Abstract
Background:
Bipolar disorder (BD) is a chronic and disabling psychiatric disorder. The treatment of BD still remains a significant clinical challenge due to the complex nature of the disease. Nutraceutical therapy as adjunctive role is a promising therapy for BD. Sulforaphane (SFN), a broccoli extract, was reported to be effective for emotional problems and cognitive impairment. However, clinical research of SFN in the treatment of BD was rare. Therefore, this study is designed to evaluate the adjuvant role of SFN in the treatment of BD.
Methods:
This is a randomized, double-blinded, placebo-controlled, parallel-group clinical trial. A total of 100 patients who meet inclusion criteria will be assigned to receive quetiapine plus SFN or quetiapine plus placebo in a 1:1 ratio. The total duration of the study will be 12 weeks including 5 follow ups. The primary outcome is in the Montgomery–Asberg depression rating scale. The secondary outcomes are the quick inventory of depressive symptomatology—self report, Hamilton anxiety rating scale, young mania rating scale, cognitive function, inflammatory factors, and intestinal flora. Any adverse events will be recorded throughout the trial.
Discussion:
This trial will provide evidences to evaluate the efficacy and safety of SFN combined with quetiapine in the treatment of BD patients, as well as the adjuvant role of SFN in combination.
Trial registration:
This study protocol was registered at the Chinese clinical trial registry (ChiCTR2000028706).
Keywords: bipolar disorder, randomized clinical trials, sulforaphane
1. Introduction
Bipolar disorder (BD) is a common psychiatric disorder characterized by manic and depressive episodes, leading cause of global disability. Bipolar depressive disorder is associated with longer illness duration and poorer response to treatment than mania, impairing quality of life, social relationship, and occupational performance.[1,2] The treatment of bipolar depression is mainly emotional stabilizer, second generation antipsychotics. Clinical recommendation supported atypical antipsychotic drugs as quetiapine, lurasidone for treatment of acute bipolar depression.[3,4] Adverse events (AEs) at recommended doses of the psychoactive agents including akathisia, somnolence, sedation, and metabolic syndrome are associated with treatment compliance and poor treatment outcomes for patient with BD.[3,5–7] Hence, for managing BD, more effective and safer interventions are urgently needed.
There is now significant evidence implicating genetic susceptibility, neurotransmitter imbalance, inflammatory oxidative stress, neurotrophic factor signal transduction deficiency, and neuroendocrine abnormality in the pathophysiology of BD.[8–13] Activated immunity and elevated biomarkers for inflammation were found in patients with BD.[14,15] In addition, compared with the healthy controls, there were inflammatory changes in the prefrontal cortex of BD patients in post-mortem studies, mainly manifested by decreased anti-inflammatory factor level and increased inflammatory factor level.[16] The widely accepted hypothesis of inflammation, oxidative stress, and abnormal immune regulation are therapeutic targets for bipolar depression.
Recently, there has been an increasing interest in the use of plant-derived natural products as complementary treatments with less toxic and fewer side effects. Sulforaphane (1-isothiocyanate-4-methylsulfonyl-butane, SFN), a common antioxidant derived from cruciferous vegetables has been used widely in cancer treatment.[17] It plays a role in the antioxidant and prooxidant activities, canonically acting through the inhibition of inflammatory cytokine production and down regulation of nuclear factor-kappa B activity which ultimately affect the occurrence and progression of mental disorders.[18–21] At present, SFN has been used to treat mental disorder in the clinical studies, such as autism, and has achieved positive results.[22,23] Shiina et al reported that SFN improved cognitive function in patients with schizophrenia.[24] What's more, SFN was proving owning a certain inhibitory effect on depression-like behavior associated with inflammation-induced depression in mice.[25–27] However, the adjuvant effect of SFN in the treatment of patients with BD has never been studied and remains unknown.
