Abstract
Background:
Over the past decade, rates of cardiac implantable electronic device (CIED) related infections have increased and been associated with increased morbidity, mortality and financial burden on healthcare systems.
Methods:
To examine the effect of an antibacterial envelope in reducing major CIED related infections, we performed a systematic review and meta-analysis by searching PubMed/MEDLINE, CENTRAL, Google scholar and Clinicaltrials.gov for studies that examined the effect of an antibiotic envelope in reducing major related CIED infections, comprising of device-related endocarditis, systemic infection requiring systemic antibiotics and or device extraction, compared to control up till February 15th, 2020. A random-effects meta-analysis was conducted by calculating risk ratios (RR) and respective 95% confidence intervals (CI).
Results:
We include 6 studies that comprise of 11,897 patients, of which 5844 received an antibiotic envelope and 6053 did not. Compared with control, utilization of an antibiotic envelope at the time of procedure was associated with a significant 74% relative risk reduction in major CIED related infections among patients at high risk for infection (RR: 0.26 [95% CI, 0.08–0.85]; P = .03), while no significant reduction was observed among patients enrolled from studies with any risk for infection (RR: 0.53 [95% CI, 0.06–4.52]; P = .56). Additionally, no reduction in mortality among patients that received an envelope compared to control was observed (RR: 1.15 [95% CI, 0.53–2.50]; P = .72).
Conclusion:
The utilization of an antibiotic envelope at the time of device implantation or upgrade reduces major CIED infections, especially if used in patients perceived to be at higher risk for infection.
Keywords: antibacterial envelope, cardiac implantable electronic device, infection, pacemaker
1. Introduction
Every year, it is estimated that over 1 million patients around the world receive a cardiac implantable electronic device (CIED).[1] With the advancement in technology and widespread accessibility to CIEDs around the world, an entity called CIED related infections has emerged. Over the last decade, the incidence of CIED related infections has been estimated to complicate around 1% to 4% of all device implantations, despite the use of prophylactic strategies such as pre-operative antibiotics and sterile surgical techniques, leading to significant morbidity, mortality and cost to health care systems.[2–8] It has been estimated that around 50% of CIED infections are attributed to 2 gram-positive organisms, such as Staphylococcus aureus and Staphylococcus epidermidis, and are believed to stem from contamination of the subcutaneous pocket that harbors the system generator at the time of device implantation.[9,10] According to one study, the mean cost of treating a patient with a major CIED related infection was well over $50,000 and the average length of hospitalization was 13 days.[11] To address these concerns, a multifilament mesh envelope that elutes two antibiotics, rifampin and minocycline, was approved by the Food and Drug Administration (FDA) for CIED stabilization with the aim of reducing CIED related infections.[12] Over the past decade, numerous observational studies[9,11,13–16] and only one large-scale randomized control trial,[17] have been conducted to evaluate the effect of an antibiotic envelope on rates of CIED infections, and have yielded variable results. To enhance the power for assessing the effect of this envelope in the prevention of CIED infections, we conducted a literature review and meta-analysis of studies examining the use of an antibacterial envelope compared with control in patients undergoing CIED implantation or upgrade.
2. Methods
2.1. Search strategy
A review of the literature of published studies that reported on prevention of CIED infections using an antimicrobial envelope was performed according to the Preferred Reporting System for Systematic statement Reviews and Meta-Analysis (PRISMA) guidelines.[18] Articles were extracted from PubMed/Medline, CENTRAL, Google Scholar and ClinicalTrials.gov from inception till February 15th, 2020. Several keywords were used including, “antimicrobial envelope,” “antibiotic envelope,” “pacemaker,” “CIED,” “defibrillator,” “TYRX,” and “AIGISRx”. Wild cards at the end of the keywords were used to broaden the search field. A manual search for published conference abstracts and posters was performed. Articles were screened initially by reviewing the title and then the abstract. Full-text evaluation was done after agreement on potentially suitable articles. This study was exempt from ethical approval as all data for this study were collected from published trials.
