Abstract
Background:
The potential benefits and safety of hepatic arterial infusion chemotherapy (HAIC) for the treatment of patients with hepatocellular carcinoma (HCC) remains inconsistent. Therefore, we conducted this meta-analysis of evaluate the efficacy and safety of HAIC in the treatment of HCC.
Methods:
A comprehensive literature search was performed using PubMed, Embase, Web of Science, and the Cochrane library to identify eligible studies that compared HAIC with other therapies for patients with HCC. The main outcomes of our interest, including overall survival (OS), disease free survival (DFS), objective response rate (ORR), disease control rate (DCR), and adverse events, were calculated using the meta-analysis. The pooled estimates were expressed with hazard ratio (HR) with 95%confidence intervals (95%CIs) or risk ratio (RR) with 95%CIs.
Results:
A total of 13 studies met the inclusion criteria and were included in this meta-analysis. Pooled estimates showed that, HAIC was associated with significantly improved OS (HR = 0.61, 95%CI: 0.48, 0.77; P < .001) and DFS (HR = 0.66, 95%CI: 0.52, 0.84; P = .001) as compared with other therapies. The ORR (RR = 2.28, 95%CI: 1.77, 2.94; P < .001) and DCR (RR = 1.47, 95%CI: 1.23, 1.77; P < .001) were also significantly higher in HAIC group than in control group. Most of the common adverse events were comparably occurred in the 2 groups, except for nausea/vomiting, hypoalbuminemia, pain, anemia and hepatic toxicity. Subgroup analysis suggested that, the improved OS and DFS associated with HAIC were only observed in patients with colorectal liver metastases (CRLM), or advanced HCC, but not in those with unresectable HCC or pancreatic liver metastases.
Conclusion:
Based on the present data, HAIC showed benefit effect in HCC patients, with pronged OS and DFS, as well as increased ORR and DCR. These benefit effects were more obvious in CRLM or advanced HCC patients. However, considering the potential limitations, more large-scale, randomized trials are needed to verify our findings.
Keywords: hepatic arterial infusion chemotherapy, hepatocellular carcinoma, meta-analysis
1. Introduction
Hepatocellular carcinoma (HCC) ranks the sixth most common cancer and the fourth most cause of cancer-related death in the world,[1] and the incidence of HCC cases is increasing worldwide.[2] Surgical resection and local ablation therapies, such as percutaneous ethanol injection and percutaneous radiofrequency ablation, are regarded as curative strategies for patients with HCC. However, most patients are not diagnosed until the disease is unresectable.[3–5] It is estimated that approximately 25% of HCC patients present with synchronous disease, and about half of HCC patients develop hepatic metastases during the course of their disease.[6] Hepatic resection is used as the only curable treatment for colorectal liver metastases (CRLM), and the 10-year survival rates reached 22%.[6] For unresectable HCC patients, various treatment options have been developed, such as transarterial chemoembolization (TACE), systemic chemotherapy, and hepatic arterial infusion chemotherapy (HAIC).[7,8]
HAIC has been applied as a palliative procedure in the treatment of unresectable CRLM. In this procedure, anticancer drugs are infused directly into the hepatic artery, which increased the intra-hepatic drug concentrations and decreased the systemic toxicity.[9] The drug most commonly used for intra-arterial applications include cisplatin (CDDP), oxaliplatin (OX), 5-fluoroouracil (5-FU), doxorubicin, epirubicin, and mitomycin-C, used individually or in combination.[10] HAIC has been used classically for unresectable CRLM,[10,11] as well as being adjuvant therapy in HCC patients after resection,[12,13] however, its role in translating improved response rates into survival benefits still remains controversial.[14] Thus, we performed the present meta-analysis to evaluate the survival rate, overall response rate (ORR), and complication rate using HAIC in the treatment of HCC patients.
2. Material and methods
Since the data analyzed in this study were derived from previously published studies, the ethical approval or patient consent was not required.
2.1. Literature search
We conducted this meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[15] Four major electronic databases, including PubMed, Embase, Web of Science, and the Cochrane library, were comprehensively searched, from their inception to November 21, 2019. The literature search terms we used were the following: ((Hepatic[All Fields] AND (“arteries”[MeSH terms] or “arteries”[all fields] or “arterial”[all fields]) and infusion[all fields] and (“drug therapy”[subheading] or (“drug”[all fields] and “therapy”[all fields]) or “drug therapy”[all fields] or “chemotherapy”[all fields] or “drug therapy”[MeSH terms] or (“drug”[all fields] and “therapy”[all fields]) or “chemotherapy”[all fields])) OR HAIC[all fields]) and ((“liver”[MeSH terms] or “liver”[all fields]) and (“neoplasm metastasis”[MeSH terms] or (“neoplasm”[all fields] and “metastasis”[all fields]) or “neoplasm metastasis”[all fields] or “metastasis”[all fields])) and (“colorectal neoplasms”[MeSH terms] or (“colorectal”[All fields] and “neoplasms”[All fields]) or “colorectal neoplasms”[all fields] or (“colorectal”[all fields] and “cancer”[all fields]) or “colorectal cancer”[all Fields]). There were no limitations on publication status or language. We also additionally searched the reference lists of included articles and reviews to identify the potential eligible studies.