In summary, SFN can influence the oxidative stress–inflammation pathways, resulting in a positive effect on cognitive and emotional development. This study aims to observe the adjuvant effects or improvement effects and safety of SFN in the quetiapine for bipolar depressive disorder, and explore the mechanisms by detecting changes in inflammatory factors and intestinal flora before and after treatment.
2. Methods
2.1. Study design and ethics approval
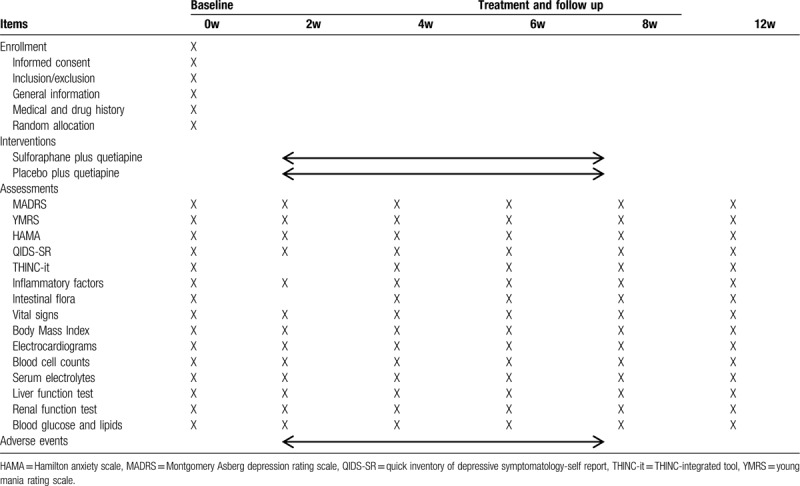
This is a randomized, double-blinded, placebo-controlled, parallel-group clinical trial. This protocol was approved by the Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (December 24th, 2019). The trial which is registered at the Chinese Clinical Registry (ChiCTR2000028706) will be conducted in the First Affiliated Hospital, College of Medicine, Zhejiang University in China. The clinic trial was supported by the grant of the National Natural Science Foundation of China (81971271). Figure 1 depicts a flow chart of the study. Patients enrolled in this study will randomly be assigned into 2 groups (with a 1:1 allocation ratio). Clinical manifestations and assessment scales, neurocognitive functions, and some of the blood biochemical tests will be checked before treatments started (baseline) and will be reexamined on week 2, 4, 8, 12 weeks of treatment to assess the effectiveness and safety. Meanwhile, inflammatory cytokines and intestinal flora were monitored during follow-up. Researchers will record and deal with the any adverse reactions and assess whether they can continue the experiment to protect the participants’ interests during the study. Specific schedules are summarized in Table 1.
Figure 1.
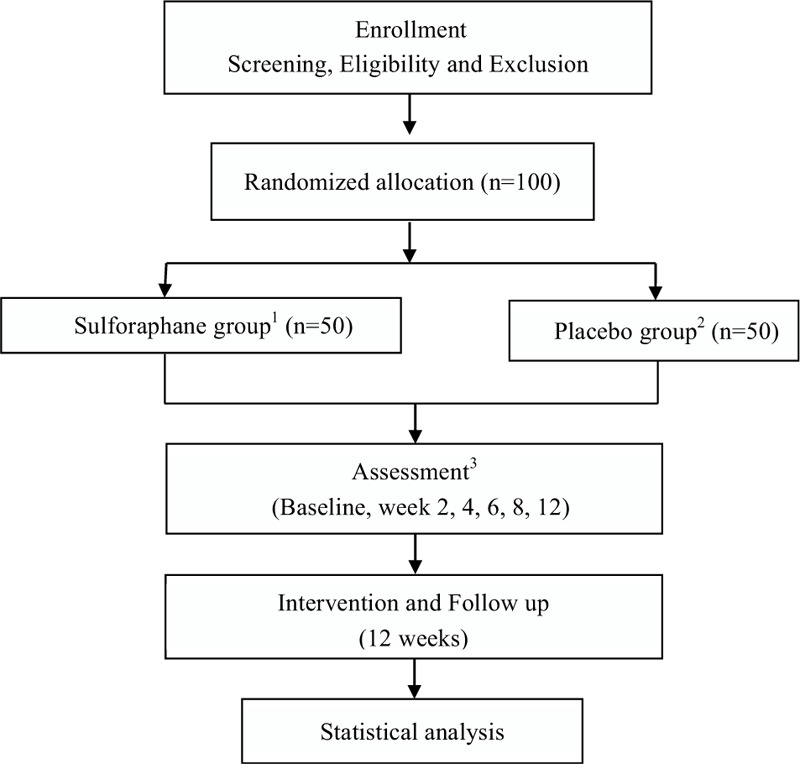
Flow chart of the study.