2.2. Study eligibility and outcomes
In our systematic review and meta-analysis, we included studies that used an antibacterial envelope to reduce CIED infections compared to matched controls. There were no restrictions on language, publication status or publication date. We excluded studies without matched controls or had inadequate follow up, ongoing trials, editorials and duplicate studies. The only antibacterial envelope used in all studies was developed by TYRX Inc. (a subsidiary of Medtronic, Inc., Minneapolis, MN) that received Food and Drug Administration clearance to market AIGISRx in 2008, now called the TYRXTM Antibacterial Envelope. Outcome information was extracted from individual studies, their online supplementary materials as well as contacting corresponding authors for detailed outcome data. The primary endpoint used in our meta-analysis was the occurrence of a major device-related infection, defined as a systemic infection or endocarditis necessitating systemic antibiotics and or device extraction following the initial procedure. We also examined the difference in overall mortality between patients that received an antibiotic envelope compared to control in studies reporting on mortality rates. Any study that did not provide adequate outcome details was excluded from the analysis.
2.3. Data extraction and study appraisal
Two independent authors (AA and SA) reviewed data using predetermined criteria and the same authors independently extracted the relevant data into a data sheet. A third investigator (MS) resolved any differences that arose while reviewing and extracting data. The risk of bias in non-randomized studies of interventions (ROBINS-I) tool was used to assess for bias in non-randomized studies and the Cochrane collaboration guidelines were used to assess for the potential risk of bias in the randomized trial.[19,20]
2.4. Statistical analyses
The outcomes of major device-related infections and overall mortality were analyzed as dichotomous variables, and risk ratios (RR) and their respective 95% confidence intervals (CI) were obtained using the Mantel-Haenszel method. As studies used in the analysis differed in sample size and population characteristics, a random-effects model was used in the initial analysis. A fixed-effects model was also used as part of the sensitivity analysis. Heterogeneity among studies was assessed using the Cochran Q test, and Higgins I2 was used to assess the degree of inconsistency in study results. A two-tailed P value < .05 was used to indicate significance. Assessment for publication bias was conducted by examination of funnel plots for asymmetry.[21] Review Manager version 5.3 (RevMan; Cochrane Collaboration) was used to analyze all study data.
3. Results
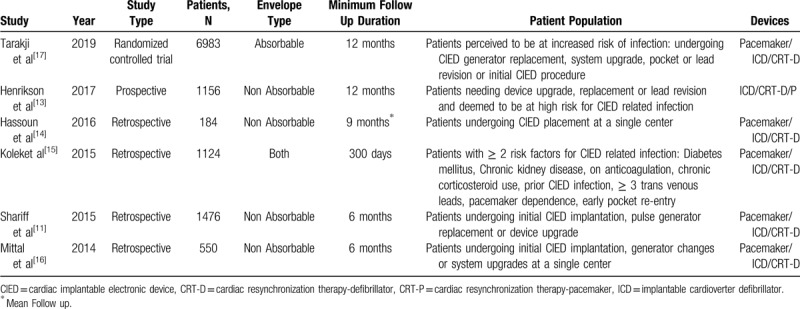
Our literature search identified 2691 studies, of which 6 full texts met our predefined inclusion criteria.[11,13–17] The PRISMA flowchart for this analysis is shown in Figure 1. A total of 11,897 patients were included in this meta-analysis (5844 patients with an antibiotic envelope and 6053 patients without an antibiotic envelope). The characteristics of individual studies are represented in Table 1. This meta-analysis included studies mainly conducted in North America; however, the WRAP-IT trial[17] also included patients recruited from centers from Asia, Africa, Australia, South America, and Europe. The minimum follow up duration was 6 months and ranged from 6 months to 1-year. In the sensitivity analysis, the fixed effects model showed no significant difference in overall results.
Figure 1.