2.2. Inclusion criteria and study selection
To be included in this meta-analysis, studies must meet the following inclusion criteria:
-
(1)
study design: randomized controlled trials (RCTs), cohort study or case-control study;
-
(2)
population: adult patients who were histologically and/or clinically diagnosed HCC;
-
(3)
intervention: neoadjuvant HAIC;
-
(4)
comparison: other therapy methods;
-
(5)
outcomes: provided 1 of the following outcome of interest: OS, DFS, ORR, DCR, or complications.
When several publications from the same trial were presented, we only included the one with the most complete data, or both if they had different outcome measures. Studies were excluded if they were non-comparative studies, or case report, case series, or did not apply HAIC for HCC patients, or did not report the data of our interest.
2.3. Data extraction and quality assessment
Using a standardized tool, 2 independent investigators performed data extraction to extract the following data from each study: first author's name, year of publication, country, sample size, baseline patient characteristics (age, sex, race, diabetes duration), disease characteristics, neoadjuvant HAIC regimen, hazard ratio (HR) with 95% confidence intervals (95%CIs) for OS and DFS, ORR, DCR, and complications.
For non-randomized trials, we used the modified Newcastle-Ottawa scale (NOS) to assess the methodological quality.[16] This method comprised of 3 items to evaluate the quality of a non-RCT trial.[16] The total score of this method was 9 points, and higher points indicated high quality. Studies with a score of more than 5 points were regarded as high quality.[16] For RCTs, we used the method recommended by Cochrane Collaboration to assess the risk of bias.[17] This method consists of the 6 items, including random sequence generation; allocation concealment; blinding of outcome participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting, and other bias.[17] Each study was regarded as “high”, “low”, or “unclear” risk of bias according to the criteria mentioned above.
2.4. Statistical analysis
Meta-analysis was analyzed using Stata version 12.0 software (Stata Corporation, College Station, TX). We used Cochrane Q and I2 statistic[18] to test the heterogeneity across included studies, in which P < .1 or I2 > 50% were considered to be significant.[18] For time-to-event variables, including OS and DFS, the HR with 95%CIs were expressed to calculate the effect estimate; for dichotomous variables, including ORR, DCR, and complication rate, risk ratio (RR) with 95%CIs were pooled to synthesize the data. Meta-analyses were performed using a fixed-effect model[19] or random-effects model[20] according to the absent or present of heterogeneity. When significant heterogeneity analysis was identified, we used sensitivity analysis to explore the potential sources of heterogeneity. Moreover, we also conducted subgroup analysis based on the study design, disease characteristics, or comparators to test whether these factors had an influence on the outcome estimate. The assessment of publication bias was evaluated by using Egger[21] and Begger[22] test. A P value less than .05 was judged as statistically significant, except where otherwise specified.
3. Results
3.1. Identification of eligible studies
The initial screening retrieved 895 publications from the databases, of which 514 were excluded because of duplicate records, leaving 381 studies. Further screening for title or abstract excluded 360 studies, leaving 21 for full-text information review. Among these studies for potential eligibility, 8 studies were excluded for a variety of reasons (single-arm trial, or HAIC in both groups, or unrelated with our topics). Finally, 13 studies[11,23–34] met the inclusion criteria and were included for the data analysis. The literature review and selection process are presented in Figure 1.
Figure 1.
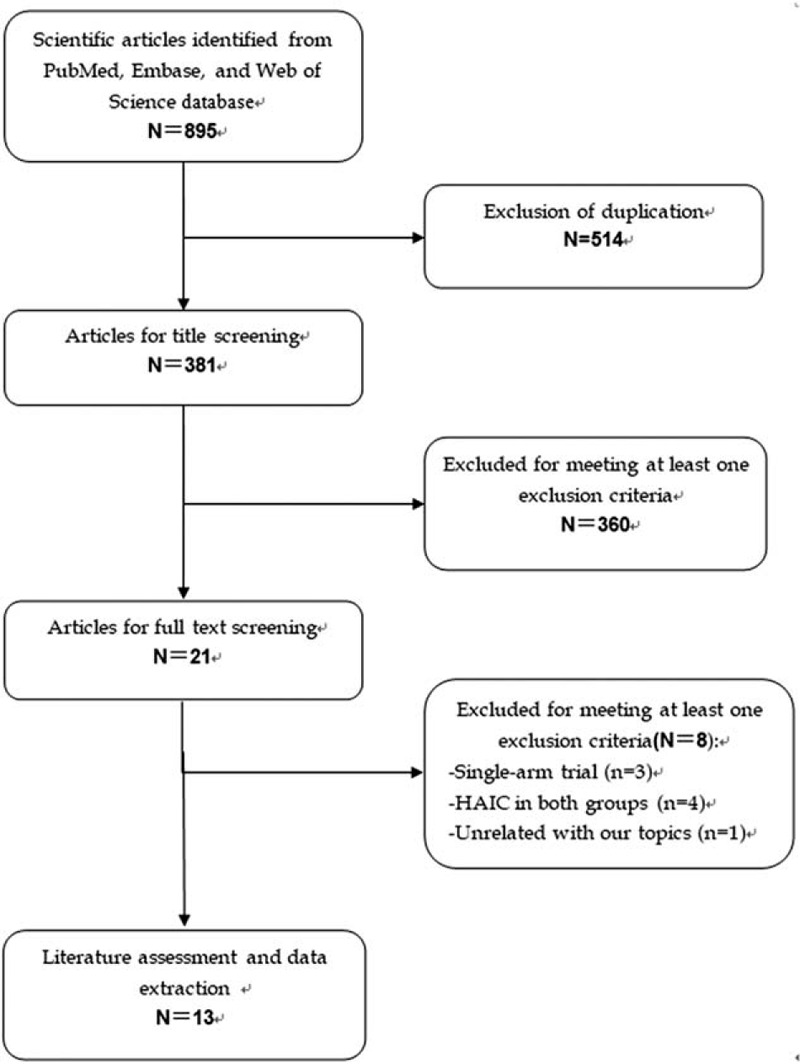
Eligibility of studies for inclusion in meta-analysis.