Table 1.
Schedule of the enrollment, intervention, and assessments in the process.
2.2. Study population
Participants who meet the eligibility criteria and sign informed consent will be enrolled in the study. Inclusion criteria for enrollment are described as follows:
-
(1)
males or females aged 16 to 65 years old;
-
(2)
agree to participate in the research and sign the informed consent form;
-
(3)
the subjects biological parents are Han;
-
(4)
diagnosed with bipolar depressive disorder according to the fourth Edition of Diagnostic and Statistical Manual of Mental Disorders diagnostic criteria;
-
(5)
score more than or equal to 17 points on the Hamilton depression scale at screening and baseline period;
-
(6)
score less than or equal to 5 points on the young mania rating scale (YMRS) at screening and baseline period;
-
(7)
no taking any drug treatment or no receiving antidepressant treatment in the last 1 month;
-
(8)
take drugs by oneself during the study period, or have a regular helper to help with the medication.
Exclusion criteria are described as follows:
-
(1)
diagnosed with other spectrum disorders according to the fourth Edition of Diagnostic and Statistical Manual of Mental Disorders diagnostic criteria;
-
(2)
mental disorder caused by substance abuse, or serious physical diseases;
-
(3)
received antibiotics within 1 month before enrollment due to respiratory tract, urinary tract, the digestive system infection;
-
(4)
subjects with a history of attempted suicide, or currently at high suicide risk, or with suicide behavior/attempt, or scoring more than or equal to 3 points on the 10th clause of Montgomery Asberg Depression Rating Scale (MADRS);
-
(5)
unwilling to take the medicine;
-
(6)
known pregnancy, lactation, or pregnant planning;
-
(7)
the subjects with contraindications of quetiapine or have used quetiapine but have poor efficacy;
-
(8)
allergic to broccoli;
-
(9)
received electroconvulsive therapy within 1 month.
Suspension criteria are as follows:
-
(1)
subjects with serious adverse drug reactions;
-
(2)
exchange drugs or receive other treatment due to unstable condition;
-
(3)
refuse to continue participating in the study;
-
(4)
lose track in follow-up;
-
(5)
other factors cause the interview to stop.
2.3. Randomization and blinding
The enrolled participates will be randomly assigned a random number sequence which generated by SAS ver.9.0 for Microsoft Windows (SAS InstituteInc, Cary, NC) in a 1:1 ratio. A sealed envelope, independently managed by a third researcher was used to allocation concealment. The researcher will not take part in follow-up and assessment. Neither assessors nor subjects will know the assigned group and the intervening measure until the study is terminated.
2.4. Groups and interventions
In this study, enrolled bipolar depressed patients are randomly assigned into 2 groups: SFN group and placebo group and will undergo 12-week quetiapine concomitantly. The initial treatment dose of quetiapine in the 2 groups are 50 mg daily, and will be added to 300 mg daily within 2 weeks. The patients in SFN group will receive 1 tablet of Avmacol Extra Strength (480 mg) daily swallowed with warm water (no more than 40 degrees) after breakfast or chewed at the same meal, and placebo group will receive a placebo in the same way. Avmacol Extra Strength contains Proprietary Sulforaphane Glucosinolate (Glucoraphanin) & Myrosinase Blend 490 mg including Broccoli Seed & Sprout Extract (containing more than 30 mg of glucoraphanin). The placebo matches Avmacol Extra Strength with size, shape, taste, and package without any active ingredient. Both of them used in this study are produced by the Nutramax Laboratories Consumer Care (Lancaster, SC, USA).