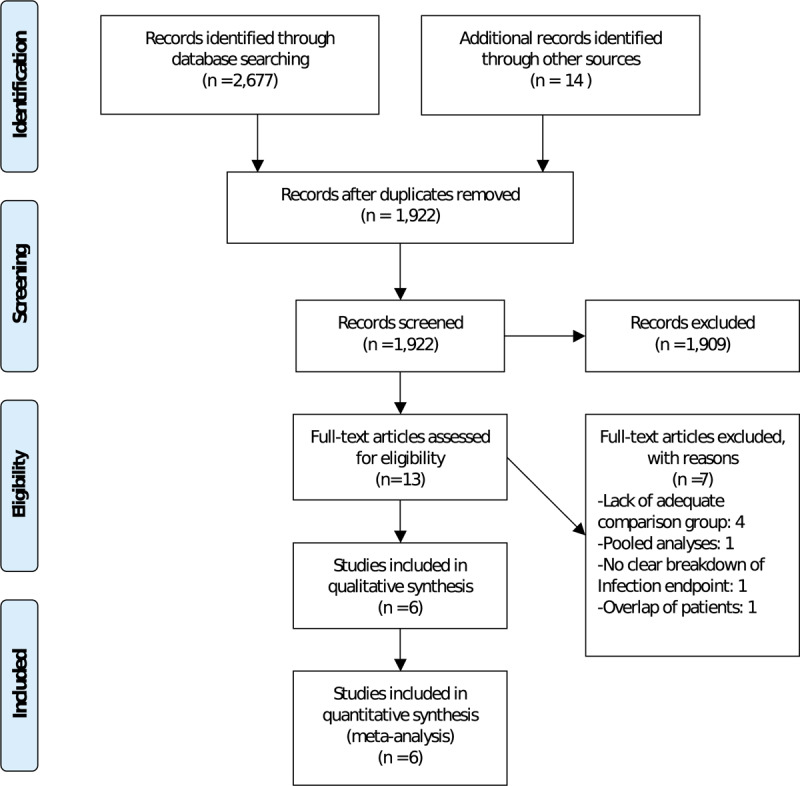
PRISMA flowchart.
Table 1.
Study characteristics.
3.1. Major CIED related infections
A total of 5 observational studies and 1 randomized control trial, a total of 11,897 patients, reported outcome data on major CIED related infections. Figure 2 indicates that patients who received an antibiotic envelope at the time of CIED implantation had a significantly lower risk of major device-related infections requiring systemic antibiotics and or device extraction compared to control (0.67% vs 1.69%; RR: 0.34 [95% CI, 0.14–0.86]; P = .02, I2 = 65%). However, when examined according to risk for infection in included studies, studies that exclusively enrolled patients at higher risk for CIED infections had a significant reduction in major infection rates (RR: 0.26 [95% CI, 0.08–0.85]; P = .03), while patients from studies that included patients at any risk for CIED related infections did not (RR: 0.53 [95% CI, 0.06–4.52]; P = .56).
Figure 2.
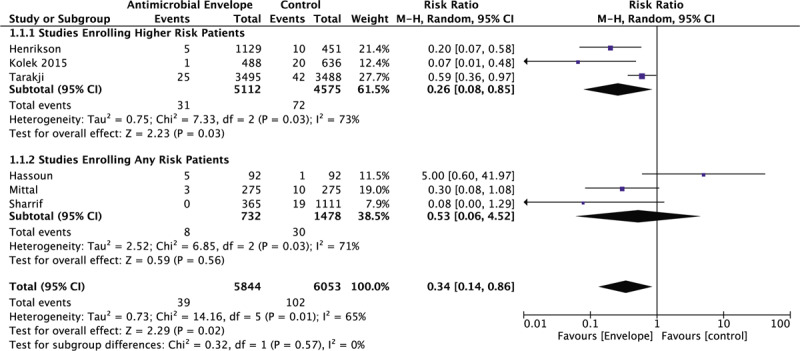
Forrest plot of the effect of an antibiotic envelope on major CIED related infections according to patient risk. CI = Confidence Interval, CIED = cardiac implantable electronic device, M-H = Mantel-Haenszel.
3.2. Randomized and propensity matched patients
Propensity matched data from 3 observational studies and data from one randomized controlled trial, a total of 8892 patients, were included in a secondary analysis for major CIED related infections (Fig. 3). Each group in individual studies was similar in baseline characteristics and risk for CIED related infections due to the inclusion of patients from a randomized controlled trial and propensity-matched patients from observational studies. A significant 70% relative risk reduction (RRR) in major CIED related infections requiring systemic antibiotics and or device extraction was observed in patients that received an antibiotic envelope compared to control (0.63% vs 1.53%, respectively; RR: 0.30 [95% CI, 0.11–0.83]; P = .02, I2 = 49%).
Figure 3.