3.2. Characteristics of eligible studies and quality assessment
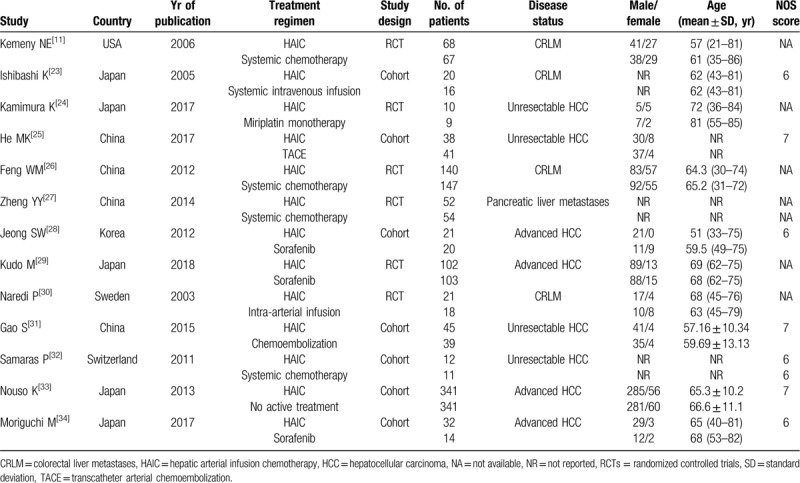
Table 1 summarizes the characteristics of the included studies. Among these studies, 5 were conducted in Japan,[23,24,29,33,34] 4 in China,[25–27,31] and each 1 in USA,[11] Sweden,[30] and Switzerland.[32] Seven studies were cohort studies,[23,25,28,31–34] and the remaining 6 were RCTs.[11,24,26,27,29,30] Sample size in each study ranged from 19 to 682, with a mean number of 137. All the enrolled patients were diagnosed with HCC, with CRLM in 4 studies,[11,23,26,30] unresectable HCC in 4 studies,[24,25,31,32] advanced HCC in 4 studies,[28,29,33,34] and pancreatic liver metastases in 1 study.[27] The comparators in each study varied greatly, with systematic chemotherapy in 4 studies,[11,26,27,32] sorafenib in 3 studies,[28,29,34] systemic intravenous infusion in 2 studies, TACE in 2 studies,[25,31] miriplatin monotherapy[24] and no active treatment[33] in each 1 study, respectively.
Table 1.
Baseline characteristics of patients in the trials included in the meta-analysis.
The methodological assessment for cohort studies showed that, the NOS score in each study was greater than 6 points, indicating that they were of high quality. Risk of bias assessment for RCTs revealed that, 3 were classified as being at low risk of bias, 2 at unclear risk of bias, and 2 at high risk of bias.
3.3. Overall survival
Eleven studies reported the data of OS. Pooled estimate suggested that, patients treated with HAIC achieved a significantly longer OS as compared with those received with other therapies (HR = 0.61, 95%CI: 0.48, 0.77; P < .001) (Fig. 2). There was significant heterogeneity among the included studies (I2 = 59.4%, P = .006). When we excluded the trial outlier,[34] the overall estimate did not change substantially (HR = 0.63, 95%CI: 0.50, 0.80; P < .001), but the heterogeneity was still present (I2 = 58.8%, P = .009). When the trial conducted by Kudo M, et al[29] was removed, the pooled data changed a little (HR = 0.52, 95%CI: 0.46, 0.59; P < .001), but no significant heterogeneity was observed (I2 = 0.3%, P = .435), which indicated that the trial by Kudo et al[29] contributed to the heterogeneity.
Figure 2.