2.5. Outcomes
Primary outcome is the change in the MADRS scores at baseline compared to each treatment time point. MADRS is a clinician-administered rating scale and widely used to assess the depression severity of patient with BD. The response was defined as at least a 50% reduction when compared to baseline in the scores.
While secondary outcomes include the scores of the quick inventory of depressive symptomatology-self report, Hamilton anxiety rating scale, and YMRS scores are used to elevate the broader affective symptoms. Besides, cognitive function which tested by THINC-integrated tool, inflammatory factors (interleukin-2, interleukin-4, interleukin-6, interleukin-10, interleukin-17A, tumor necrosis factor and interferon-γ) and intestinal flora are used to investigate the underlying biological factors.
2.6. Safety assessment
Vital signs, body mass index, the liver and kidney function, levels of serum electrolytes, blood glucose and lipids, blood cell counts, and electrocardiograms will be conducted on all participants. At each participant's visit, AEs will be assessed. The severity of AEs were assign as mild, moderate, severe, or serious. We will record the onset date, end date, severity, the relationship with the clinical trial, the outcomes, and the measures adopted in the AEs form and provide appropriate treatment if any AEs occur during the study process. The trial will be stopped in case of treatment-related suspected unexpected serious AEs.
2.7. Sample size
It was absent of direct evidences to support t our hypothesis, consequently, estimating the sample size is difficult. At an 8-week study, the response rate of quetiapine in BD patients was 58.9%, and the remission rate was 42.1%.[28] Combination therapy has been shown to be more effective. We estimated the effective rate of SFN combined with quetiapine in the treatment of bipolar depressive disorder to be about 70%. The sample size with 80% power at a significance level of 0.05 was calculated in this study. Anticipating a 10% dropout rate, a total of 100 patients, with 50 patients in each group, will be needed.
2.8. Statistical analysis
All statistical analyses will be performed using SPSS 19.0 software (IBM, Armonk, NY) in a blinded manner. Outcomes will be analyzed on the basis of intention-to-treat principle. Missing values will be handled by the practical guide described by Jakobsen et al. The comparison of primary outcomes and secondary outcomes between 2 groups will be performed by t test for continuous variables and χ2 test for categorical variables, or with Wilcoxon Mann–Whitney test. The paired t test will be used for analysis in each group. P < .05 will be considered significant
3. Discussion
As a chronic and complex mood disorder, the treatment of BD frequently required combinations of therapies. A number of pharmacotherapeutic treatments with side effects induce low adherence of patient and poor outcomes. Nutraceutical agents with higher acceptability are more and more considered to improve treatment for the symptoms of BD as adjunctive therapies.[29] In the treating mental disorders, SFN has been proved have a significant improvement in mood and cognitive function by regulating peroxidation reactions.[25,30] The aim of this randomized clinical trial is to investigate the effect of SFN as an adjuvant role in the treatment of bipolar depressive disorder.
In this study, besides using the MADRS, Hamilton depression scale, Hamilton anxiety rating scale, quick inventory of depressive symptomatology-self report, and YMRS scales to assess the clinical curative effect on emotional problem, THINC-integrated tool will be introduced to evaluate neurological status. In addition, we monitor inflammatory factors including serum concentration of and intestinal flora before and after treatment to explore the possible mechanism of SFN combined with quetiapine on treating bipolar depressive disorder. In summary, we expected that our findings will provide evidence for efficacy and safety of SFN as an adjuvant treatment of BD.