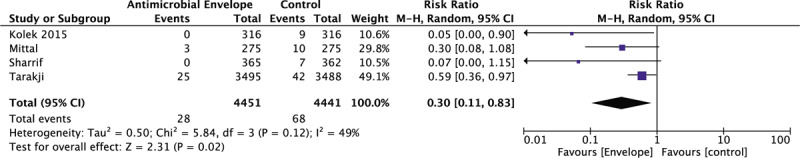
Forrest plot of the effect of an antibiotic envelope on major CIED related infections among randomized and propensity score-matched patients. CI = confidence interval, CIED = cardiac implantable electronic device, M-H = Mantel-Haenszel.
3.3. Overall mortality
A total of 4 studies, comprising of 9799 patients, reported outcome data on mortality in patients undergoing CIED procedures with and without antibiotic envelopes. Figure 4 shows that patients who received an antibiotic envelope at the time of CIED implantation had no significant mortality benefit compared with control (9.32% vs 8.39%; RR: 1.15 [95% CI, 0.53–2.50]; P = .72, I2 = 87%).
Figure 4.
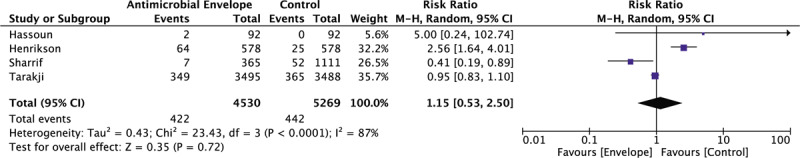
Forrest plot of the effect of an antibiotic envelope on overall mortality. CI = confidence interval, CIED = cardiac implantable electronic device, M-H = Mantel-Haenszel.
4. Discussion
This meta-analysis demonstrates several important findings regarding the use of an antibiotic envelope to reduce major CIED related infections. Collectively, the risk of major CIED related infection was significantly reduced by 66% in patients receiving an antibiotic envelope when compared to control. In a subgroup analysis including studies that exclusively enrolled patients at high risk for CIED related infections, the use of an antibiotic envelope was associated with a 74% RRR in major CIED related infections. However, among studies that enrolled patients at any risk for infection, there was no significant reduction in major CIED infections in the envelope group compared to control. To more accurately estimate the reduction in risk of major CIED related infection, we performed a secondary analysis only including randomized or propensity-matched patients, which showed a significant 70% RRR in major CIED infections in the envelope group compared to control irrespective of predetermined risk for infection. However, this reduction in major CIED related infections was not translated into any significant reduction in overall mortality in patients receiving an antibiotic envelope compared to control. Our results differ from an earlier meta-analysis of observational studies, published prior to results of the WRAP-IT trial,[17] that concluded that an antibiotic envelope reduces major CIED infections by 71% regardless of device type or patient risk.[22]
CIED related infections are rare but serious events that require an extended duration of systemic antibiotics, device extraction and have even culminated into death.[23] In 2009, a large randomized controlled trial using cefazolin compared to placebo prior to CIED implantation demonstrated that the risk of device-related infections was reduced dramatically by 81% in the group receiving antibiotics compared to control.[3] Despite the utilization of standard sterile surgical techniques and the administration of pre-procedure antibiotics the risk of CIED related infections remains high.[2,4] In 2008, the FDA approved the use of an antibiotic envelope coated with minocycline and rifampin intending to reduce device-related infections among patients undergoing de-novo CIED implantation or device upgrade. Since the emergence of the antibiotic envelope in clinical practice, there have been varying conclusions regarding its efficacy in reducing device-related infections.
One of the earliest studies to report their results on the use of an antibiotic envelope was conducted by Bloom et al,[12] in which 624 patients from multiple centers undergoing a CIED procedure all received an antibiotic envelope. After a short follow up period of 1.9 ± 2.4 months, there were only 3 device-related infections and they exclusively occurred among patients at higher risk of infection, either undergoing device replacement or revision. In a retrospective study of 184 patients that underwent CIED implantation by Hassoun et al,[14] there was a 5 times higher incidence of major device-related infections among the envelope group compared to control (5.4% vs 1.1%). However, in this study, the decision to use an antibiotic envelope or not was made by the implanting cardiologist without any predefined criteria. Also, compared to the control group, a higher proportion of patients in the envelope group were chronically using corticosteroids, undergoing device replacements or revisions (51.1% vs 8.7%) and had devices with > 2 intra-cardiac leads (42.4% vs 29.3%).