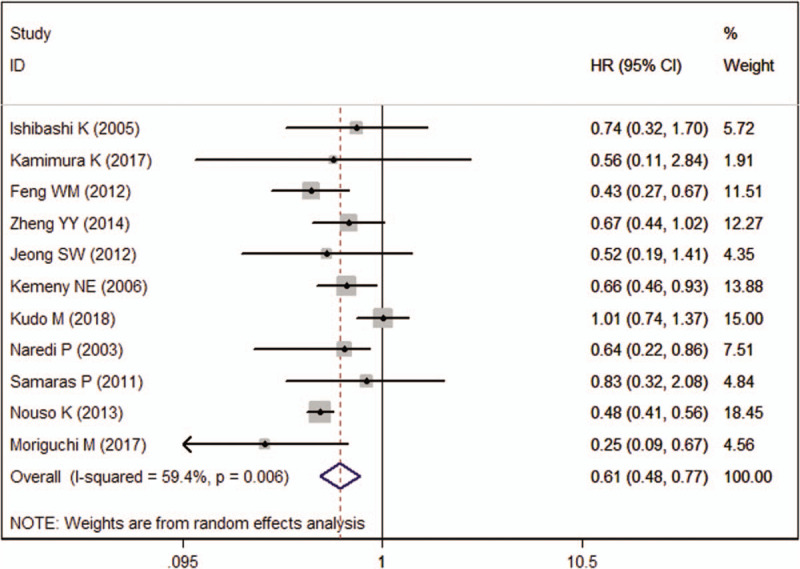
Forest plot showing the effect of hepatic arterial infusion chemotherapy on overall survival.
Subgroup analysis based on study design showed that, the prolonged OS associated with HAIC was observed in both RCTs (HR = 0.68, 95%CI: 0.51, 0.89; P = .005) and cohort studies (HR = 0.49, 95%CI: 0.41, 0.58; P < .001).
Subgroup analysis based on disease characteristics revealed that, the improved OS associated with HAIC was only observed in patients with CRLM (HR = 0.59, 95%CI: 0.46, 0.75; P < .001) and advanced HCC (HR = 0.55, 95%CI: 0.32, 0.96; P = .036), but not in those with unresectable HCC (HR = 0.75, 95%CI: 0.33, 1.69; P = .492), or pancreatic liver metastases (HR = 0.67, 95%CI: 0.44, 1.02; P = .062).
3.4. Disease-free survival
Ten studies reported the data of DFS. The aggregated results indicated that, patients in HAIC group achieved a significantly prolonged DFS than those in other therapy group (HR = 0.66, 95%CI: 0.52, 0.84; P = .001) (Fig. 3). The test for heterogeneity was significant (I2 = 50.5%, P = .033). Sensitivity analysis by excluding the trial of Samaras P, et al[32] showed that, the pooled data did not alter substantially (HR = 0.63, 95%CI: 0.52, 0.78; P < .001), and the evidence of heterogeneity was not present (I2 = 31.8%, P = .164). This indicated that the trial of Samaras et al[32] was responsible for the absent of heterogeneity.
Figure 3.
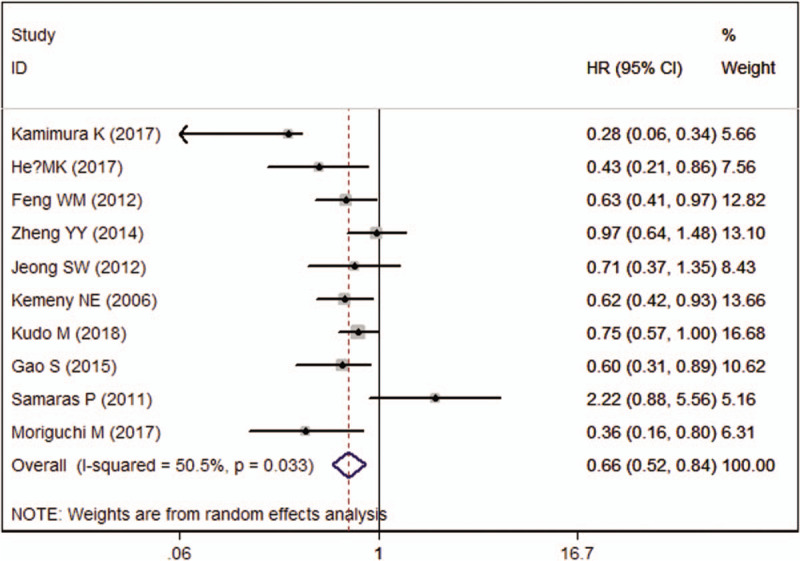
Forest plot showing the effect of hepatic arterial infusion chemotherapy on disease free survival.
Subgroup analysis based on study design suggested that, the prolonged DFS associated with HAIC was only observed in RCT (HR = 0.68, 95%CI: 0.52, 0.86; P = .004), but not in cohort studies (HR = 0.65, 95%CI: 0.39, 1.08; P = .097).
Subgroup analysis based on disease characteristics revealed that, the improved DFS associated with HAIC was found in patients who had CRLM (HR = 0.62, 95%CI: 0.47, 0.84; P = .002) or advanced HCC (HR = 0.66, 95%CI: 0.46, 0.94; P = .023), but not in unresectable HCC (HR = 0.62, 95%CI: 0.30, 1.27; P = .189) or pancreatic liver metastases (HR = 0.97, 95%CI: 0.64, 1.48; P = .887).