This study also has some limitations. First, this is an exploratory trial with evaluating the intervention by various outcomes and process measures from multiple perspectives, the results should be cautiously interpreted. Besides, as a nutritional supplement, the dose of SFN in the preliminary study is conventional, but perhaps that's not necessarily a most effective dose. Thus, further study is required to explore an optimal dose plan for SFN as an adjuvant in the treatment of bipolar depressive disorder.
In summary, this trial is expected to provide initial findings on the synergistic effect of SFN in the treatment of BD. If, as expected, combination of quetiapine and SFN therapy could have more positive effects, it will provide an opportunity to increase the availability of evidence treatment for patients with BD.
Author contributions
Conceptualization: Xingyang Chen.
Supervision: Shaohua Hu, Yi Xu.
Writing – original draft: Congchong Wu.
Writing – review & editing: Jianbo Lai.
Footnotes
Abbreviations: AEs = adverse events, BD = bipolar disorder, HAMA = Hamilton anxiety scale, MADRS = Montgomery Asberg depression rating scale, SFN = sulforaphane, YMRS = young mania rating scale.
How to cite this article: Wu C, Chen X, Lai J, Xu Y, Hu S. The efficacy and safety of sulforaphane as an adjuvant in the treatment of bipolar depressive disorder: study protocol for a randomized, double-blinded, placebo-controlled, parallel-group clinical trial. Medicine. 2020;99:26(e20981).
CW, XC contributed equally to this study.
Placebo preparation for the trial is ongoing at the time of submission. Recruitment has not yet begun.
The clinical trial was supported by the grant of the National Natural Science Foundation of China (81971271).
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Harrison PJ, Geddes JR, Tunbridge EM. The emerging neurobiology of bipolar disorder. Trends Neurosci 2018;41:18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hu SH, Han YQ, Mou TT, et al. Association of genetic polymorphisms with age at onset in Han Chinese patients with bipolar disorder. Neurosci Bull 2019;35:591–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Garay RP, Llorca PM, Young AH, et al. Bipolar disorder: recent clinical trials and emerging therapies for depressive episodes and maintenance treatment. Drug Discov Today 2014;19:1792–800. [DOI] [PubMed] [Google Scholar]
- [4].Suttajit S, Srisurapanont M, Maneeton N, et al. Quetiapine for acute bipolar depression: a systematic review and meta-analysis. Drug Des Devel Ther 2014;8:827–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tamayo JM, Zarate CA, Vieta E, et al. Level of response and safety of pharmacological monotherapy in the treatment of acute bipolar I disorder phases: systematic review and meta-analysis. Int J Neuropsychopharmacol 2010;13:813–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Young AH, McElroy SL, Bauer M, et al. Double-blind, placebo-controlled study (EMBOLDEN I) of quietapine and lithium monotherapy in adults in the acute phase of bipolar depression. J Clin Psychiatry 2010;71:150–62. [DOI] [PubMed] [Google Scholar]
- [7].Centorrino F, Masters GA, Talamo A, et al. Metabolic syndrome in psychiatrically hospitalized patients treated with antipsychotics and other psychotropics. Hum Psychopharmacol 2012;27:521–6. [DOI] [PubMed] [Google Scholar]
- [8].Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet 2012;13:537–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rosenblat JD, Cha DS, Mansur RB, et al. Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog Neuropsychopharmacol Biol Psychiatry 2014;53:23–34. [DOI] [PubMed] [Google Scholar]
- [10].Zhao F, Yang J, Cui R. Effect of hypoxic injury in mood disorder. Neural Plast 2017;2017:6986983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Grande I, Berk M, Birmaher B, et al. Bipolar disorder. Lancet 2016;387:1561–72. [DOI] [PubMed] [Google Scholar]