With the aim to better estimate the effect of the antibiotic envelope, two studies by Kolek et al retrospectively studied the effect of an antibiotic envelope on reducing CIED related infections among patients deemed to be at high risk for CIED infection.[9,15] According to their studies, risk factors for infection were defined as having >2 of the following; diabetes mellitus, chronic kidney disease, use of anticoagulant, chronic corticosteroid use, prior CIED related infection, ≥3 lead devices or ≥1 abandoned lead, pacemaker dependence and pocket re-entry within 2 weeks. In their earlier study,[9] 260 patients received an antibiotic envelope and 639 site-matched controls did not. After 90 days, 1 patient from the envelope group had a major CIED infection compared to 19 in the control group (OR: 0.13, 95% CI: 0.02–0.95, P = .04). In order to effectively eliminate any differences between patients and controls at baseline, 209 patients from the envelope group were propensity score-matched with 209 patients that did not receive an envelope. The beneficial effect of the envelope in reducing major CIED infections persisted despite propensity score matching (OR: 0.11, 95%CI: 0.01–0.85; P = .035).[9] Results from their second study,[15] were in line with results from their previous study,[9] further emphasizing the benefit of the use of an antibiotic envelope among high-risk patients for CIED infection.
To efficiently utilize the antibiotic envelope in clinical practice, Mittal et al conducted a regression model to identify independent risk factors for CIED infections.[16] Based on their retrospective analysis of 2891 patients that underwent a CIED procedure, 7 independent risk factors for CIED infection were identified; male sex, heart failure, hypertension, glomerular filtration rate <60 mL/min, diabetes mellitus, early pocket re-exploration and undergoing a device upgrade. Additionally, they stratified their patients into 3 groups based on their composite risk score that ranged from 0 to 25; low risk (0–7) with 1% infection risk; medium risk (8–14) with 3.4% infection risk; and high risk (≥15) with >11% risk for infection. They were also able to conclude that an envelope was unnecessary when implanting a permanent pacemaker (PPM) regardless of risk group and in one-third of implantable cardioverter defibrillators / cardiac resynchronization therapy-defibrillator (ICD)/(CRT-D) procedures in the low risk (score 0–7) group.
One of the initial large-scale multicenter prospective cohort studies to determine the efficacy of a non-absorbable antimicrobial envelope in patients deemed to be at high risk for CIED infections was conducted by Henrikson et al.[13] Their study was comprised of 2 prospective registries, the Centurion and Citadel registries, enrolling patients undergoing CRT or ICD replacement/upgrade, respectively. Three separate control groups, site-matched, previously published and Medicare-database comorbidity matched controls, were compared to patients from their registries. Compared to a previously published benchmark infection rate of 2.2%, patients from both registries had less than one-fifth of the rate of major CIED infections (0.4%; P value = .002). At 12 months of follow-up of CRT related procedures, rates of major CIED infections were 0.7% compared to 1.0% in site-matched controls (P = .38) and 1.3% among Medicare-database comorbidity matched controls (P = .02). Additionally, patients undergoing an ICD related procedure with an envelope had around 90% less major CIED infections compared to published controls (0.2% vs 2.2%; P value = .005).
The WRAP-IT trial is the only randomized controlled trial and the largest study to date that utilized antibiotic envelopes intending to reduce major device-related infections.[17] In 6983 patients deemed to be at increased risk for CIED related infections, including patients undergoing generator replacements, device upgrade or revisions and those undergoing an initial CRT-D, 3,495 patients were randomized to the envelope group. After 12 months of follow up, there were 30 major CIED infections in the envelope group compared to 45 among controls, a 40% reduction in risk of major CIED infections was observed (hazard ratio, 0.60; 95% CI, 0.36–0.98; P = .04). However, after 36 months of follow-up, no observed overall mortality benefit was observed between the envelope and control groups, 17.4% and 17.8%, respectively (hazard ratio, 0.96; 95% CI, 0.83–1.11).
Ideal and effective management of major CIED related infections generally requires device extraction and re-implantation leading to significant health care costs.[7] A retrospective analysis of 1476 CIED procedures, in which 365 patients received an antibiotic envelope by Shariff et al,[11] estimated the cost-effectiveness of using an antibiotic envelope in clinical practice. In their study, 19 patients were hospitalized for CIED related infections and they were all among the group that did not receive an antibiotic envelope. The cost of treating these patients summed to $1,043,592, with an average of > $50,000 per patient and a mean of 13 days of inpatient care. Based on their data, at an infection rate of 1.59%, the cost to treat and care for these patients would be balanced by the cost of using an envelope in every patient in their center.