3.5. Overall response rate and disease control rate
Eight studies reported the data of ORR. The ORR in HAIC and other therapy groups was 44.04% and 19.33%, respectively. Pooling the data showed that patients treated with HAIC achieved a significantly higher ORR than those with other therapies (RR = 2.28, 95%CI: 1.77, 2.94; P < .001) (Fig. 4).
Figure 4.
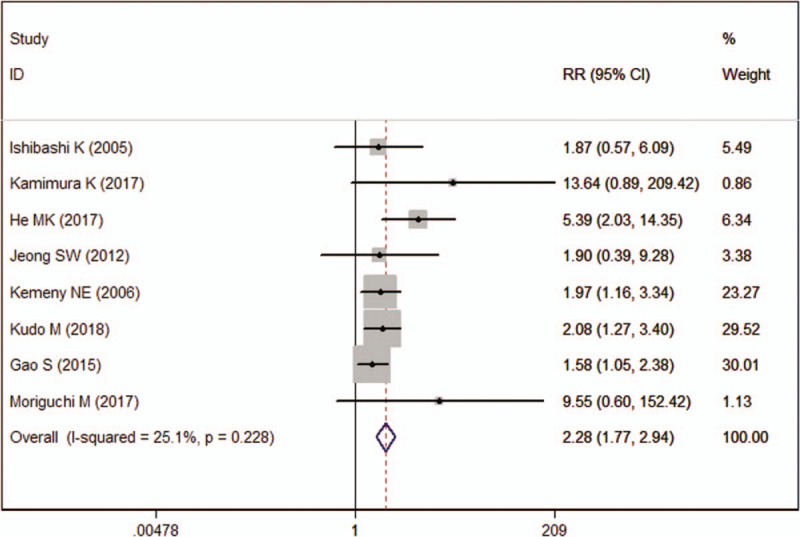
Forest plot showing the effect of hepatic arterial infusion chemotherapy on objective response rate.
The DCR in HAIC group was 72.29% as compared with 50.36% in other therapy group. The pooled estimate showed that DCR was significantly higher in HAIC group than in other therapy group (RR = 1.47, 95%CI: 1.23, 1.77; P < .001) (Fig. 5).
Figure 5.
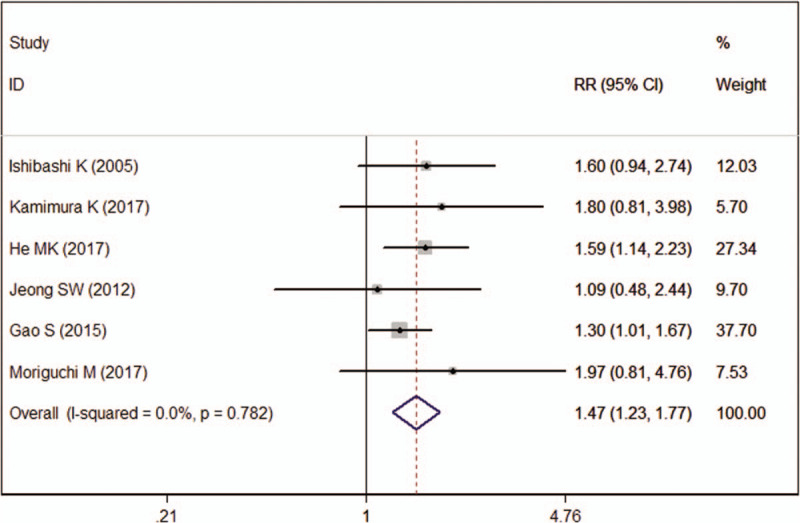
Forest plot showing the effect of hepatic arterial infusion chemotherapy on disease control rate.
3.6. Complications
All the included studies reported the data of complications, however, only 9 presented available data for meta-analysis. Overall, most of the complications were comparatively occurred between the 2 treatment regimens. However, HAIC was associated with significantly higher rates of nausea/vomiting (RR = 1.37, 95%CI: 1.05, 1.79; P = .022), hypoalbuminemia (RR = 1.36, 95%CI: 1.07, 1.74; P = .013), pain (RR = 1.23, 95%CI: 1.02, 1.49; P = .029), anemia (RR = 1.11, 95%CI: 1.01, 1.22; P = .031), and hepatic toxicity (RR = 1.32, 95%CI: 1.04, 1.67; P = .022), and lower rates of elevated ALT level (RR = 0.81, 95%CI: 0.68, 0.97; P = .018), and hoarseness (RR = 0.49, 95%CI: 0.28, 0.86; P = .012), as compared with other therapies.
Whereas, for grade 3 and 4 level adverse events, all these commonly developed complications were comparable between the 2 treatment regimens, except for pain (RR = 3.99, 95%CI: 1.12, 14.19; P = .037) and decreased white blood count (RR = 2.69, 95%CI: 1.03, 7.07; P = .044), the incidences of which were higher in HAIC group than in the control group.