- [12].Castren E, Kojima M. Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol Dis 2017;97:119–26. [DOI] [PubMed] [Google Scholar]
- [13].Linkowski P. Neuroendocrine profiles in mood disorders. Int J Neuropsychopharmacol 2003;6:191–7. [DOI] [PubMed] [Google Scholar]
- [14].Anderson G, Maes M. Bipolar disorder: role of immune-inflammatory cytokines, oxidative and nitrosative stress and tryptophan catabolites. Curr Psychiatry Rep 2015;17:541. [DOI] [PubMed] [Google Scholar]
- [15].Culmsee C, Michels S, Scheu S, et al. Microglia and immune system—how are they linked in affective disorder? Front Psychiatry 2019;9:739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bezchlibnyk YB, Wang JF, McQueen GM, et al. Gene expression differences in bipolar disorder revealed by cDNA array analysis of post-mortem frontal cortex. J Neurochem 2001;79:826–34. [DOI] [PubMed] [Google Scholar]
- [17].Tortorella SM, Royce SG, Licciardi PV, et al. Dietary sulforaphane in cancer chemoprevention: the role of epigenetic regulation and HDAC inhibition. Antioxid Redox Signal 2015;22:1382–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dinkova-Kostova AT, Fahey JW, Kostov RV, et al. KEAP1 and done? Targeting the NRF2 pathway with sulforaphane. Trends Food Sci Technol 2017;69:257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fahey JW, Wade KL, Wehage SL, et al. Stabilized sulforaphane for clinical use: phytochemical delivery efficiency. Mol Nutr Food Res 2017;61:1600766. [DOI] [PubMed] [Google Scholar]
- [20].Staurengo-Ferrari L, Badaro-Garcia S, Hohmann MSN, et al. Contribution of Nrf2 modulation to the mechanism of action of analgesic and anti-inflammatory drugs in pre-clinical and clinical stages. Front Pharmacol 2018;9:1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang JC, Yao W, Dong C, et al. Keap1- Nrf2 signaling pathway confers resilience versus susceptibility to inescapable electric stress. Eur Arch Psychiatry Clin Neurosci 2018;268:865–70. [DOI] [PubMed] [Google Scholar]
- [22].Bent S, Lawton B, Warren T, et al. Identification of urinary metabolites that correlate with clinical improvements in children with autism treated with sulforaphane from broccoli. Mol Autism 2018;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Singh K, Connors SL, Macklin EA, et al. Sulforaphane treatment of autism spectrum disorder (ASD). Proc Natl Acad Sci USA 2014;111:15550–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shiina A, Kanahara N, Sasaki T, et al. An open study of sulforaphane-rich broccoli sprout extract in patients with schizophrenia. Clin Psychopharmacol Neurosci 2015;13:62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang JC, Yao W, Dong C, et al. Prophylactic effects of sulforaphane on depression-like behavior and dendritic changes in mice after inflammation. J Nutri Biochem 2017;39:134–44. [DOI] [PubMed] [Google Scholar]
- [26].Ferreira-Chamorro P, Redondo A, Riego G, et al. Sulforaphane inhibited the nociceptive responses, anxiety- and depressive-like behaviors associated with neuropathic pain and improved the anti-allodynic effects of morphine in mice. Front Pharmacol 2018;9:1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wu S, Gao Q, Zhao P, et al. Sulforaphane produces antidepressant- and anxiolytic-like effects in adult mice. Behav Brain Res 2016;301:55–62. [DOI] [PubMed] [Google Scholar]
- [28].Jeong JH, Bahk WM, Woo YS, et al. Efficacy of quetiapine in patients with bipolar I and II depression: a multicenter, prospective, open-label, observational study. Neuropsychiatr Dis Treat 2013;9:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dean OM, Gliddon E, Van RTE, et al. An update on adjunctive treatment options for bipolar disorder. Bipolar Disord 2018;20:87–96. [DOI] [PubMed] [Google Scholar]
- [30].Li S, Yang C, Fang X, et al. Role of Keap1-Nrf2 signaling in anhedonia symptoms in a rat model of chronic neuropathic pain: improvement with sulforaphane. Front Pharmacol 2018;9:887. [DOI] [PMC free article] [PubMed] [Google Scholar]