Several studies have reported on the effect of an antibiotic envelope and CIED infection rates according to CIED type.[16,17] Based on results from the WRAP-IT trial[17] there was nearly a 50% reduction in risk of CIED infection in patients that received an envelope undergoing a high power device procedure (ICD or CRT-D) compared to control (HR 0.51; 95% CI: 0.29–0.90); however, there was an equal amount of CIED infections in either group of patients undergoing implantation of a lower power device (PPM or CRT-P), which is in line with the conclusion derived by Mittal et al.[16] A recent meta-analysis combining data from Mittal et al[16] and the WRAP-IT trial[17] concluded that patients undergoing high power device implantation with an antibiotic envelope had a significant 66% reduction in risk of major CIED infection compared to controls. Meanwhile, there was no derived significant reduction observed among patients receiving a low power device.[24]
This meta-analysis has several limitations. First, data was derived at the study level for assessment of outcomes and not at the individual level. Also, the majority of studies included are observational in nature with only one being a randomized controlled trial. There was significant heterogeneity within the meta-analysis attributed to varying patient populations and the number of participants in each study and funnel plot inspection for the individual analyses was visually asymmetrical, indicating the possible presence of publication bias (Fig. 5). Additionally, a majority of the envelopes used in the studies included in the meta-analysis used a non-absorbable envelope, for the exception of the WRAP-IT trial[17] and 135 patients from the study conducted by Kolek et al.[15]
Figure 5.
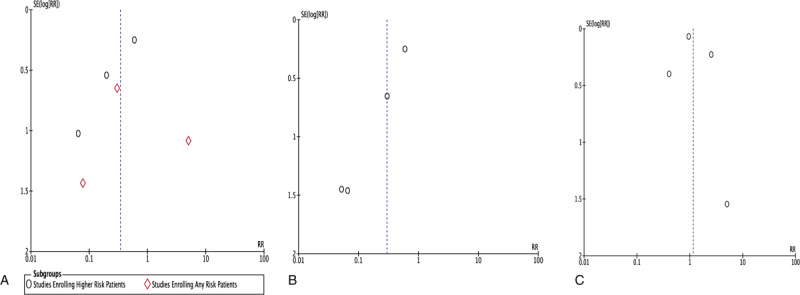
Funnel plots for individual analyses. Panel A: Funnel plot for major CIED related infections according to patient risk; Panel B: Funnel plot for major CIED related infections among randomized and propensity score-matched patients; Panel C: Funnel plot for overall mortality.
5. Conclusion
In conclusion, our meta-analysis indicates that the use of an antibiotic envelope at the time of CIED implantation leads to a significant reduction in major device-related infections compared to control, specifically among patients at higher risk for device-related infections. Additionally, to derive the greatest benefit of the antibiotic envelope, the decision to use it at the time of CIED implantation should be individualized based on the predetermined risk for infection in each patient.
Author contributions
AA and SA conceived the study. AA and SA screened and extracted data from relevant studies. MA and MS overlooked the search and extraction of data procedure. AA performed the analysis and wrote the first draft of the paper. All authors contributed with regards to interpretation of results. The final draft was critically revised and approved by all authors.
Footnotes
Abbreviations: CIED = cardiac implantable electronic device, CRT-D = cardiac resynchronization therapy-defibrillator, CRT-P = cardiac resynchronization therapy- Pacemaker, ICD = implantable cardioverter defibrillator, PPM = permanent pacemaker.
How to cite this article: Asbeutah AA, Salem MH, Asbeutah SA, Abu-Assi MA. The role of an antibiotic envelope in the prevention of major cardiac implantable electronic device infections: a systematic review and meta-analysis. Medicine. 2020;99:26(e20834).