3.7. Publication bias
Assessment of publication bias using Egger and Begg test showed that there was no potential publication bias among the included studies (Egger test, P = .558; Begg test, P = .621).
4. Discussion
In the present study, we assessed the efficacy and safety of HAIC in the treatment of HCC patients. We found that HAIC was associated a significantly prolonged OS and DFS, as compared with other therapies. Moreover, HAIC also improved ORR and DCR, and had similar incidence of adverse events, when compared with other therapies.
In HAIC, greater concentrations of chemotherapeutic agents can be directly to the tumor, which resulted in significant response rates.[35] Moreover, the drugs used for HAIC, such as 5-FU and floxuridine (FUDR), have a first-pass hepatic clearance effect. These drugs are primarily metabolized in the liver and have a short half-life, which lead to low drug concentrations in the peripheral blood.[36] This not only maintains a higher exposure of chemotherapy to malignant cells, but also reduces the risk of systemic adverse events.[36] 5-FU has been used as the preferred agent in Europe.[37] This is because of its short half-life, high first-pass extraction rate (95%), up to 400-fold estimated increase in tumor exposure, and low incidence of systemic toxicity.[38] Oxaliplatin has been widely used in the HAIC therapy and shows favorable results.[39–41] In a multicenter phase 2 clinical trial, CRLM patients who received HAIC oxaliplatin achieved a response rate of 63%, even if the local concentration of oxaliplation is less than that of FUDR.[39,41]
HAIC has presented with a significantly longer survival and time to progression than other therapies, suggesting that HAIC might have great survival effects in HCC patients. The prolonged OS achieved in HCC patients with HAIC is similar to the findings reported in the previous studies of CRLM patients.[11,26,30] For example, in the randomized trial of efficacy, quality, and molecular markers (CALGB9481),[11] OS was significantly longer in HAIC group (24.4 months) than in systemic bolus fluorouracil and leucovorin treatment group (20.0 months) (P = .0034). Moreover, patients in HAIC group achieved a significantly higher probability of 2-year survival of 51%, as compared with 35% in systemic treatment group.[11] Similarly, in a prospective RCT of patients with CRLM, the mean survival was longer in HAIC group than in 5-FU infusion (HAO) group (19 vs 13 months, P = .0147).[30] The median time to progression in the 2 groups was 7 (1–23) months and 4 (1–22) months, respectively.[30] The better effect of HAIC suggest that, CRLM patients would benefit from HAIC in terms of prolonged OS and time to progression than other therapies.
Except for CRLM patients, advanced HCC patients were also found to achieve significantly longer OS and DFS when they were treated with HAIC. The better effect of HAIC in survival profile observed in advanced HCC patients was in consistent with the previous cohort studies.[33,34] Nouso et al[33] reported the effect results of HAIC of 5-FU and cisplatin for advanced HCC in Nationwide Survey of Primary Liver Cancer in Japan. In that study, 475 patients with advanced HCC who underwent HAIC were compared with 1466 patients who did not receive active therapy.[33] Results from propensity score-matched analysis (n = 682) showed that, the median OS time was significantly longer for patients with HAIC (14.0 months) than for patients without therapy (5.2 months, P < .0001).[33] Similar results were observed in studies comparing HAIC versus sorafenib for HCC patients with portal vein tumor thrombosis.[34] The median OS for HAIC and sorafenib group was 309 and 120 days, respectively (P = .022).[34] These results suggest that HAIC might improve the OS in patients with advanced HCC when compared with other therapies.
In this meta-analysis, we found that HAIC was associated with significantly higher ORR and DCR than other treatments for patients with HCC. In the HAIC group, 44.04% of patients achieved an overall response and 72.29% of patients achieved disease control, as compared to 19.33% and 50.36% of patients in other treatment group, respectively. Similar findings were observed in another studies.[11,25,29,31] He et al[25] performed a prospective, non-randomized, phase 2 trial to compare the efficacy of HAIC with modified FOLFOX (mFOLFOX) in patients with massive unresectable HCC. They reported that, the ORR and DCR were significantly higher in HAIC group than that in mFOLFOX group (54.1% vs 9.8%, P < .001; 83.8% vs 52.5%, P = .004).[25] Gao et al[31] compared HAIC combined chemoembolization with chemoembolization alone for inoperable HCC patients. Their results suggested that, the ORR in combination group was 68.9% as compared to 45.9% in chemoembolization group (P = .036). The DCR was 86.7% in combination group as compared with 70.3% in chemoembolization group; however, the difference between the two groups was not significant (P = .068).[31]
With regard to adverse events, our results showed that, most of the common complications were comparably occurred between the 2 treatment regimens. However, for some complications, such as nausea, hypoalbuminemia, pain, anemia, and hepatic toxicity, they were more frequently seen among patients who were treated with HAIC. For grade 3 and 4 level adverse events, the incidence of pain and decreased white blood count was found to be significantly higher in HAIC group than in other treatment group. Kudo et al[29] reported the safety results of sorafenib plus low-dose cisplatin and fluorouracil HAIC as compared with sorafenib alone for patients with advanced HCC. Their results showed that, grade 3 to 4 adverse events, including anemia, neutropenia, thrombocytopenia and anorexia, were more frequently occurred in combination group than in the sorafenib alone group.[29] Despite these adverse events might be related to cytotoxic agents cisplatin and fluorouracil, they were managed with treatment interruption or dose modification.
