterations in leg extensor muscle-tendon unit biomechanical properties with ageing and mechanical loading, Front. Physiol 9 (2018) 1–7. 10.3389/fphys.2018.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