The authors have no funding and conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009-a world society of arrhythmias project. Pacing Clin Electrophysiol 2011;34:1013–27. [DOI] [PubMed] [Google Scholar]
- [2].Da Costa A, Kirkorian G, Cucherat M, et al. Antibiotic prophylaxis for permanent pacemaker implantation. Circulation 1998;97:1796–801. [DOI] [PubMed] [Google Scholar]
- [3].De Oliveira JC, Martinelli M, Nishioka SAD, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators. Circ Arrhythm Electrophysiol 2009;2:29–34. [DOI] [PubMed] [Google Scholar]
- [4].Cabell CH, Heidenreich PA, Chu VH, et al. Increasing rates of cardiac device infections among medicare beneficiaries: 1990-1999. Am Heart J 2004;147:582–6. [DOI] [PubMed] [Google Scholar]
- [5].Voigt A, Shalaby A, Saba S. Rising rates of cardiac rhythm management device infections in the United States: 1996 through 2003. J Am Coll Cardiol 2006;48:590–1. [DOI] [PubMed] [Google Scholar]
- [6].Voigt A, Shalaby A, Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: Temporal trends and causative insights. Pacing Clin Electrophysiol 2010;33:414–9. [DOI] [PubMed] [Google Scholar]
- [7].Greenspon AJ, Patel JD, Lau E, et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States. J Am Coll Cardiol 2011;58:1001–6. [DOI] [PubMed] [Google Scholar]
- [8].Kay G, Eby EL, Brown B, et al. Cost-effectiveness of TYRX absorbable antibacterial envelope for prevention of cardiovascular implantable electronic device infection. J Med Econ 2018;21:294–300. [DOI] [PubMed] [Google Scholar]
- [9].Kolek MJ, Dresen WF, Wells QS, et al. Use of an antibacterial envelope is associated with reduced cardiac implantable electronic device infections in high-risk patients. Pacing Clin Electrophysiol 2013;36:354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tarakji KG, Wilkoff BL. Management of cardiac implantable electronic device infections: the challenges of understanding the scope of the problem and its associated mortality. Expert Rev Cardiovasc Ther 2013;11:607–16. [DOI] [PubMed] [Google Scholar]
- [11].Shariff N, Eby E, Adelstein E, et al. Health and economic outcomes associated with use of an antimicrobial envelope as a standard of care for cardiac implantable electronic device implantation. J Cardiovasc Electrophysiol 2015;26:783–9. [DOI] [PubMed] [Google Scholar]
- [12].Bloom HL, Constantin L, Dan D, et al. Implantation success and infection in cardiovascular implantable electronic device procedures utilizing an antibacterial envelope. Pacing Clin Electrophysiol 2010;34:133–42. [DOI] [PubMed] [Google Scholar]
- [13].Henrikson CA, Sohail MR, Acosta H, et al. Antibacterial envelope is associated with low infection rates after implantable cardioverter-defibrillator and cardiac resynchronization therapy device replacement. JACC Clin Electrophysiol 2017;3:1158–67. [DOI] [PubMed] [Google Scholar]
- [14].Hassoun A, Thottacherry E, Raja M, et al. Retrospective comparative analysis of cardiovascular implantable electronic device infections with and without the use of antibacterial envelopes. J Hosp Infect 2017;95:286–91. [DOI] [PubMed] [Google Scholar]
- [15].Kolek MJ, Patel NJ, Clair WK, et al. Efficacy of a bio-absorbable antibacterial envelope to prevent cardiac implantable electronic device infections in high-risk subjects. J Cardiovasc Electrophysiol 2015;26:1111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mittal S, Shaw R, Michel K, et al. Cardiac implantable electronic device infections: incidence, risk factors, and the effect of the AigisRx antibacterial envelope. Heart Rhythm 2014;11:595–601. [DOI] [PubMed] [Google Scholar]
- [17].Tarakji KG, Mittal S, Kennergren C, et al. Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med 2019;380:1895–905. [DOI] [PubMed] [Google Scholar]
- [18].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [19].Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaborations tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ 2007;176:1091–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ali S, Kanjwal Y, Bruhl SR, et al. A meta-analysis of antibacterial envelope use in prevention of cardiovascular implantable electronic device infection. Ther Adv Infect Dis 2017;4:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kusumoto FM, Schoenfeld MH, Wilkoff BL, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017;14:e503–51. [DOI] [PubMed] [Google Scholar]
- [24].Pranata R, Tondas AE, Vania R, et al. Antibiotic envelope is associated with reduction in cardiac implantable electronic devices infections especially for high-power device-Systematic review and meta-analysis. J Arrhythm 2019;36:166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]