There are several potential limitations to note when interpreting our findings. First, moderate or substantial heterogeneity was observed among the included studies in this study. However, the source for heterogeneity was identified after the sensitivity analysis was performed. We think the heterogeneity might be related with the following factors: study design, patient characteristics, duration of following-up, chemotherapeutic drugs and doses, and comparators. Second, some of the included articles were cohort studies, and patients were not enrolled on a randomized basis, which might result in selection bias to the results and inevitably over/underestimation of the measured effects. Third, the sample size in several studies was relatively small. It is assumed that studies with small sample size were more likely to overestimate the treatment effect as compared with larger trials. Thus, large-scale trials are needed to draw definitive conclusions.
In conclusion, the present study demonstrated that HAIC might be a generally safe treatment, and it is more effective in prolonging the OS and DFS, and improving ORR and DCR for patients with HCC. These benefit effects were more obvious in CRLM or advanced HCC patients. Based on the promising results and potential limitations, more large-scale, randomized trials are needed to verify our findings.
Author contributions
Data curation: Guan-Bao Long, Chao-Wen Xiao.
Formal analysis: Chao-Wen Xiao, Xin-Yang Zhao.
Methodology: Guan-Bao Long, Chao-Wen Xiao, Xin-Yang Zhao, Jun Zhang, Xin Li.
Writing – original draft: Guan-Bao Long.
Writing – review and editing: Jun Zhang, Xin Li.
Footnotes
Abbreviations: 5-Fu = 5-fluoroouracil, CDDP = cisplatin, CIs = confidence intervals, CRLM = colorectal liver metastases, DCR = disease control rate, DFS = disease free survival, FUDR = floxuridine, HAIC = hepatic arterial infusion chemotherapy, HCC = hepatocellular carcinoma, HR = hazard ratio, NOS = Newcastle-Ottawa scale, ORR = objective response rate, OS = overall survival, OX = oxaliplatin, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCTs = randomized controlled trials, RR = risk ratio, TACE = transarterial chemoembolization.
How to cite this article: Long G-B, Xiao C-W, Zhao X-Y, Zhang J, Li X. Effects of hepatic arterial infusion chemotherapy in the treatment of hepatocellular carcinoma: a meta-analysis. Medicine. 2020;99:26(e20745).
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Parkin DM, Bray F, Ferlay J, et al. Estimating the world cancer burden: Globocan 2000. Int J Cancer V 94 2001;153–6. [DOI] [PubMed] [Google Scholar]
- [3].Donadon M, Solbiati L, Dawson L, et al. Hepatocellular carcinoma: the role of interventional oncology. Liver Cancer 2016;6:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lanza E, Donadon M, Poretti D, et al. Transarterial therapies for hepatocellular carcinoma. Liver Cancer 2016;6:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kudo M, Izumi N, Sakamoto M, et al. Survival analysis over 28 years of 173,378 patients with hepatocellular carcinoma in Japan. Liver Cancer 2016;5:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Adam R, De Gramont A, Figueras J, et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist 2012;17:1225–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 2016;150:835–53. [DOI] [PubMed] [Google Scholar]
- [8].Kudo M, Trevisani F, Abou-Alfa GK, et al. Hepatocellular carcinoma: therapeutic guidelines and medical treatment. Liver Cancer 2016;6:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ando E, Tanaka M, Yamashita F, et al. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer 2002;95:588–95. [DOI] [PubMed] [Google Scholar]
- [10].Baek YH, Kim KT, Lee SW, et al. Efficacy of hepatic arterial infusion chemotherapy in advanced hepatocellular carcinoma. World J Gastroenterol 2012;18:3426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kemeny NE, Niedzwiecki D, Hollis DR, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (calgb 9481). J Clin Oncol 2006;24:1395–403. [DOI] [PubMed] [Google Scholar]
- [12].Clavien PA, Selzner N, Morse M, et al. Downstaging of hepatocellular carcinoma and liver metastases from colorectal cancer by selective intra-arterial chemotherapy. Surgery 2002;131:433–42. [DOI] [PubMed] [Google Scholar]
- [13].Tang ZY, Zhou XD, Ma ZC, et al. Downstaging followed by resection plays a role in improving prognosis of unresectable hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2004;3:495–8. [PubMed] [Google Scholar]
- [14].Piedbois P, Buyse M, Kemeny N, et al. Reappraisal of hepatic arterial infusion in the treatment of nonresectable liver metastases from colorectal cancer. J Natl Cancer Inst 1996;88:252–8. [DOI] [PubMed] [Google Scholar]
- [15].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical research ed) 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wells GA, Shea B, O’Connell D, et al. (2011) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Available: www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 25 November 2012. [Google Scholar]
- [17].Higgins JP, Altman DG, Gotzsche PC, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed) 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [20].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [21].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ (clinical research ed) 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [23].Ishibashi K, Yoshimatsu K, Yokomizo H, et al. Low-dose leucovorin and 5-fluorouracil for unresectable multiple liver metastasis from colorectal cancer. Anticancer Res 2005;25:4747–52. [PubMed] [Google Scholar]
- [24].Kamimura K, Suda T, Yokoo T, et al. Transhepatic arterial infusion chemotherapy using a combination of miriplatin and CDDP powder versus miriplatin alone in the treatment of hepatocellular carcinoma: a randomized controlled trial. BMC Cancer 2017;17:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].He MK, Le Y, Li QJ, et al. Hepatic artery infusion chemotherapy using mfolfox versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study. Chin J Cancer 2017;36:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Feng WM, Tang CW, Huang SX, et al. Prophylactic adjuvant hepatic arterial infusion chemotherapy reduced hepatic metastases from stage III colorectal cancer after curative resection. Hepatogastroenterology 2012;59:1087–90. [DOI] [PubMed] [Google Scholar]
- [27].Zheng YY, Tang CW, Xu YQ, et al. Hepatic arterial infusion chemotherapy reduced hepatic metastases from pancreatic cancer after pancreatectomy. Hepatogastroenterology 2014;61:1415–20. [PubMed] [Google Scholar]
- [28].Jeong SW, Jang JY, Lee JE, et al. The efficacy of hepatic arterial infusion chemotherapy as an alternative to sorafenib in advanced hepatocellular carcinoma. Asia Pac J Clin Oncol 2012;8:164–71. [DOI] [PubMed] [Google Scholar]
- [29].Kudo M, Ueshima K, Yokosuka O, et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (silius): a randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol 2018;3:424–32. [DOI] [PubMed] [Google Scholar]
- [30].Naredi P, Oman M, Blind PJ, et al. A comparison between hepatic artery ligation and portal 5-FU infusion versus 5-FU intra arterial infusion for colorectal liver metastases. Eur J Surg Oncol V 29 2003;459–66. [DOI] [PubMed] [Google Scholar]
- [31].Gao S, Zhang PJ, Guo JH, et al. Chemoembolization alone vs combined chemoembolization and hepatic arterial infusion chemotherapy in inoperable hepatocellular carcinoma patients. World J Gastroenterol 2015;21:10443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Samaras P, Breitenstein S, Haile SR, et al. Selective intra-arterial chemotherapy with floxuridine as second- or third-line approach in patients with unresectable colorectal liver metastases. Ann Surg Oncol 2011;18:1924–31. [DOI] [PubMed] [Google Scholar]
- [33].Nouso K, Miyahara K, Uchida D, et al. Effect of hepatic arterial infusion chemotherapy of 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma in the nationwide survey of primary liver cancer in Japan. Br J Cancer 2013;109:1904–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Moriguchi M, Aramaki T, Nishiofuku H, et al. Sorafenib versus hepatic arterial infusion chemotherapy as initial treatment for hepatocellular carcinoma with advanced portal vein tumor thrombosis. Liver Cancer 2017;6:275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liang YH, Shao YY, Chen JY, et al. Modern prospection for hepatic arterial infusion chemotherapy in malignancies with liver metastases. Int J Hepatol 2013;2013:141590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sadahiro S, Suzuki T, Tanaka A, et al. Clinical significance of and future perspectives for hepatic arterial infusion chemotherapy in patients with liver metastases from colorectal cancer. Surg today 2013;43:1088–94. [DOI] [PubMed] [Google Scholar]
- [37].Kerr DJ, McArdle CS, Ledermann J, et al. Intrahepatic arterial versus intravenous fluorouracil and folinic acid for colorectal cancer liver metastases: a multicentre randomised trial. Lancet (London, England) 2003;361:368–73. [DOI] [PubMed] [Google Scholar]
- [38].Kingham TP, D’Angelica M, Kemeny NE. Role of intra-arterial hepatic chemotherapy in the treatment of colorectal cancer metastases. J Surg Oncol 2010;102:988–95. [DOI] [PubMed] [Google Scholar]
- [39].Ducreux M, Ychou M, Laplanche A, et al. Hepatic arterial oxaliplatin infusion plus intravenous chemotherapy in colorectal cancer with inoperable hepatic metastases: a trial of the gastrointestinal group of the Federation Nationale Des Centres De Lutte Contre Le Cancer. J Clin Oncol 2005;23:4881–7. [DOI] [PubMed] [Google Scholar]
- [40].Boige V, Malka D, Elias D, et al. Hepatic arterial infusion of oxaliplatin and intravenous LV5FU2 in unresectable liver metastases from colorectal cancer after systemic chemotherapy failure. Ann Surg Oncol 2008;15:219–26. [DOI] [PubMed] [Google Scholar]
- [41].Kern W, Beckert B, Lang N, et al. Phase I and pharmacokinetic study of hepatic arterial infusion with oxaliplatin in combination with folinic acid and 5-fluorouracil in patients with hepatic metastases from colorectal cancer. Ann Oncol 2001;12:599–603. [DOI] [PubMed] [Google Scholar]


